Patents
Literature
68 results about "Mouse skin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Self-healing hydrogel capable of promoting wound healing and cancer therapy and preparation method thereof
ActiveCN108434091AGood biocompatibilityImprove antibacterial propertiesEnergy modified materialsAerosol deliveryBenzaldehydeSelf-healing hydrogels
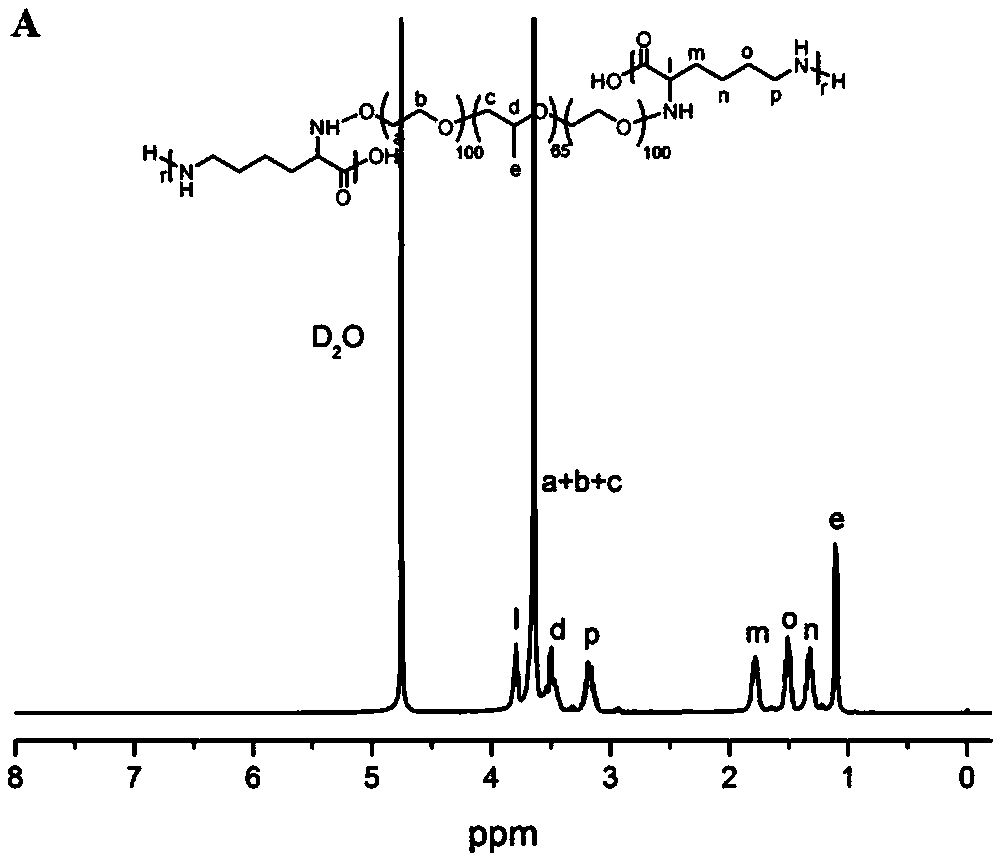
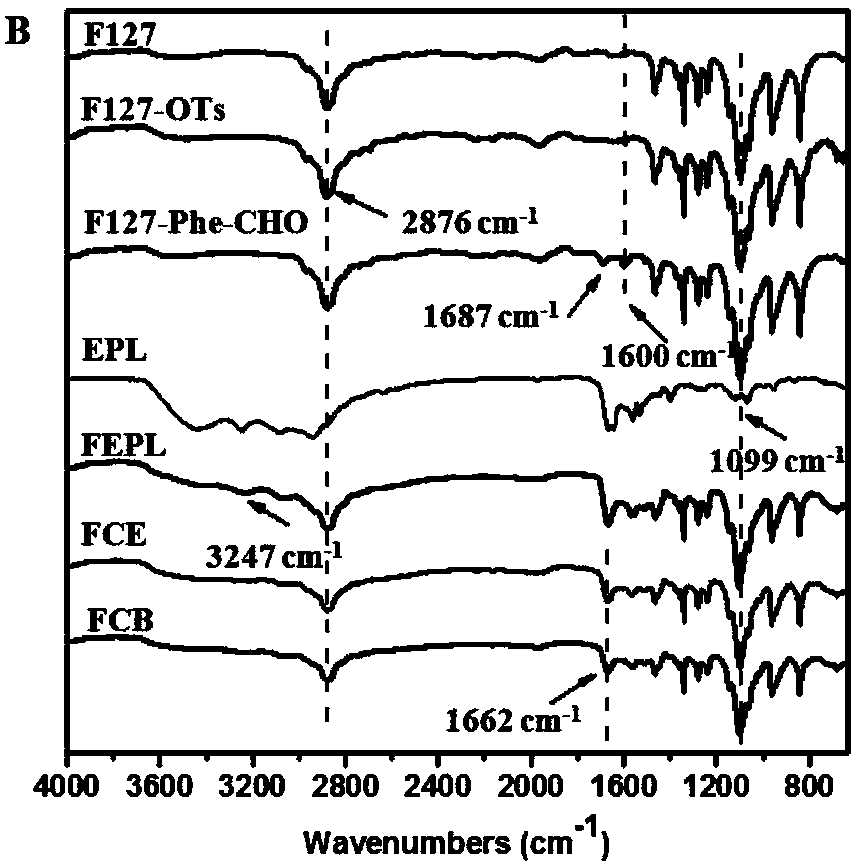
The invention discloses self-healing hydrogel capable of promoting wound healing and cancer therapy and preparation method thereof. The self-healing hydrogel is FCB hydrogel which is prepared by Schiff base reaction among a polyether-cationic polymer water solution with a mass concentration as 8% to 50%, a polyether-p-hydroxy benzaldehyde water solution with a mass concentration as 10% to 40% anda dopamine modified biological active inorganic material water solution with a mass concentration as 2% to 10%, the water solutions are mixed according to a volume ratio of (22 to 28) to (7 to 10) to(4 to 10), the F is polyether-cationic polymer, the C is polyether-p-hydroxy benzaldehyde, and the B is a dopamine modified biological active inorganic material. The self-healing hydrogel has good biological compatibility, better photo-thermal character and stronger antibacterial property; under laser radiation, the self-healing hydrogel can effectively inhibit animal cancer growth; furthermore, the self-healing hydrogel can promote mouse skin damage repair. The preparation method has the advantages of simpleness, no organic solvent residue, environment-friendly synthesizing method, convenience in operation and low raw material cost.
Owner:XI AN JIAOTONG UNIV
Chinese medicine extracting liquid eye plaster and preparation thereof
ActiveCN101264131ARelieve eye fatigueVisual fatigue hasSenses disorderHydroxy compound active ingredientsAcute toxicity testingIrritation
The invention relates to eye mask for healthcare product for eyes and the preparation method, which is characterized in that: the eye mask comprises non woven fabric adding medical solution; following raw material of weight are added into each 1000 ml of medical liquor: ginsenoside 2 to 3g, lucid ganoderma and spores powder 0.08 to 0.12g, pearl 2 to 3g, puerarin 0.4 to 0.6g, wild chrysanthemum flower 18 to 25g, mint 15 to 18g, musk 0.06 to 0.08g and borneol 0.2 to 0.6g. Through test of Health Supervision Test Center of Jilin Province: acute toxicity experiment of mouse skin shows that the eye mask does not have toxin; acute eye irritation experiment of rabbit shows the does not have irritation; test of 40 tested volunteers with fatigable eyesight shows that the eye mask has the function of relieving fatigue of eyesight.
Owner:力神药业(吉林)有限公司
Organogenesis from dissociated cells
A method assay for rapidly and reproducibly generating hair follicles from dissociated epithelial and mesenchymal cells is disclosed. The method serves both as a tool for measuring the trichogenic (i.e., hair growth-inducing) property of cells and for studying the mechanisms dissociated cells employ to assemble an organ. In a method of this application dissociated cells, isolated from newborn mouse skin, are injected into adult mouse truncal skin, hair follicles develop. This process involves the aggregation of epithelial cells to form clusters which are sculpted by apoptosis to generate “infundibular cysts”. From the “infundibular cysts” hair germs form followed by follicular buds and then pegs which grow asymmetrically to differentiate into cycling mature pilosebaceous structures. Using various techniques, exposure of the “infundibular cysts” by puncturing, piercing, or scratching the skin and, in an approach, covering the exposed cysts with a wound dressing material produced egressing hair follicles.
Owner:ADERANS RES INST
Paeonol niosome emulsifiable paste for external use and preparation method thereof
ActiveCN105748436AImprove permeabilityBoost rateAerosol deliveryOintment deliveryAllergic dermatitisBenzoic acid
The invention relates to paeonol niosome emulsifiable paste. The paste is prepared by the following steps: paeonol, a nonionic surfactant, cholesterol and the like are used for preparing a niosome suspension by a self assembly mode; the suspension, paraffin, glyceryl monostearate, vaseline, propylene glycol, ethyl p-hydroxybenzoate and other auxiliary materials are used for preparing a niosome emulsion; the paste can be partially applied to skin, and can treat allergic dermatitis and eczema with skin whitening effects. The prepared niosomes have round forms, uniform particle size distribution, and high encapsulation efficiency. The paeonol niosome emulsifiable paste has good uniformity and stable properties, and in vitro rat and mouse skin experiment results prove that intracutaneous retention volume of medicaments is substantially increased with slow releasing effects. The partially applied position of the paeonol niosome emulsifiable paste has a high medicament utilization rate and substantial medicament reservoir slow releasing effects, and curative effects are improved.
