Medicament composition for improving stability of Ulinastatin
A technology of ulinastatin and composition, applied in the field of pharmaceutical composition, capable of solving problems such as easy inactivation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1: the preparation of ulinastatin freeze-dried powder injection
[0024] Prescriptions 1-9 are as follows:
[0025]
(10,000 units) Mannitol
(g) Hydrolyzed Ming
Glue (g) Dextrose
(g) Ulinastatin: excipients (million
Unit: g) Prescription 1 10000 5 —— —— 0.05 10:0.005 Prescription 2 10000 5 5 —— 0.05 10:0.01 Prescription 3 10000 5 5 5 0.05 10:0.015 Prescription 4 10000 15 —— —— 0.1 10:0.015 Prescription 5 10000 15 10 —— 0.1 10:0.025 Prescription 6 10000 15 —— 10 0.1 10:0.025 Prescription 7 10000 15 10 5 0.09 10:0.03 Prescription 8 10000 15 10 10 0.08 10:0.035 Prescription 9 10000 0 0 0 0 10:0
[0026] Preparation:
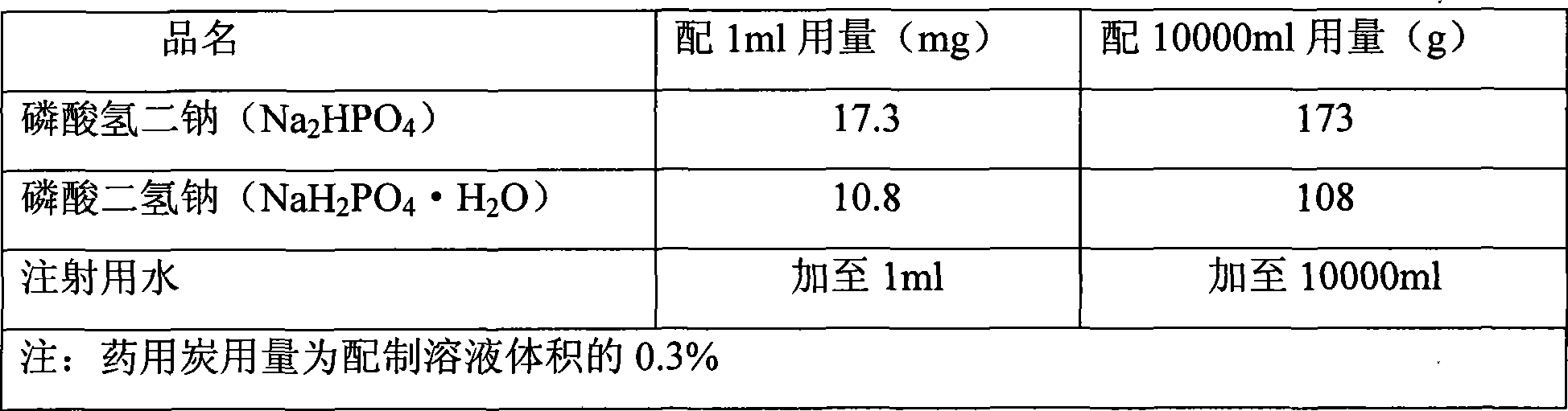
[0027] (1) pH7.0 phosphate buffer preparation:
[0028] 0.2mol / L phosphate buffer solution, pH7.0, the preparation method is as follows:
[0029] Benchmark prepar...
Embodiment 2
[0039] Embodiment 2: Stability test of ulinastatin in glucose infusion
[0040] Take one 100,000 unit ulinastatin freeze-dried powder injection prepared according to the method of prescription 1-9 in Example 1, pour into 100 ml of 5% glucose isotonic solution, shake well, place at 25°C, and place at 2 and 4 respectively. , 8, 12, 16, and 24 hours (h) sampling and determination of activity residual rate, the results are as follows:
[0041] Table 1 The active residual rate (%) of ulinastatin prepared by prescription 1-10 in glucose infusion
[0042] |Sampling time (h) 0 2 4 8 12 16 24
[0043] Prescription 1 100 100 99.3 98.9 96.8 94.2 93.5 Prescription 2 100 100 99.1 97.5 93.5 92.8 90.0 Prescription 3 100 100 99.2 98.6 95.7 93.6 92.3 Prescription 4 100 100 100 100 99.9 99.8 99.4 Prescription 5 100 100 99.9 98.7 97.2 96.4 95.2 Prescription 6 100 100 99.9 98.6 96.9 95.7 94.2 Pr...
Embodiment 3
[0044] Example 3 Stability Test of Ulinastatin in Sodium Chloride Infusion
[0045] Take one 100,000 unit ulinastatin freeze-dried powder injection prepared according to the method of prescription 1-9 in Example 1, pour into 100 milliliters of 0.9% sodium chloride solution and shake well, place it at 25°C, and place it at 2 and 4 respectively. , 8, 12, 16, and 24 hours (h) sampling and determination of activity residual rate, the results are as follows:
[0046] Table 2 The active residual rate (%) of Ulinastatin prepared in Examples 1-4 in large sodium chloride infusions
[0047] Sampling time (h) 0 2 4 8 12 16 24 Prescription 1 100 99.8 98.6 96.8 94.6 92.7 91.8 Prescription 2 100 99.8 98.9 97.0 95.2 93.6 92.5 Prescription 3 100 99.8 98.9 97.5 95.6 94.7 93.6 Prescription 4 100 100 100 100 99.8 99.8 99.5 Prescription 5 100 100 99.9 98.9 97.6 95.9 94.2 Prescription 6 100 100 99.8 98.7 97.9 96...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com