Application of Ulinastatin in preparing drug for curing autoimmune encephalomyelitis and pharmaceutical composition thereof
An autoimmune, ulinastatin technology, applied in the field of medicinal chemistry, can solve problems such as side effects infection, increased neuron loss, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Embodiment 1 prepares ulinastatin dry powder injection
[0013] Take 100 million units of filtered sterilized ulinastatin aqueous solution, add 20 grams of mannitol to dissolve, adjust the pH to neutral, add water for injection to 2000 ml, add sodium chloride to adjust isotonicity, filter aseptically, and pack in 1000 pieces In a vial, freeze-dried under sterile conditions, to obtain.
Embodiment 2
[0014] Embodiment 2 prepares ulinastatin injection
[0015] Take 100 million units of filtered sterilized ulinastatin aqueous solution, add 20 grams of mannitol to dissolve, adjust the pH to neutral, add water for injection to 2000 ml, add sodium chloride to adjust isotonicity, filter aseptically, and pack 1000 pieces Get it in a vial.
Embodiment 3
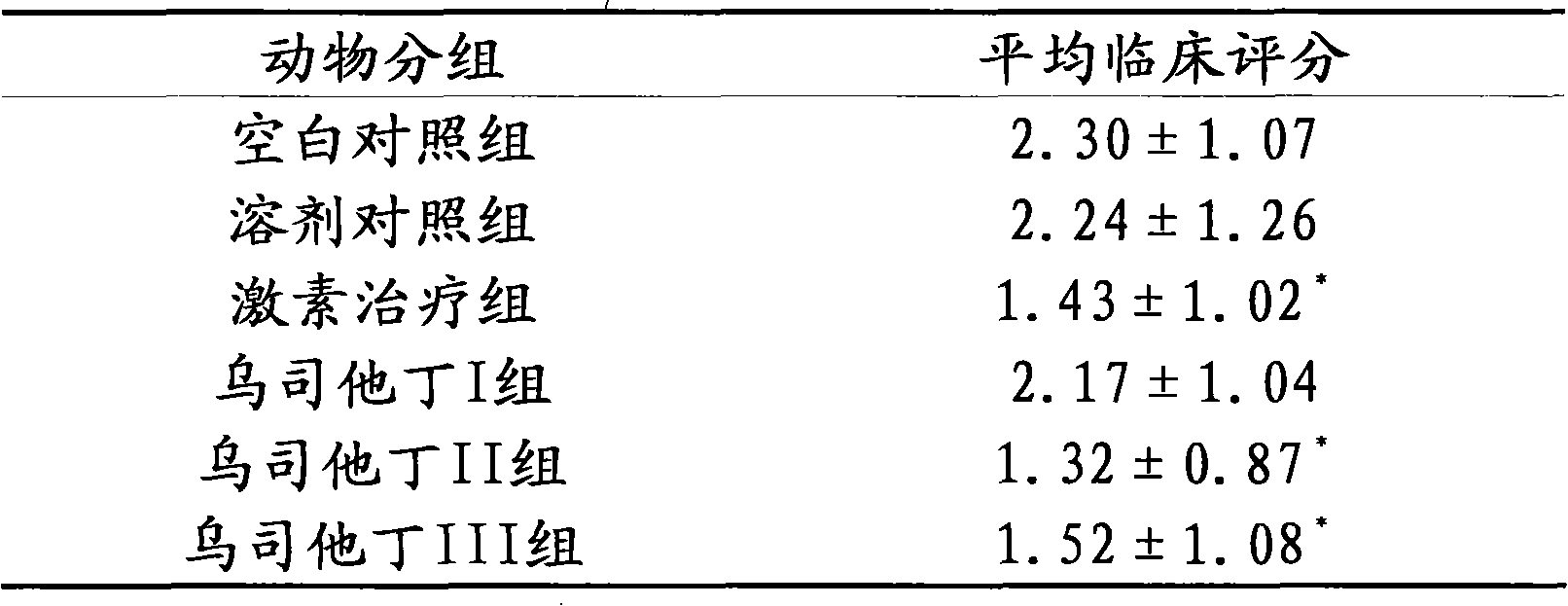
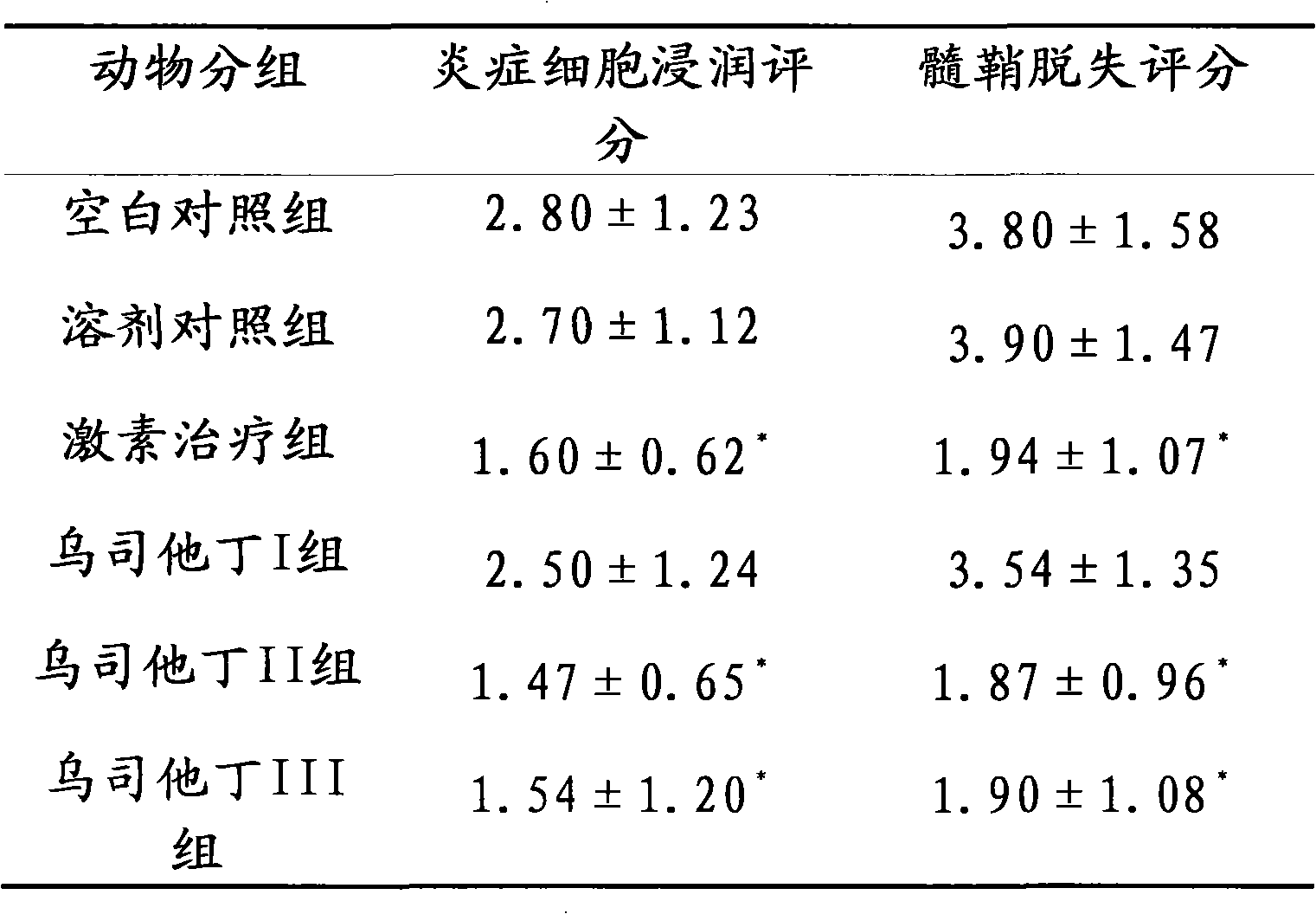
[0016] Embodiment 3 Ulinastatin is to experimental autoimmune encephalomyelitis
[0017] Therapeutic effect of (experimental autoimmune encephalomyelitis, EAE) mice
[0018] Part 1: Establishment of EAE mouse model
[0019] Take 6-8 weeks old C57BL / 6 female mice, weighing (18-20) g, first prepare the following preparations:
[0020] Solution A: MOG35-55, 0.2 mg per mouse, dissolved in sterile endoin-free PBS.
[0021] Solution B: tuberculin H37RA, 0.4 mg per mouse, dissolved in complete Freund's adjuvant containing tuberculin H37RA.
[0022] Liquid A is emulsified in liquid B with an emulsifier for more than 30 minutes, and fully emulsified to make MOG emulsion.
[0023] After the mouse skin was disinfected, the emulsion was injected subcutaneously at multiple points in the lower abdomen on both sides. On day 0 and 48 hours, each mouse was injected with pertussis toxin, 0.4 μg (dissolved in 0.1 ml of PBS), and on day 7, an equal amount of MOG emulsion was subcutaneously in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com