Owner:HEFEI LIFEON PHARMA
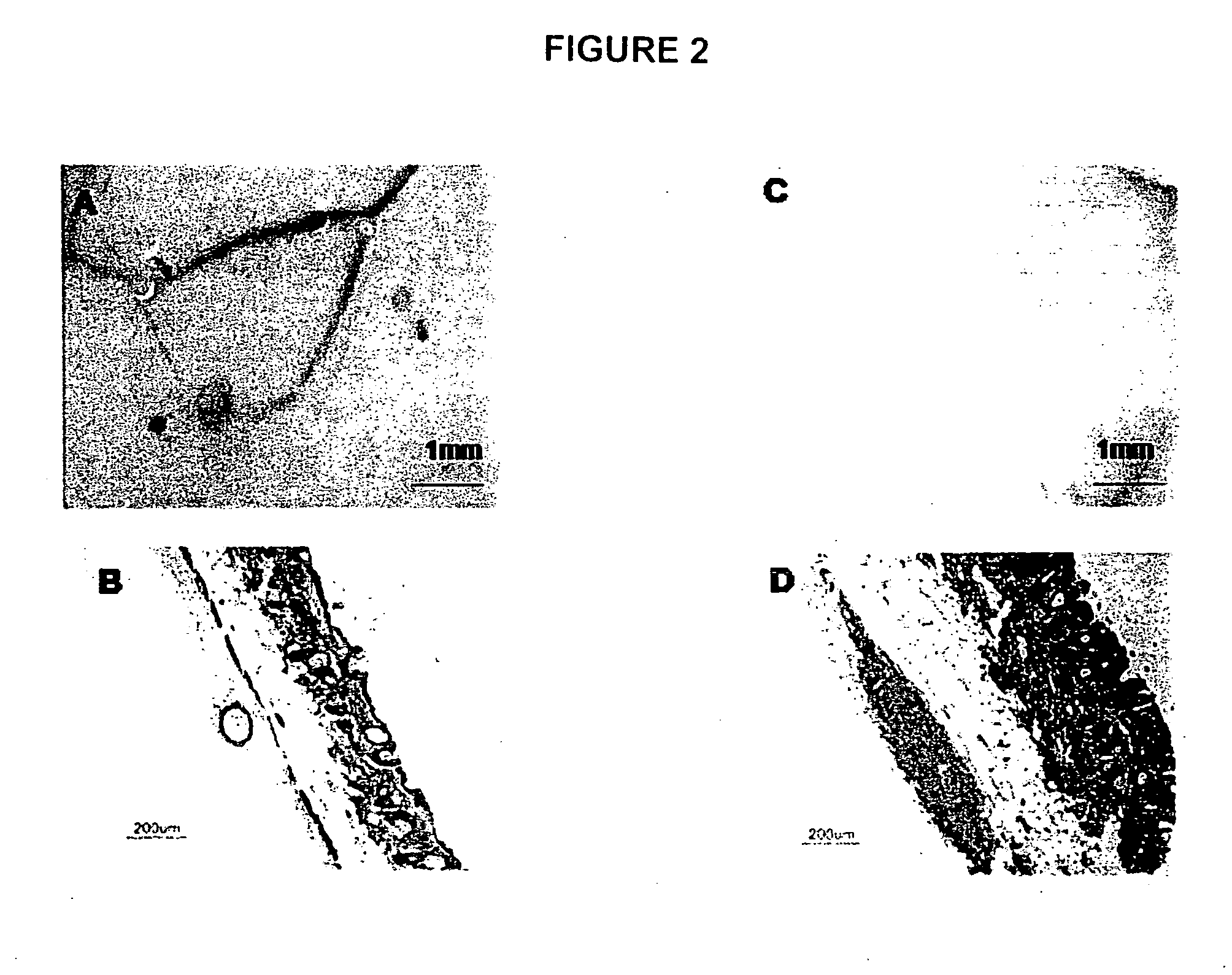
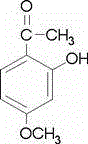
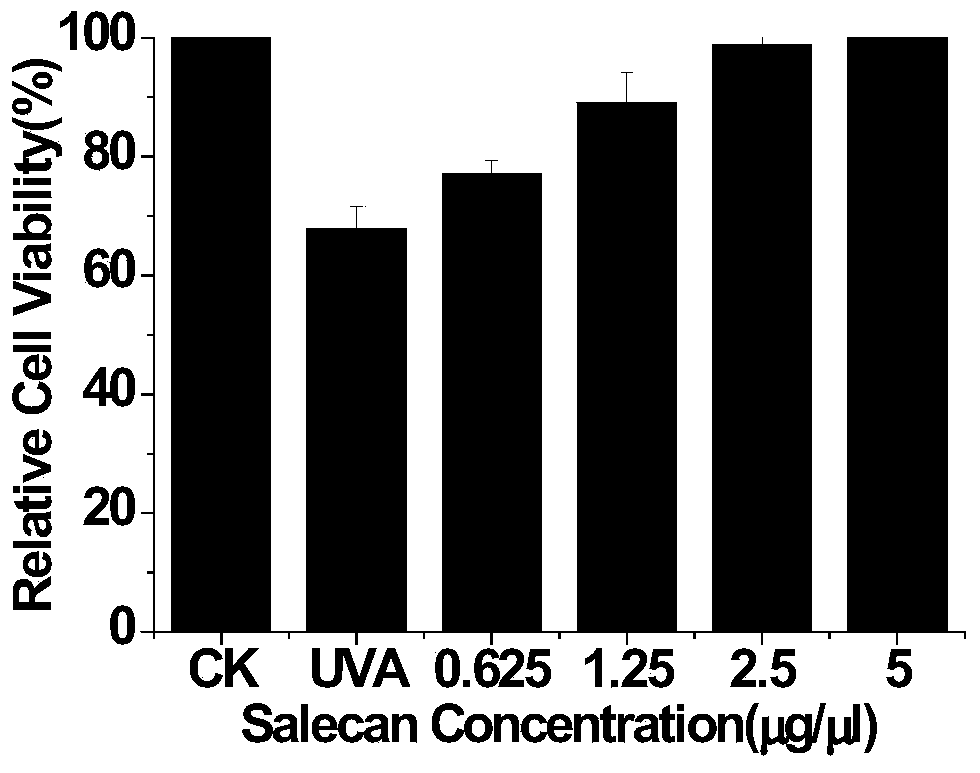
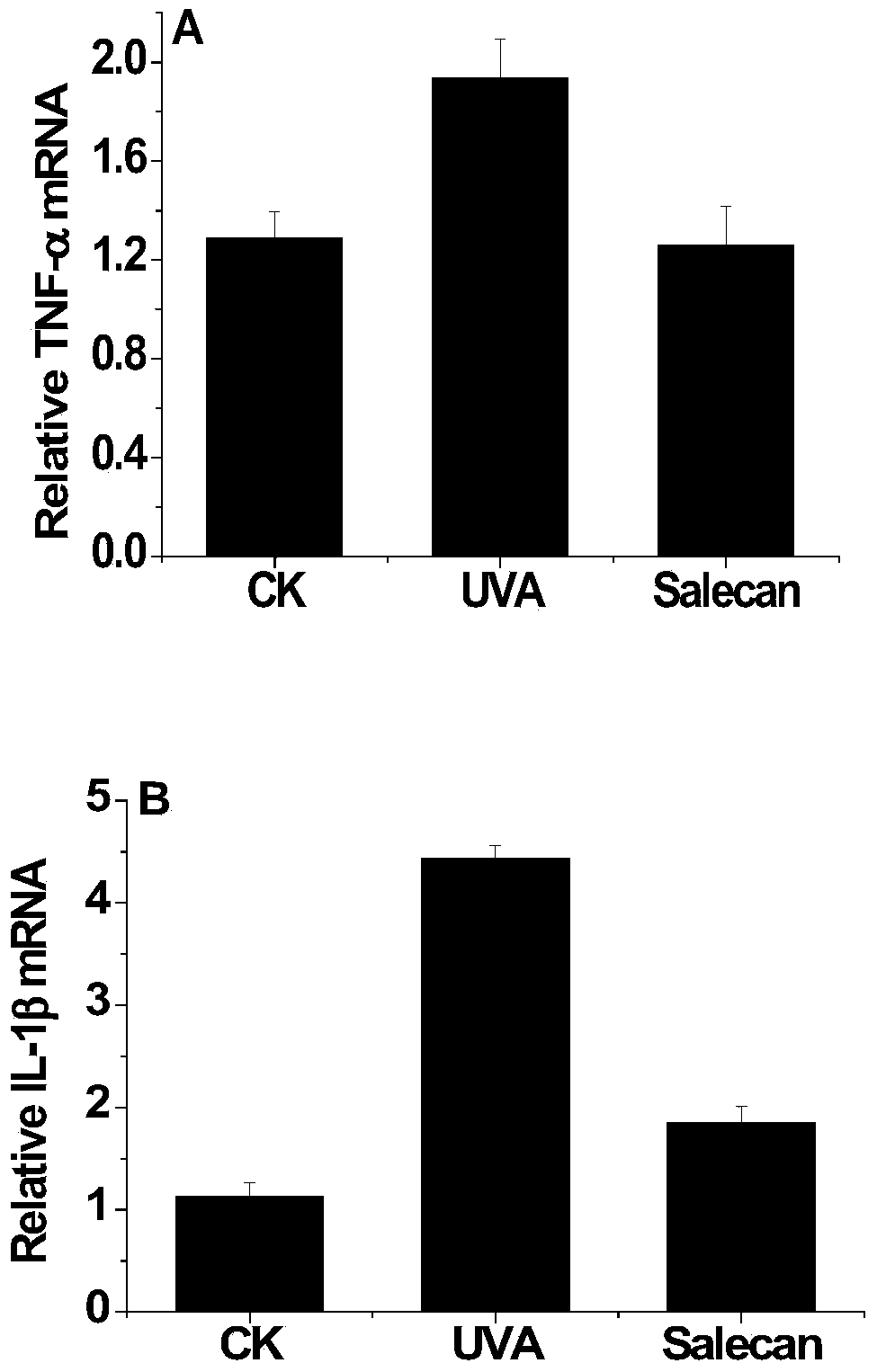
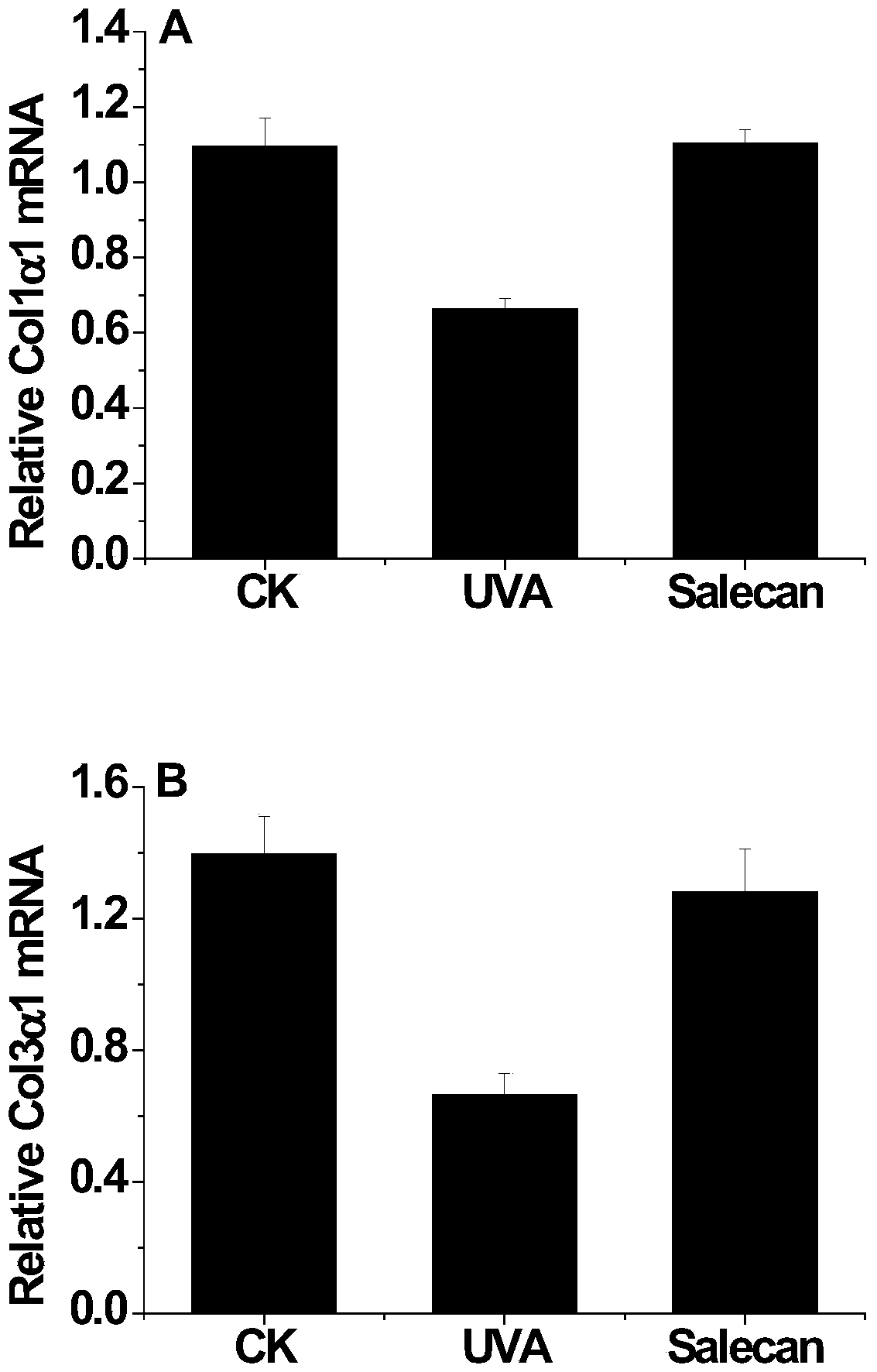
Application of soracan gum in inhibiting skin injury of ultraviolet ray
InactiveCN103735566ASimple extraction methodGood UV resistanceOrganic active ingredientsCosmetic preparationsUltravioletUltraviolet A Radiation
The invention discloses application of soracan gum in inhibiting skin injury of ultraviolet ray, and relates to application of soracan gum with different mass concentrations in protecting skin from injury due to ultraviolet ray UVA. A cell model adopted in the invention is an ultraviolet ray UVA induced cell injury model, wherein the dosage of soracan gum is 0.625-5 mu g / mu l; a mouse model adopted in the invention is an ultraviolet ray UVA induced mouse skin injury model, and the dosage of the soracan gum is 200mu l of 1 percent solution, the soracan gum can be used for improving the cell survival rate of cells in ultraviolet radiation, reducing inflammation factors in mouse skin and improving gene expression of synthetic collagen, so that skin injury caused by ultraviolet ray UVA can be inhibited. Cell and animal experiments prove that soracan gum has a remarkable effect of inhibiting skin injury of ultraviolet ray, so that soracan gum can be used in processes of preventing and repairing skin injury due to ultraviolet ray UVA, and can be used for preparing anti-ultraviolet radiation injury medicines and anti-ultraviolet radiation cosmetics.
Owner:NANJING UNIV OF SCI & TECH
Construction method for mouse skin allergy animal model
InactiveCN1634598AGood reproducibilityOvercoming the need for pre-prepared antiseraIn-vivo testing preparationsAntigenSkin sensitization
The invention belongs to medical evaluation and detection technology field. It concerns in particular to the construction method of animal model for skin allergy therapeutic drug screen and skin allergy mechanism study. The skin allergic mouse model is built by injecting anti DNP IgE monoclonal antibody to BALB / c mouse caudal vein and provoking the action by making 2,4 dinitroflurobenzene as antigen. The model can imitate the skin allergy dynamic reaction whole process favorably, therefore, it can be used for the screening of skin allergy treating drug and study of skin allergy pathogenesis and drug action mechanism.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
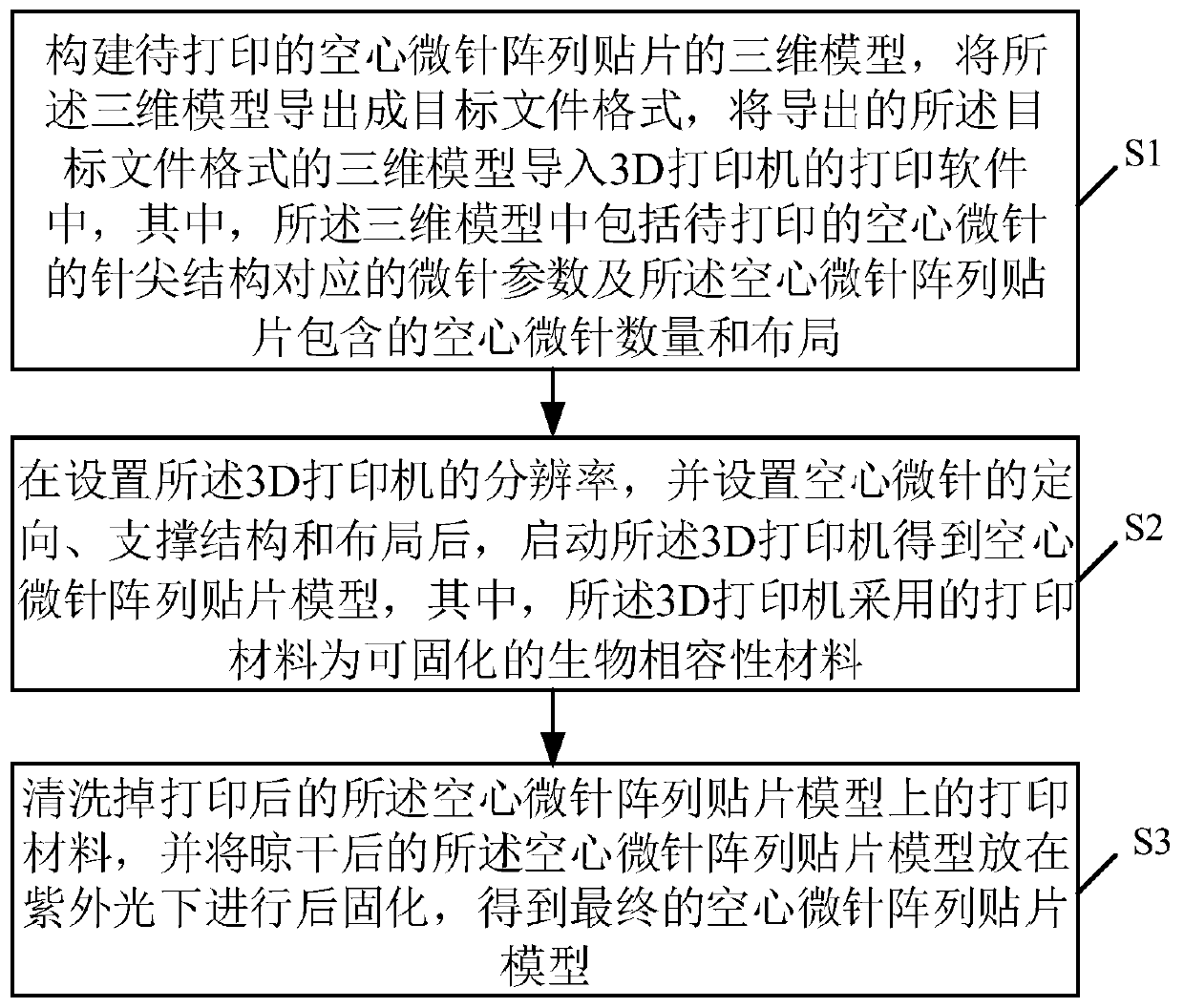
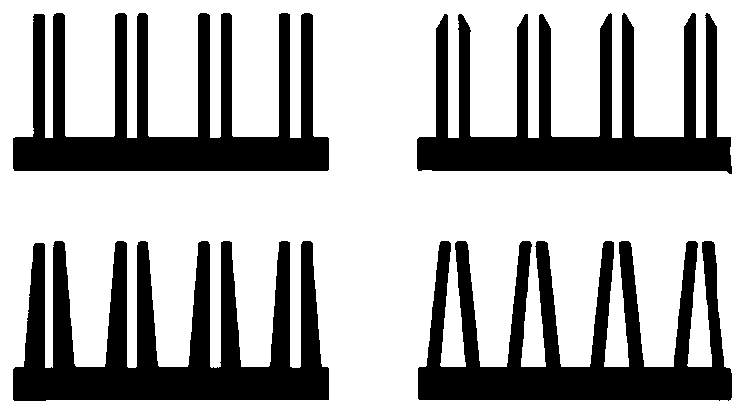
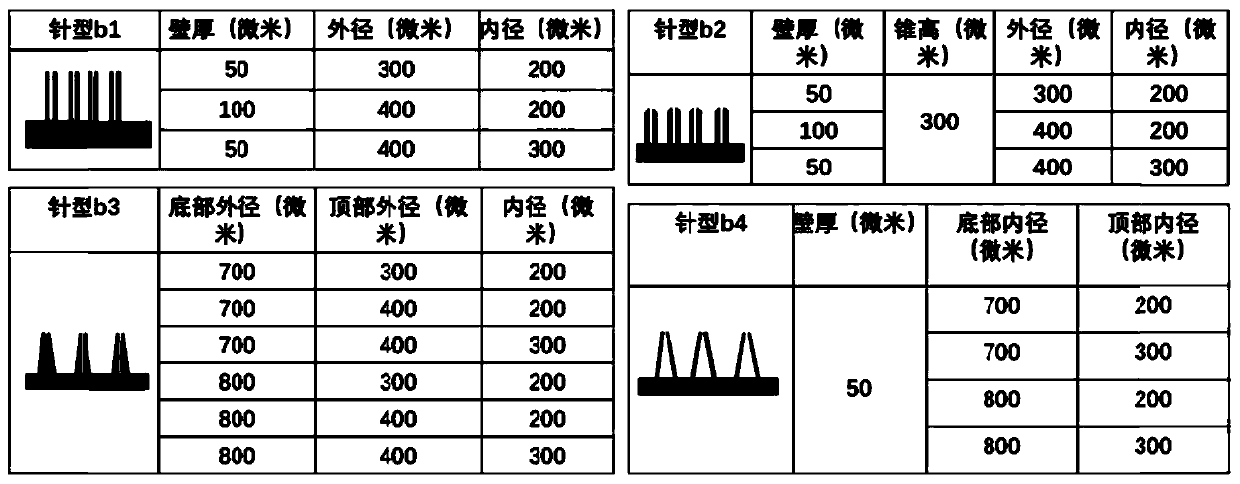
Manufacturing method and application of 3D printing hollow microneedles
InactiveCN110435139AEasy to operateImprove efficiencyAdditive manufacturing apparatusMicroneedlesInsulin infusionBiocompatibility Testing
The invention discloses a manufacturing method and application of 3D printing hollow microneedles, and belongs to the field of biological materials. Through the micro stereoscopic photocuring formingtechnology in the 3D printing technology, a light-sensitive material with good biocompatibility serves as a printing material, and a Form 2 photocuring printing machine is utilized for printing out hollow microneedle arrays of different needle point structures and different parameters. Through parameter analog computation and optimization, the hollow microneedle arrays high in mechanical performance and high in agent discharge rate are selected out to be used for experiments. Inner hole diameters of the optimized 3D printing hollow microneedle arrays can be observed under a microscopic device,and mouse skins can be punctured successfully. The 3D printing hollow microneedles, a piezoelectric micropump and the like are packaged and integrated into a minimally invasive insulin infusion system, the minimally invasive insulin infusion system is used for intelligent in-vivo blood glucose regulation, the 3D printing hollow microneedle arrays can achieve controllable insulin release under theaction of the piezoelectric micropump, and in-vivo blood glucose is successfully regulated.
Owner:WUHAN UNIV
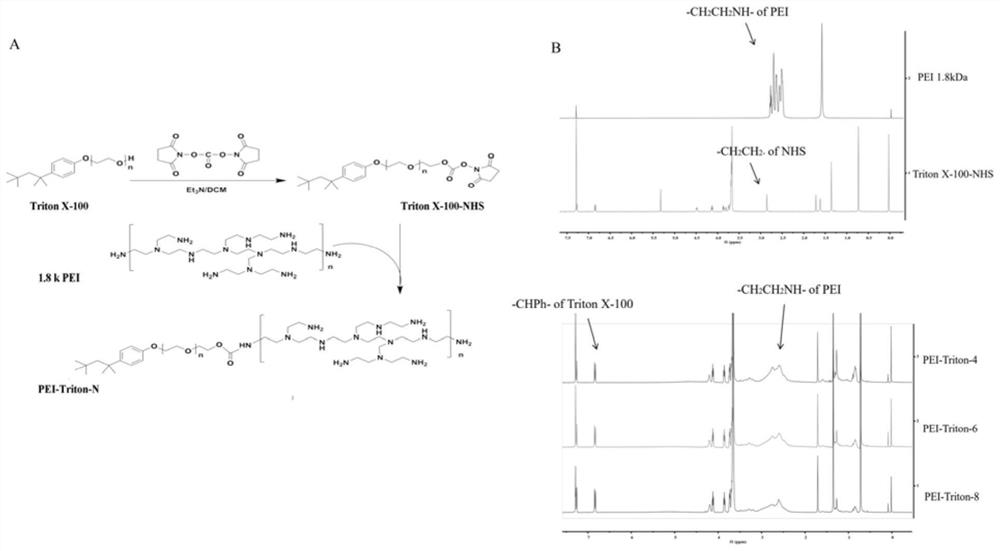
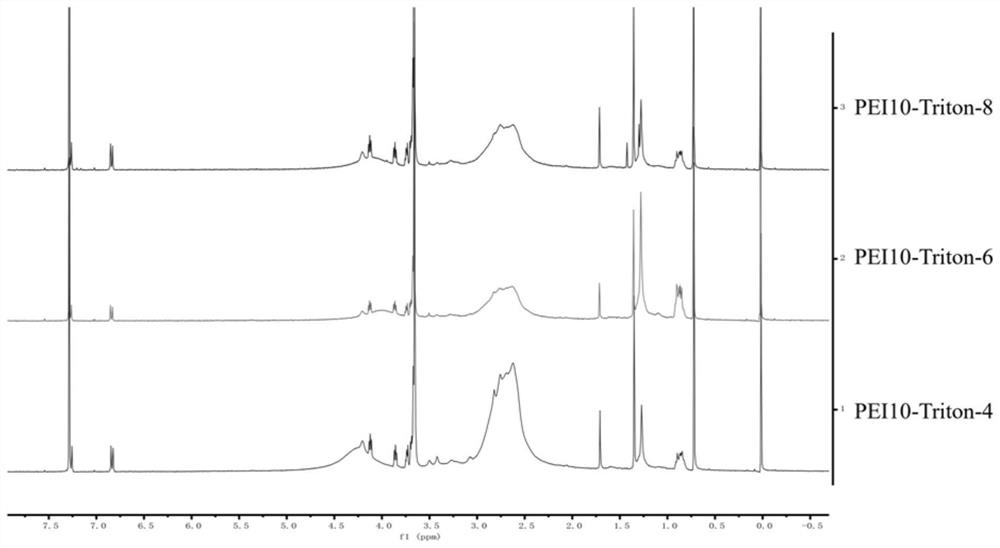
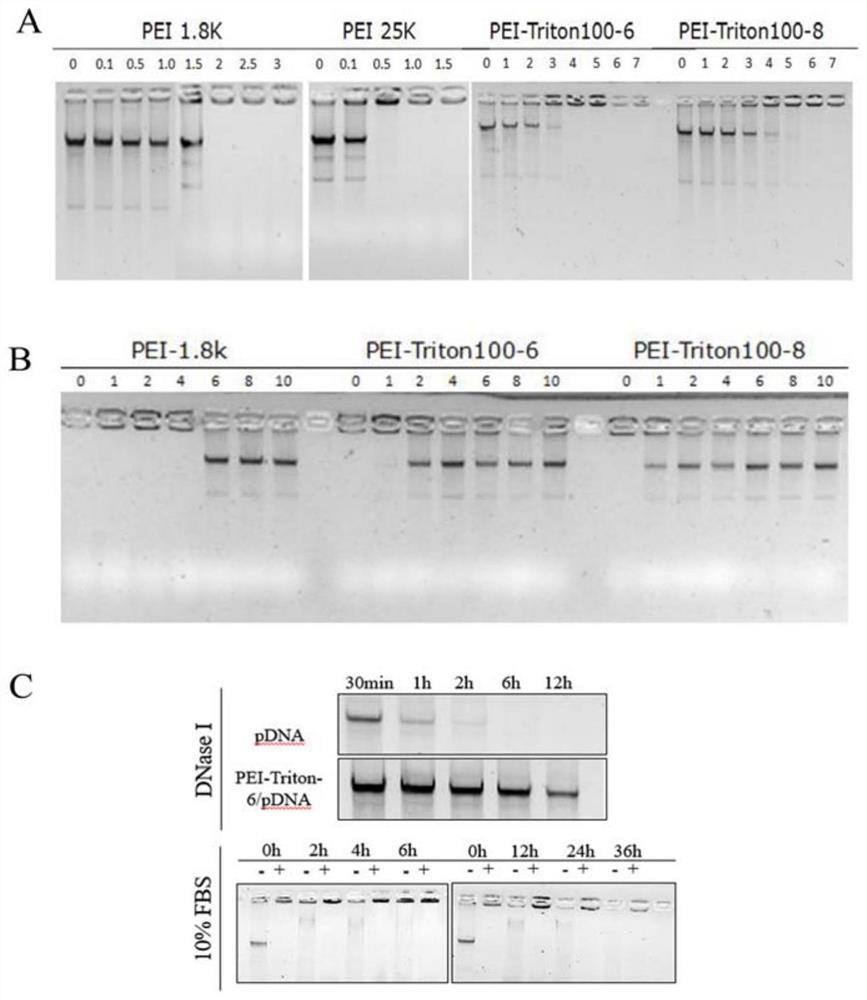
Modified polyethyleneimine derivative as well as synthesis method and application thereof
InactiveCN112142972AImprove transfection efficiencyGood gene delivery capability for local administrationPharmaceutical non-active ingredientsGene therapyCell EvaluationPerylene derivatives
The invention discloses a modified polyethyleneimine derivative as well as a synthesis method and application thereof. According to the invention, Triton X-100 is conjugated to polyethyleneimine, suchthat a PEI-Triton-N carrier is obtained. In-vitro cell evaluation shows that the carrier shows good safety and stability; cell transfection effect experiments show that the carrier shows efficient and low-toxicity gene delivery capacity, can significantly improve the gene transfection efficiency of various cells including skin immortalized cells HaCaT difficult to transfect, and is an excellent in-vitro transfection reagent; an in-vivo toxicity experiment shows that the carrier has no obvious systemic toxicity; and a gene delivery experiment result of mouse skin local administration shows that the carrier has good local administration gene delivery capability and is a good local administration gene delivery vector.
Owner:PEKING UNIV
Application of protein tyrosine phosphatase SHP2 inhibitor in preparation of medicine for treating psoriasis
ActiveCN111265529AEasy to prepareReduce the overall heightOrganic active ingredientsDermatological disorderInflammatory factorsDisease
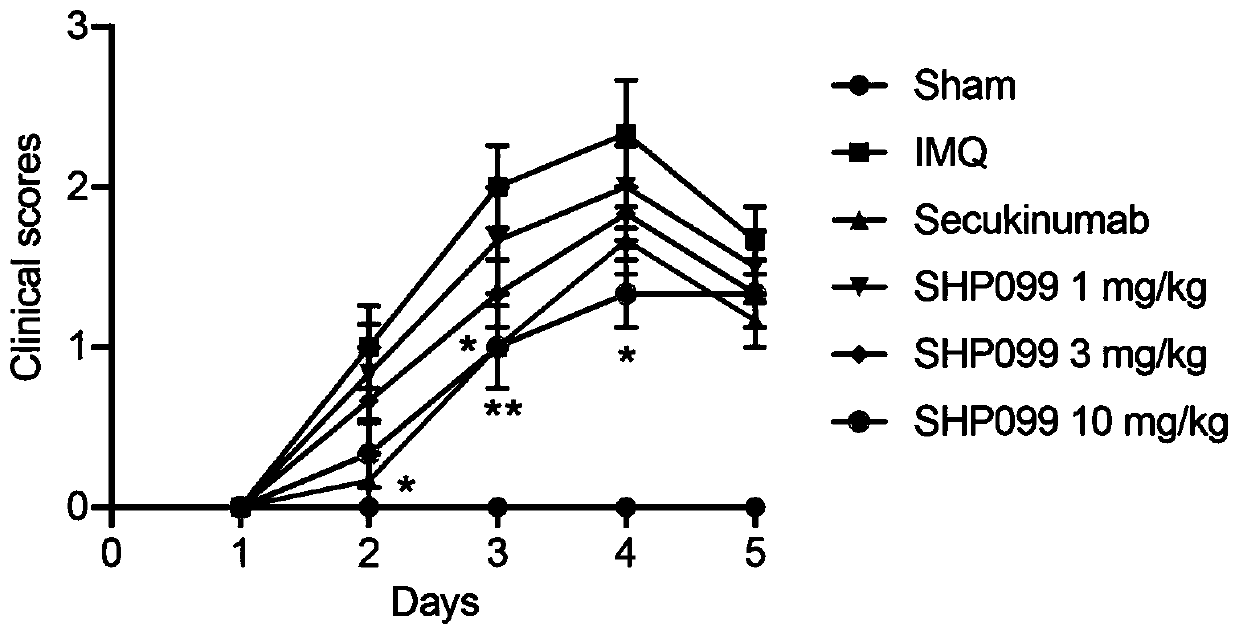
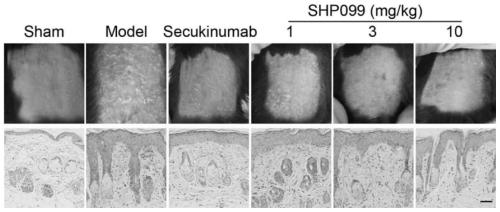
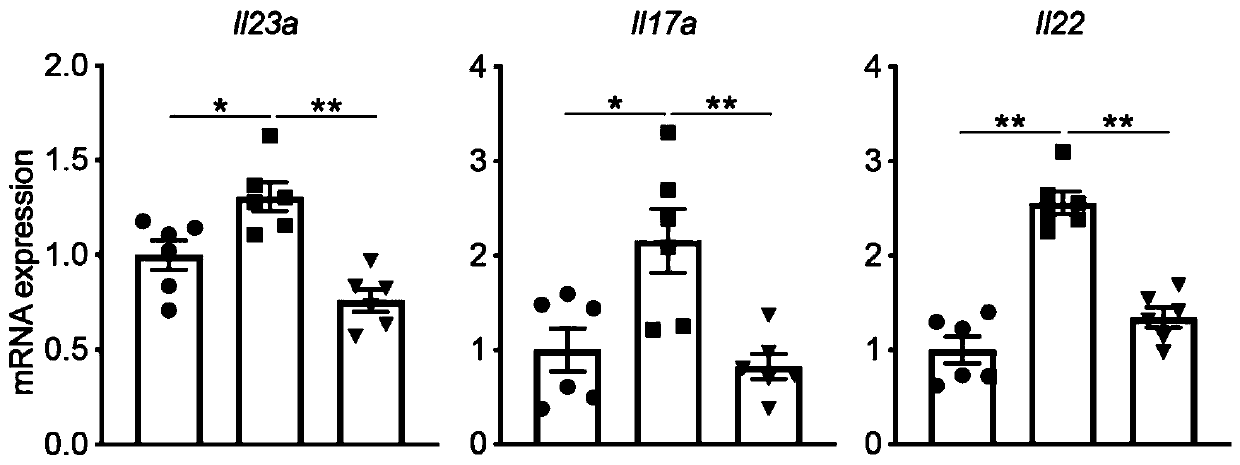
The invention belongs to the technical field of pharmacy. According to the application of a protein tyrosine phosphatase SHP2 inhibitor SHP099 in preparation of the medicine for treating psoriasis, aiming at an imiquimod-induced mouse psoriasis-like disease model, the SHP099 can effectively improve epidermal thickening of mouse skin lesion tissues, inflammatory cell infiltration and the level of inflammatory factors in mouse serum. At the cellular level, the SHP099 can significantly reduce the release of inflammatory factors related to psoriasis.
Owner:NANJING UNIV
Construction method and applications of engineering strain for delivering mammalian cell protein
InactiveCN107099495AEasy to injectEfficient injectionBacteriaMicroorganism based processesReprogrammingCytotoxicity
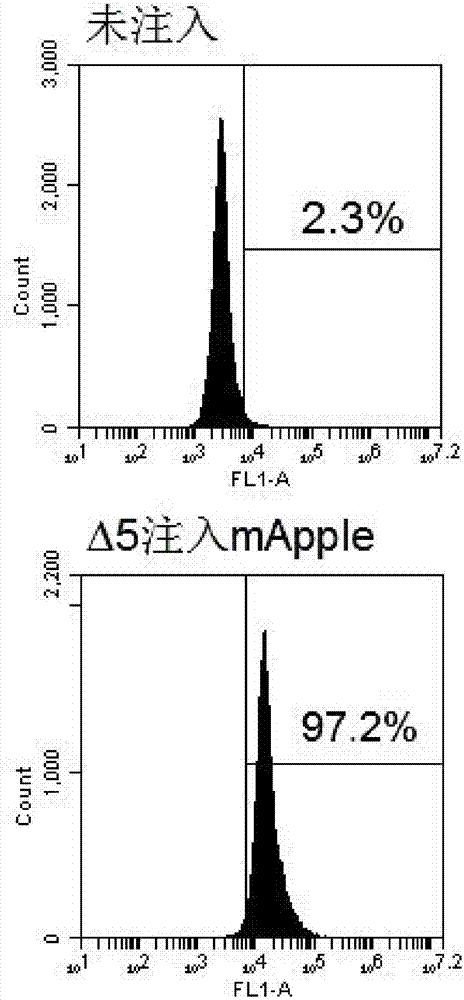
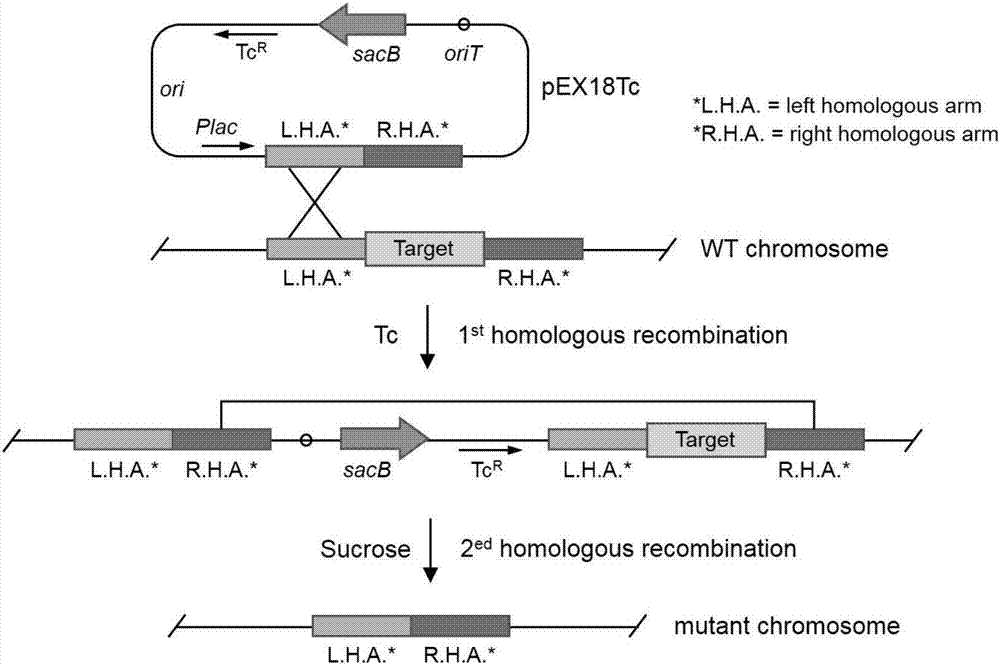
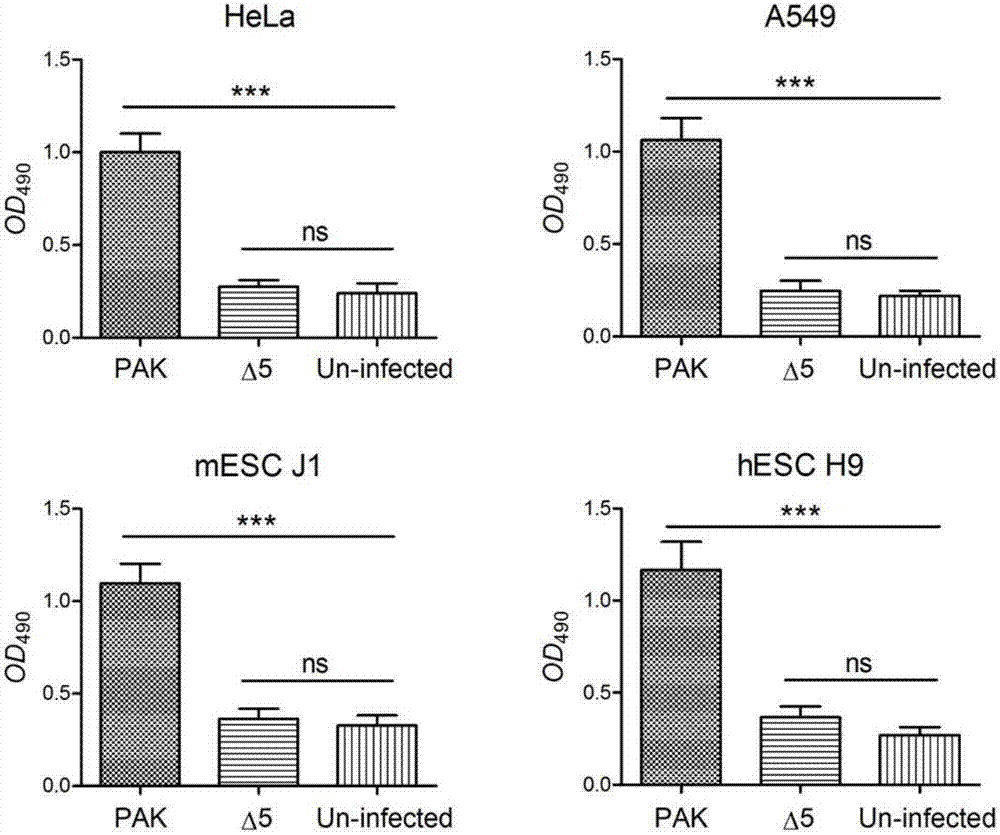
The invention relates to a construction method and applications of engineering strain for delivering mammalian cell protein. The construction method comprises the following steps: deleting four virulence factors including exoS, exoT, exoY and ndk genes from pseudomonas aeruginosa PAK strain genome, so as to have no cytotoxicity to mammalian cells; further deleting popN gene, for inhibiting bacterial III type secretory system (T3SS), from chromosome, so as to remarkably increase the injection quantity of T3SS-mediated protein, thus constructing the engineering strain delta5. The constructed engineering strain delta5 can be used for delivering proteins of various mammalian cell linings, performing gene editing, cell reprogramming, protein function research and other work. The types of delivered cells are wide, including human and mice skin cells, muscle cells, intestinal cells, liver cells, multipotent stem cells and the like.
Owner:NANKAI UNIV
Medicinal extract ointment for treating skin scald
ActiveCN102935224ASimple componentsGood treatment effectOrganic active ingredientsPeptide/protein ingredientsMedicinal herbsTreatment effect
The invention relates to a medicinal extract ointment for treating skin scald, and belongs to the technical field of pharmacy. The ointment is characterized by consisting of the following materials in a ratio: sturgeon collagen protein extract, bletilla polysaccharide extract, ginkgo leaf polysaccharide extract, stearyl alcohol, lauryl sodium sulfate, albolene, methyl hydroxybenzoate, propyl hydroxybenzoate, propylene glycol and distilled water. The prepared ointment has the functions of astringing sore, stopping bleeding, transforming stasis, dispersing swelling, eliminating putridity, engendering flesh and the like. The sturgeon collagen protein extract, the bletilla polysaccharide extract and the ginkgo leaf polysaccharide extract are used as active ingredients of the ointment, so the ointment prepared from the ingredients can promote wound healing of burns and scalds and improve the recovery rate of mouse skin injury and the treatment effect of a scald medicament, and can be used for treating skin scald; and the components of the ointment are simple, and the medicinal materials are cheap and readily available.
Owner:JIANGSU UNIV
In-vitro building method of cell photoaging model
InactiveCN103421763AWide variety of sourcesVigorousVertebrate cellsArtificial cell constructsFiberPhotoaging
The invention belongs to the field of medicine and cytobiology and relates to an in-vitro building method of a cell photoaging model. The method includes the following steps: (1), obtaining newborn mouse skin fibroblast primary cells, and using DMEM+10%FBS for culture in vitro; (2), when the mouse skin fibroblast primary cells grow to 80%, going down to the future generation, allowing the cells to adherently grow for 24 hours, performing first-time medium-wave ultraviolet irradiation, repeatedly irradiating for four times in each 12 hours, finally irradiating for 24-72 hours to obtain a mouse skin fibroblast cell photoaging model. Mouse-source cells are adopted, so that a lot of primary cells can be obtained only by one-time material taking; the defects of repeated cryopreseration and shortage of activity of cell lines are overcome; cell activity is good in in-vitro culture, so that no interference can be generated on a UVB-induced aging phenotype within limited generations; time needed for modeling is short, only two days are needed; effect is stable, and the aging phenotype can be observed for 24 hours after final irradiation.
Owner:HUADONG HOSPITAL
Method and application of large proliferation of newborn mouse skin terminally isolated cells
PendingCN108865977AMeet Restoration StudiesMeet the requirements of cell volumeCell dissociation methodsEpidermal cells/skin cellsPrimary cellDigestion
The invention belongs to the technical field of animal cell culture, and relates to a method and application of large proliferation of newborn mouse skin terminally isolated cells. The method comprises the following steps that a newborn mouse skin sample is pretreated; an enzyme digestive liquid is prepared; the sample is treated by the enzyme digestive liquid, and terminally isolated cell suspension liquid is obtained; primary cells of the newborn mouse skin terminally isolated cells are prepared; passage and expansion culture is conducted on the primary cells; passage cells are prepared; andthe skin terminally isolated cells are screened and purified by a c-kit+ / CD34+ flow cytometry, the cell sap is resuspended and subjected to expansion culture, and the skin terminally isolated cells with the high purity are prepared. The method adopts collagenase II and trypsin with the concentration being both 0.05% to separate and culture the skin terminally isolated cells by combining with a digestion method, the number of the obtained primary terminally isolated cells is large, the purity is high, the activity is good, the cells can be rapidly and largely proliferated after being screenedby the flow cytometry, and the proliferated cells are stable in morphology and high in activity, and can be widely applied to morphological detection and function research of the follow-up related terminally isolated cells.
Owner:XINXIANG MEDICAL UNIV
Mouse models to study cachexia
Embodiments of the invention provide mouse tumor models involving either the B16 mouse melanoma or MXT mouse mammary tumor, untreated or treated with chemotherapy and / or anti-cachectic agents, for the study of tumor-generated or cancer therapy-generated cachexia-inducing signals and mechanisms. Other embodiments of the invention also provide additional models, including pre-treatment of mouse skin with anti-cachectic proteins prior to tumor implantation, for the study of anti-cachectic signals and mechanisms generated by the skin. To reduce or reverse tumor- and chemotherapy-induced cachexia, embodiments of the invention use the human proteins placental alkaline phosphatase, transferrin, α1-antitrypsin preparations or combinations thereof as well as chemically synthesized CCDTHT or N,N-diethyl-N-methyl-2-[(9-oxo-9H-thioxanthen-2-yl)methoxy]-ethanaminium iodide or CCDTHT-like compounds.
Owner:ZOLTAN LAB
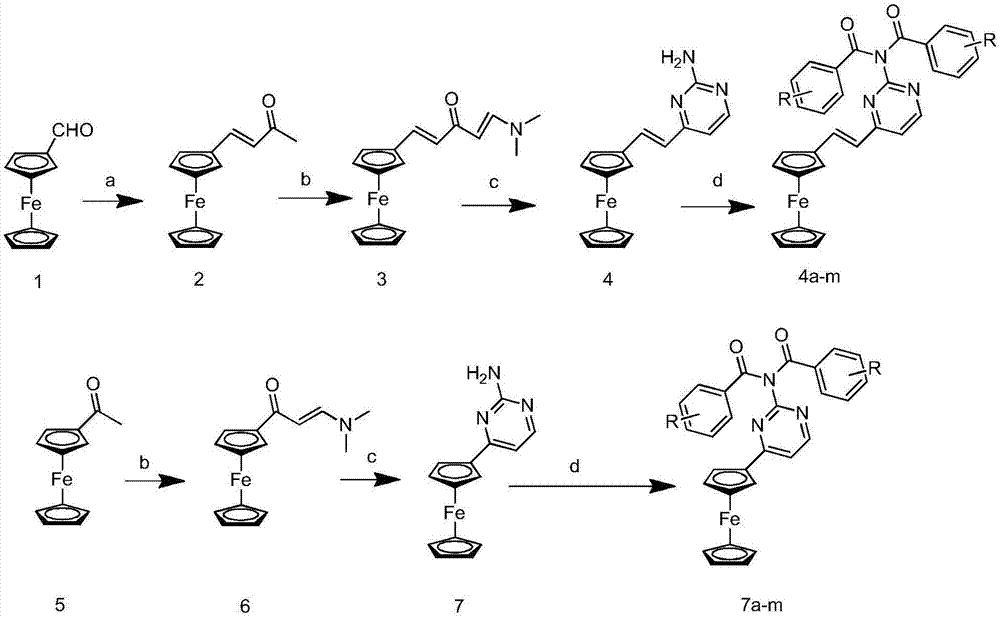
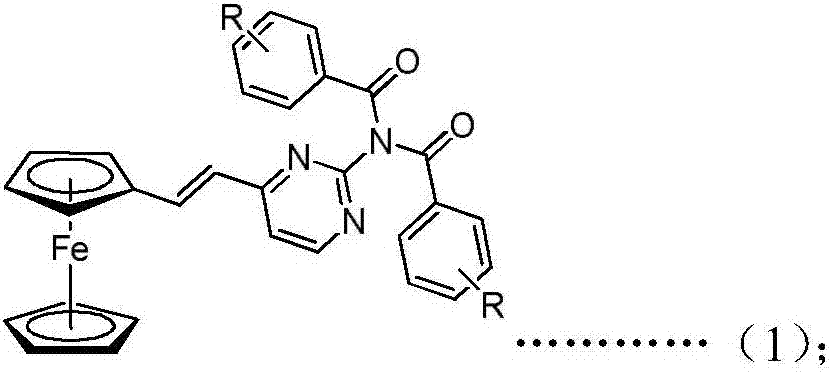
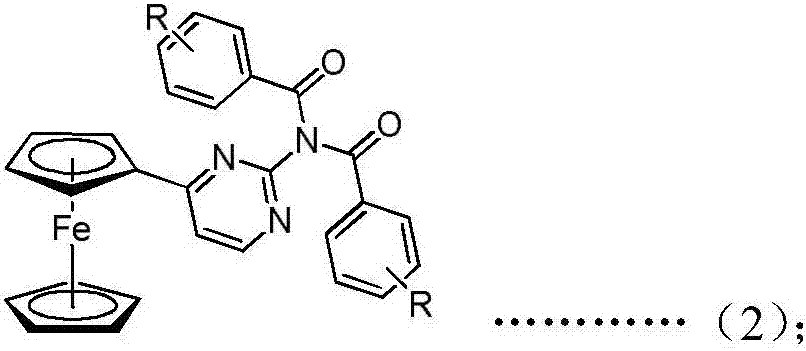
Ferrocenyl amide derivative containing pyrimidine ring and preparation method and appliation thereof
InactiveCN106905380AStrong growth inhibitory effectOrganic active ingredientsAntineoplastic agentsHydrogenBromine
The invention discloses a ferrocenyl amide derivative containing a pyrimidine ring and a preparation method and application thereof. The structure of the ferrocenyl amides derivative containing the pyrimidine ring is as shown in a general formula (1) or a general formula (2) as shown in the specification, wherein R is selected from 4-chlorine, 4-methoxyl, 4-methyl, 3-chlorine, 3,5-dimethoxy, 3,5-dichlorine, 4-ethyl, 4-chlorine, 4-bromine, 3-bromine, 3-methyl, 4-trifloromethoxyl or hydrogen. The ferrocenyl amide derivative containing the pyrimidine ring has an obvious growth inhibiting effect on mice skin melanoma cells (B16-F10) and human lung cancer cell strains (A549), and can be used for preparing antineoplastic pharmaceuticals.
Owner:HEFEI UNIV OF TECH
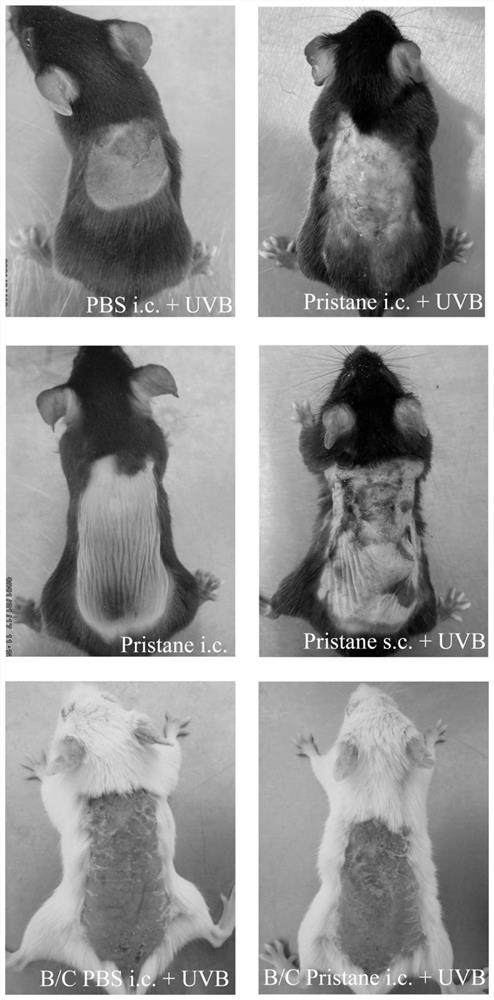
Skin type lupus erythematosus mouse model and construction method and application thereof
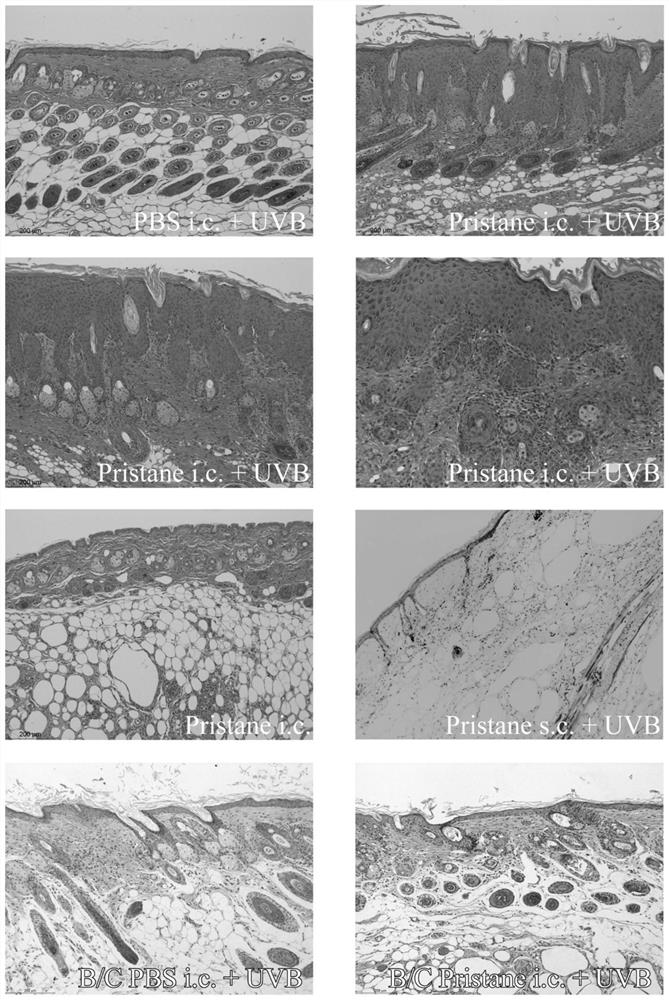
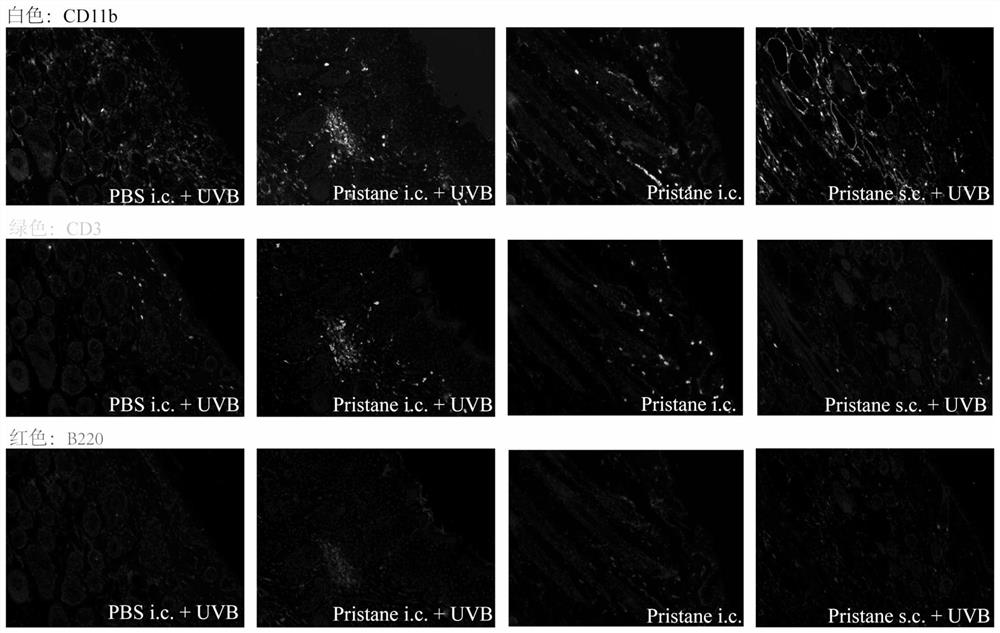
ActiveCN113412813AAccelerate the clinical transformation of scientific researchLight therapyVeterinary instrumentsDiseaseSystemic lupus erythematosus
The invention provides a skin type lupus erythematosus mouse model and a construction method and application thereof. According to the skin type lupus erythematosus mouse model constructed by the construction method, pristane is combined with ultraviolet (UVB) stimulation to construct the skin type lupus erythematosus mouse model. A C57BL / 6 mouse is continuously irradiated by a proper amount of UVB after being subjected to Pristane intradermal immune treatment. The skin type lupus erythematosus mouse model better simulates immune response generated by abnormal activation of mouse skin immune cells under UVB stimulation. The model can be used for discussing the occurrence mechanism of the disease progress of lupus erythematosus, researching and developing drugs for targeting lupus skin lesion and the like, and is of great significance in the aspect of accelerating and promoting scientific research and clinical transformation.
Owner:THE SECOND XIANGYA HOSPITAL OF CENT SOUTH UNIV
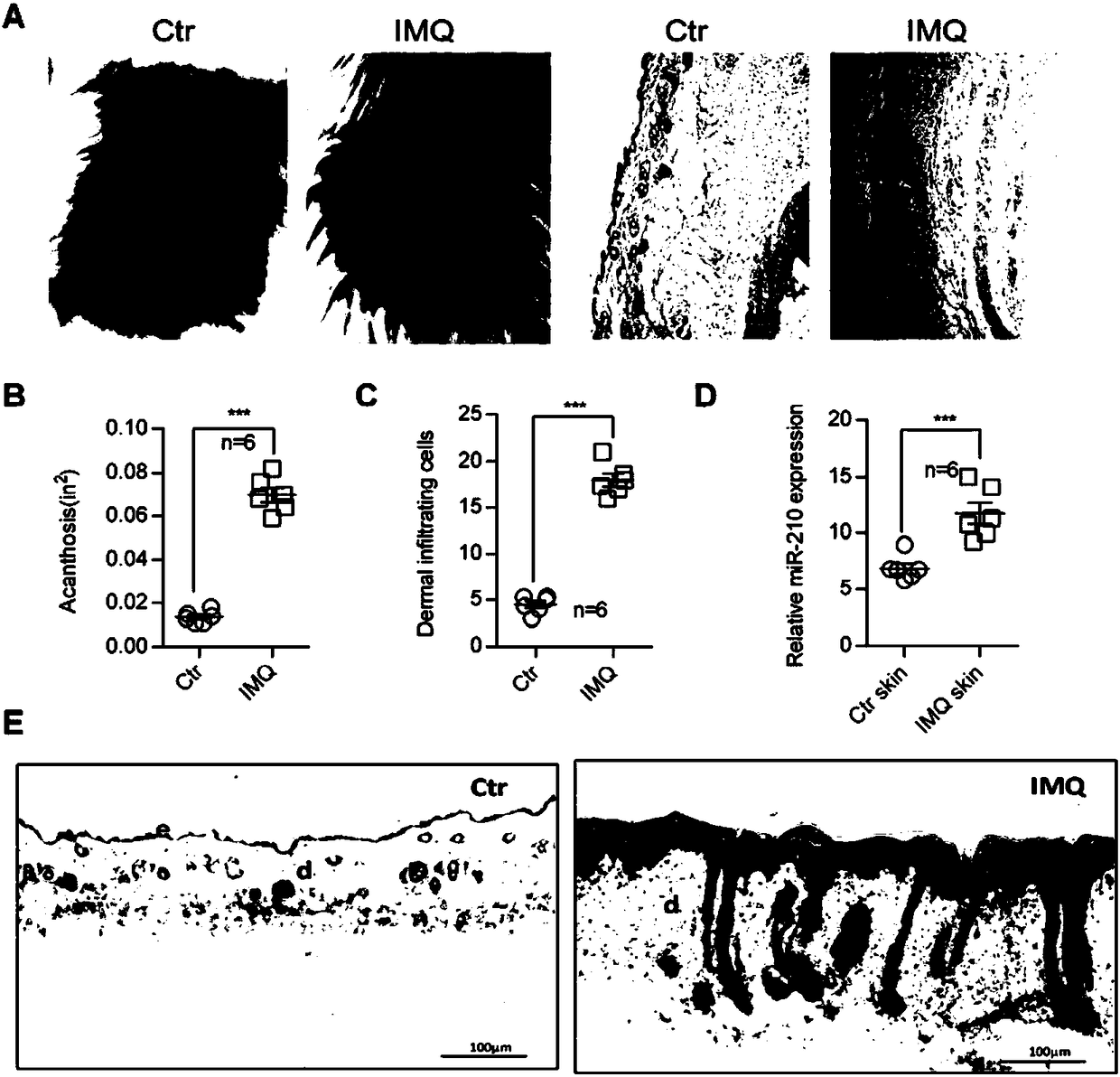
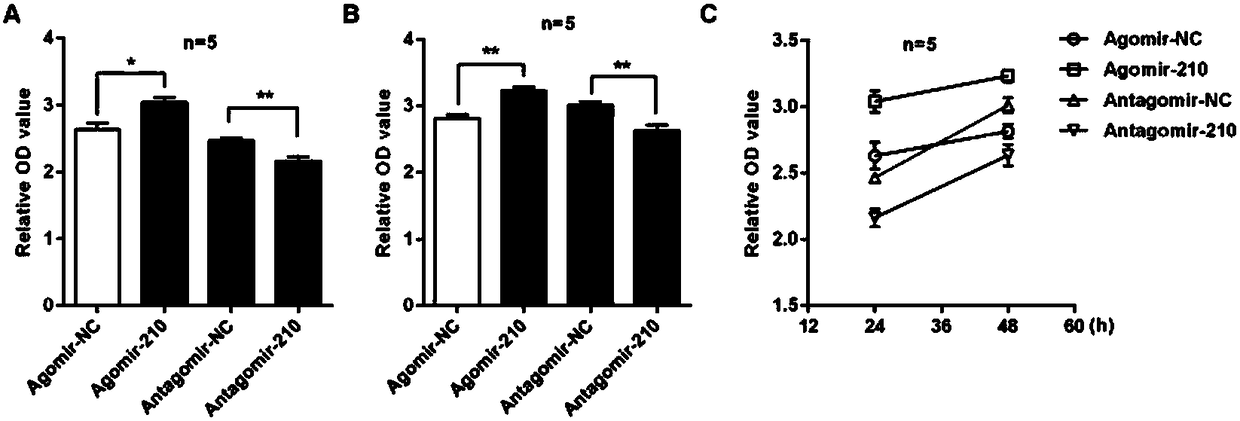
Application of microRNA-210 inhibitor in preparing medicine treating inflammatory dermatosis
ActiveCN108144061AImprove inflammationPromote infiltrationOrganic active ingredientsPolymorphism usesCholesterolInflammatory dermatosis
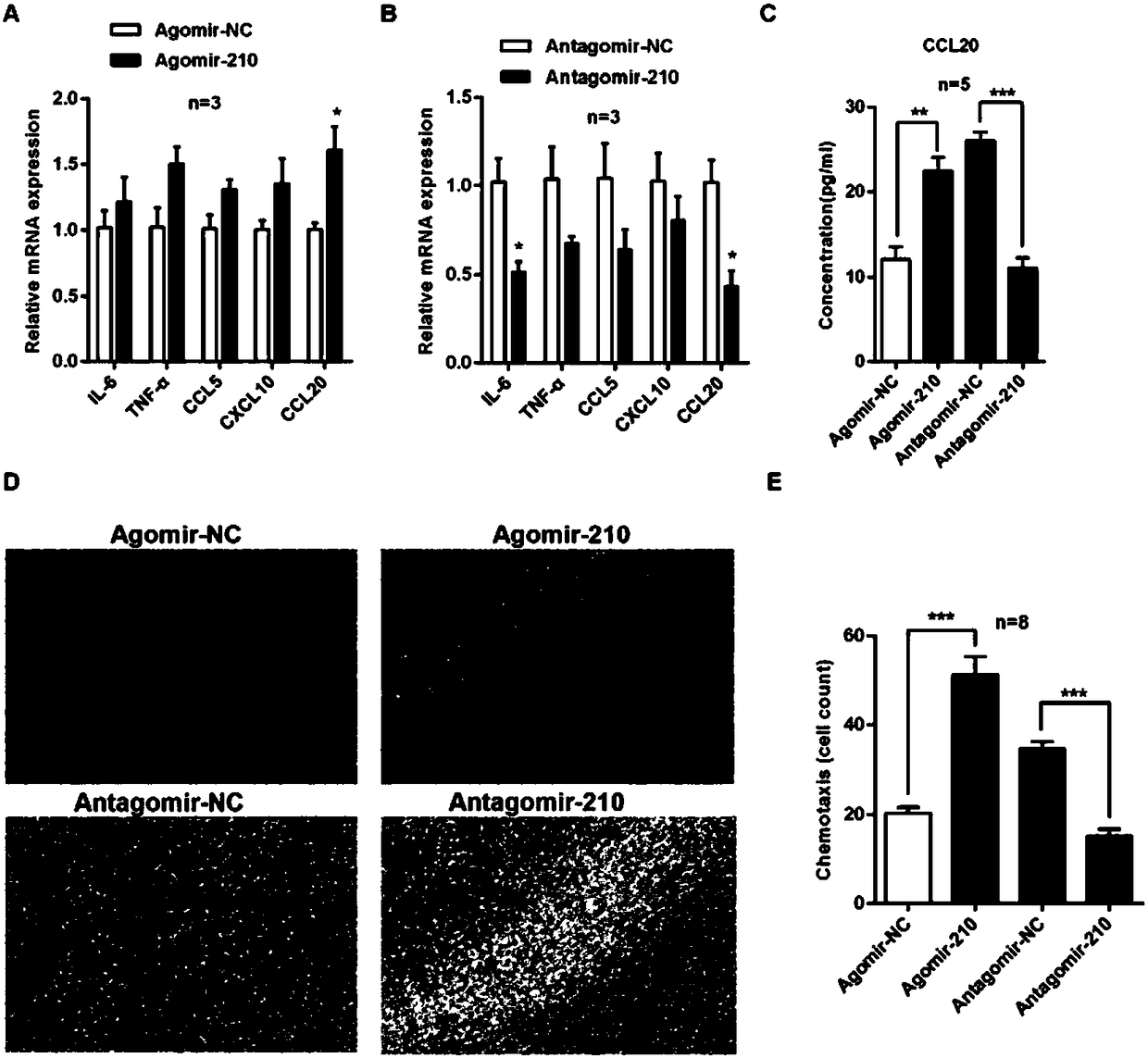
The invention discloses application of microRNA-210 inhibitor in preparing medicine treating inflammatory dermatosis. A large quantity of experiments prove that in-vitro inhibiting of expression of microRNA-210 can obviously improve the expression of a target gene STAT6 of microRNA-210, thus the formation of cell proliferation by cutin and secretion of chemokine CCL20 are inhibited, migration of chemotaxis T cells of chemokine CCL20 to the skin lesion parts is further inhibited, and meanwhile, differentiation of TH1 and TH17 can be inhibited. MicroRNA-210 knockout and injection of the microRNA-210 inhibitor (antagomiR-210 modified by cholesterol) given into the skin lesion specifically inhibits the expression of microRNA-210, which can both obviously inhibit mouse skin inflammation and improve T cell immune imbalance. A novel pathophysiology mechanism is provided for inflammatory dermatosis, and a new strategy capable of being used for preparing the medicine treating inflammatory dermatosis is provided.
Owner:THE SECOND XIANGYA HOSPITAL OF CENT SOUTH UNIV
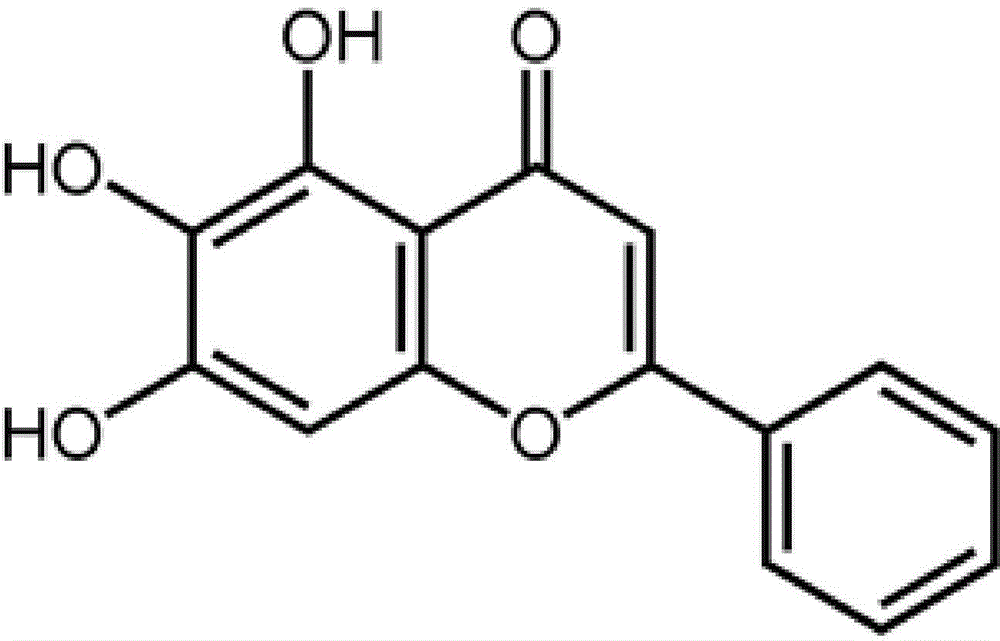
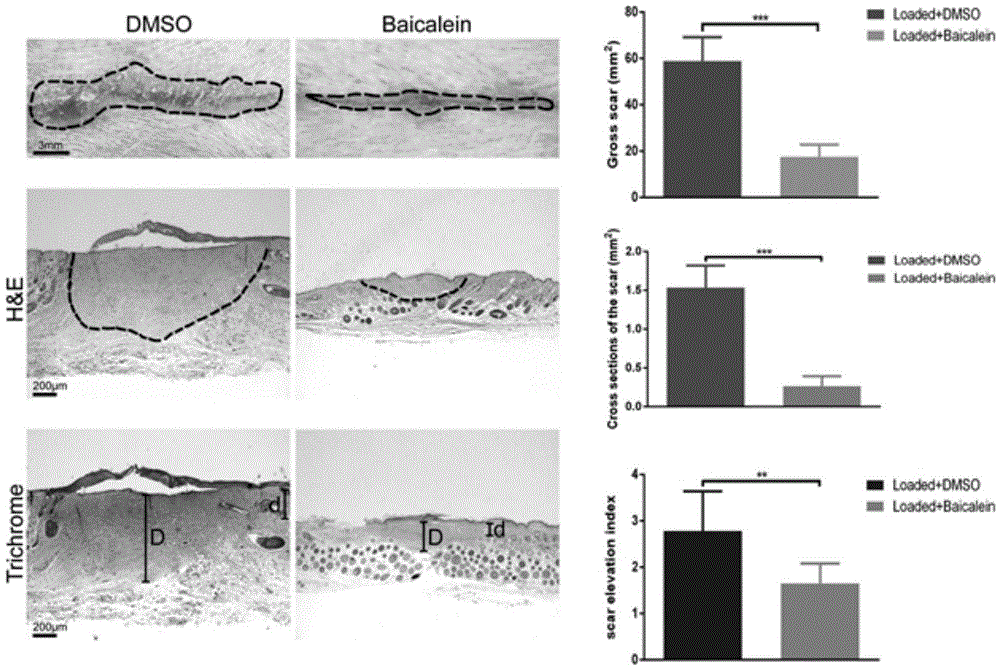
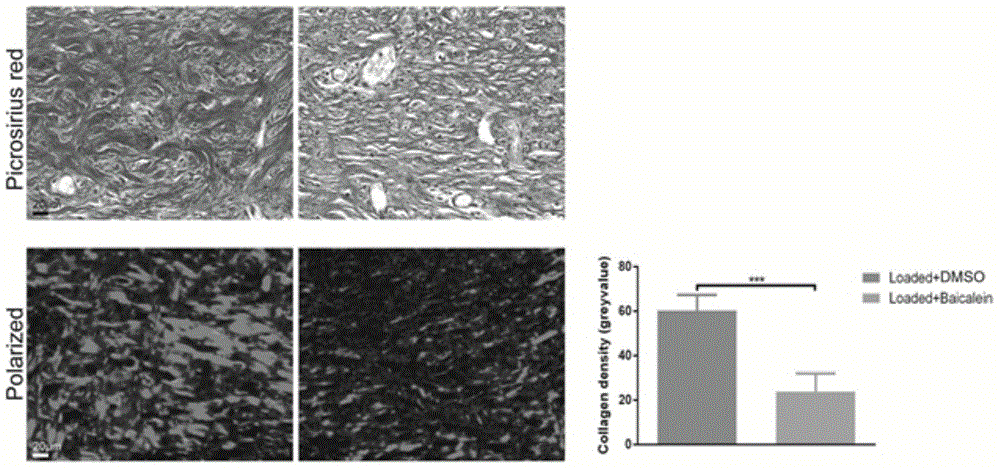
Application of flavonoids compound baicalein in skin cicatrisation inhibition
InactiveCN104922104AHigh molecular weightHigh fat solubilityOrganic active ingredientsDermatological disorderFibroblastIn vivo experiment
The invention relates to the application of flavonoids compound baicalein in skin cicatrisation inhibition. The invention verifies the fact that baicalein can be combined with TGFbetaRI(ALK5) to inhibit a TGFbeta1 signal channel in a cell, and accordingly abnormal proliferation, activation, shrinking and migration ability of human cicatrisation fibroblast are inhibited. An animal in-vivo experiment verifies the fact that after baicalein has an effect on mouse skin, mouse skin cicatrisation induced by tension can be inhibited. Baicalein is small in molecular weight and high in fat solubility, transdermal absorption capacity is good, accordingly, the molecules can be used for preparing medicine for skin cicatrisation inhibition, and the baicalein has wide application prospect in the field of medical treatment, plastic surgery and cosmetics.
Owner:SHANGHAI NINTH PEOPLES HOSPITAL SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
EGF mesenchymal stem cell exosome as well as preparation method and application thereof
InactiveCN113088496AGood treatment effectPromote proliferationCosmetic preparationsToilet preparationsTissue repairCell membrane
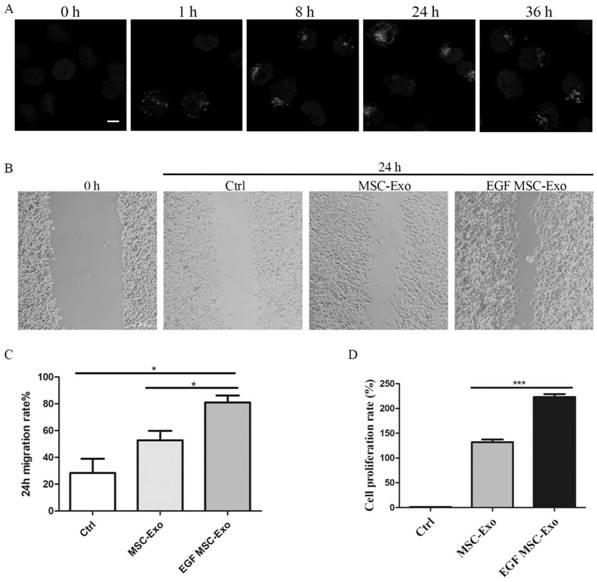
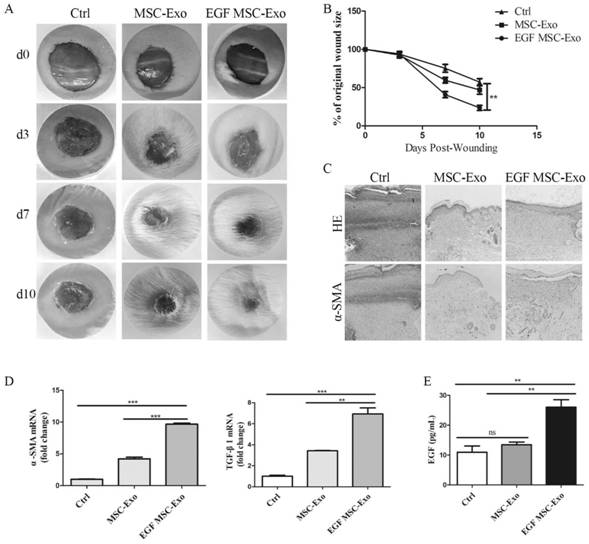
The invention relates to an EGF mesenchymal stem cell exosome, which is secreted by mesenchymal stem cells and can express EGF fusion protein on a mesenchymal stem cell membrane. The EGF fusion protein sequentially comprises an N-terminal signal peptide, a target EGF protein, a connecting peptide and a mesenchymal stem cell transmembrane region from an N terminal, the EGF fusion protein is obtained by transforming a growth factor into a membrane protein and anchoring the membrane protein in a cell membrane of a mesenchymal stem cell for overexpression, the exosome of the overexpression membrane protein EGF mesenchymal stem cells is collected, and the application of the exosome in tissue repair is researched. The cell level and animal experiment level verify that EGF protein can be expressed in a large amount, and the exosome which expresses EGF and is derived from mesenchymal stem cells can promote proliferation and migration of epidermal cells and accelerate healing of mouse skin wounds, and has a good treatment effect on tissue wounds.
Owner:广州远想生物科技股份有限公司
Small RNA used for psoriasis treatment, and derivatives and medicinal preparations thereof
InactiveCN103736102AReduces thickening of the epidermisImprove keratinizationGenetic material ingredientsDermatological disorderLesion skinRelated gene
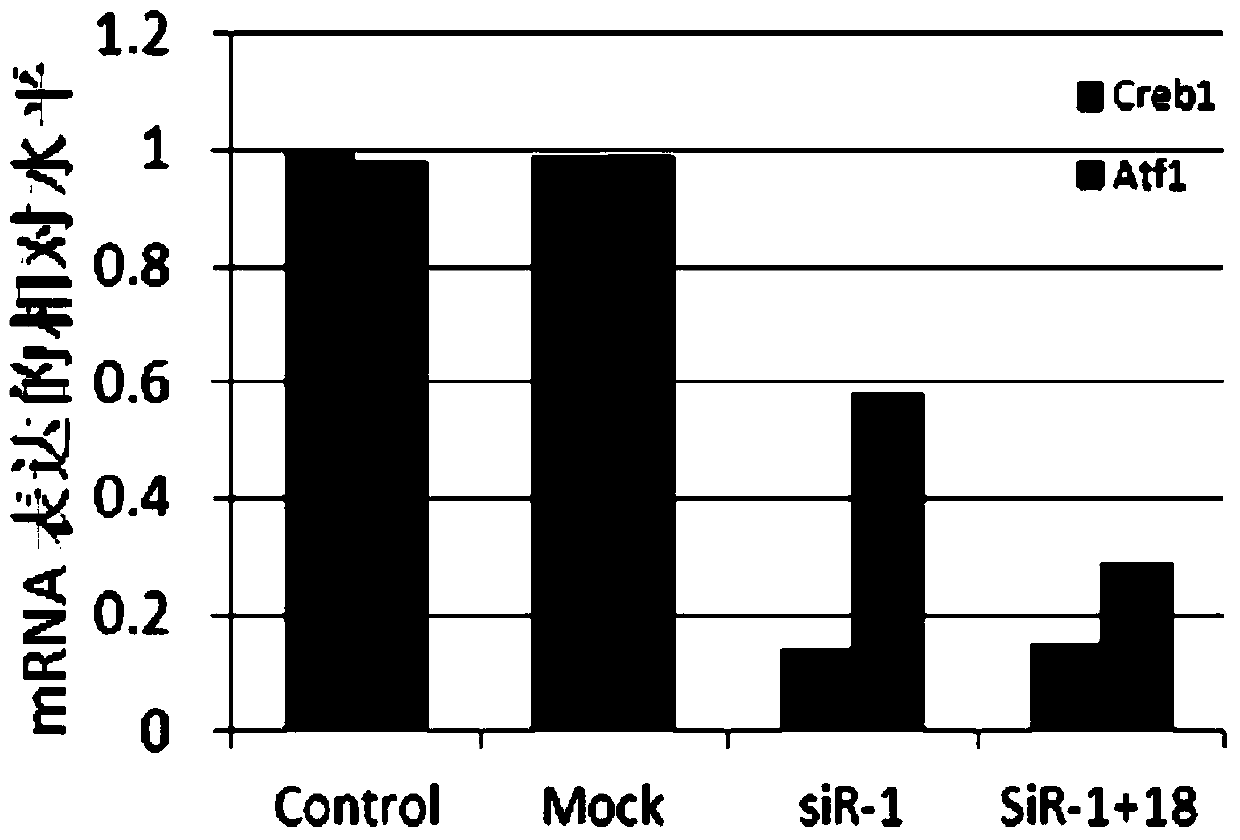
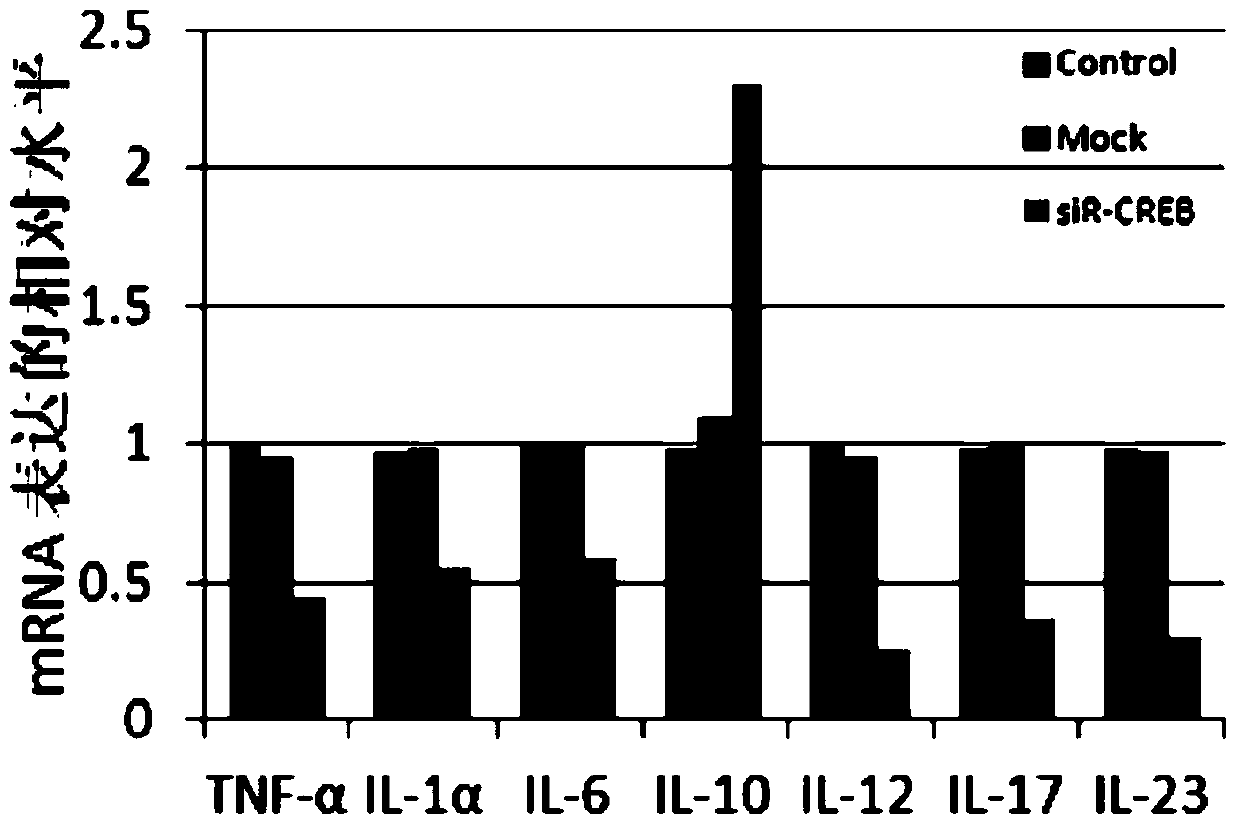
The invention provides a small RNA used for psoriasis treatment, and derivatives and medicinal preparations thereof. The small RNA inhibits the expression of psoriasis pathopoiesis related gene products comprising TNF alpha, IL-1a, IL6 and IL by inhibiting the expression of CREB1 and ATF1. The above small RNA medicines can substantially improve the psoriasis model mouse skin symptom formed after smearing mice with a 5% Imiquimod cream in an external use mode, can mitigate the cuticle thickening degree of pathologic skins, can improve the skin keratinization, can promote the hair growth of the pathologic skins, and has a certain treatment effect on the psoriasis.
Owner:黄兵
Ointment for treating skin scalds
ActiveCN102940881ASimple componentsMedicinal materials are cheap and easy to getOrganic active ingredientsPeptide/protein ingredientsMedicinal herbsTreatment effect
The invention relates to an ointment for treating skin scalds and belongs to the technical field of pharmacy. The ointment is characterized by comprising sturgeon collagen extracts, Bletilla striata polysaccharide extracts, mushroom polysaccharide extracts, stearyl alcohol, lauryl sodium sulfate, albolene, methylparaben, propyl hydroxybenzoate, propylene glycol and distilled water. The ointment has the functions of being capable of converging wounds, stopping bleeding, dispersing blood stasis, subsiding swelling, removing necrotic tissues, promoting granulation and the like. Active components in the ointment comprises sturgeon collagen extracts, Bletilla striata polysaccharide extracts and mushroom polysaccharide extracts, the ointment made of active components can facilitate healing of scalded wounds, can improve the restoration ratio of mouse skin wounds and the treating effect of scald medicines, is applicable to treating of skin scalds and is simple in component, and medicine materials are low in price and easy to obtain.
Owner:JIANGSU UNIV
Use of shea butter as transdermal osmosis promoter
The invention relates to the technical field of medicinal materials, and in particular discloses use of shea butter as a transdermal osmosis promoter. Mouse skin stimulus and sensitization trials and in-vitro transdermal trials prove that the shea butter has a good transdermal osmosis effect, has a stronger transdermal osmosis capability compared with azone, does not irritate skin and sensitize, and can be used as the new transdermal osmosis promoter.
Owner:HENAN UNIV OF SCI & TECH
Application of 6-hydroxy dopamine (6-OHDA) to building of depigmentation model and method
The invention relates to application of 6-hydroxy dopamine (6-OHDA) to building of a depigmentation model and a method for building the depigmentation model through the 6-OHDA. The 6-OHDA-induced mouse skin depigmentation model can be used for analyzing the pathogenesis of depigmentation diseases (including but not specific to vitiligo) and screening medicines for curing the diseases. Application of the 6-OHDA to building of the depigmentation model and the method for building the depigmentation model through the 6-OHDA have the beneficial effects that the 6-OHDA can be used for successfully building the depigmentation model and providing potential animal models for vitiligo basic and clinic research, and a foundation is provided for screening of antioxidized medicines and anti-vitiligo medicines.
Owner:HANGZHOU THIRD HOSPITAL
Constructing method of model of lacking of Langerhans cells of small rat skin
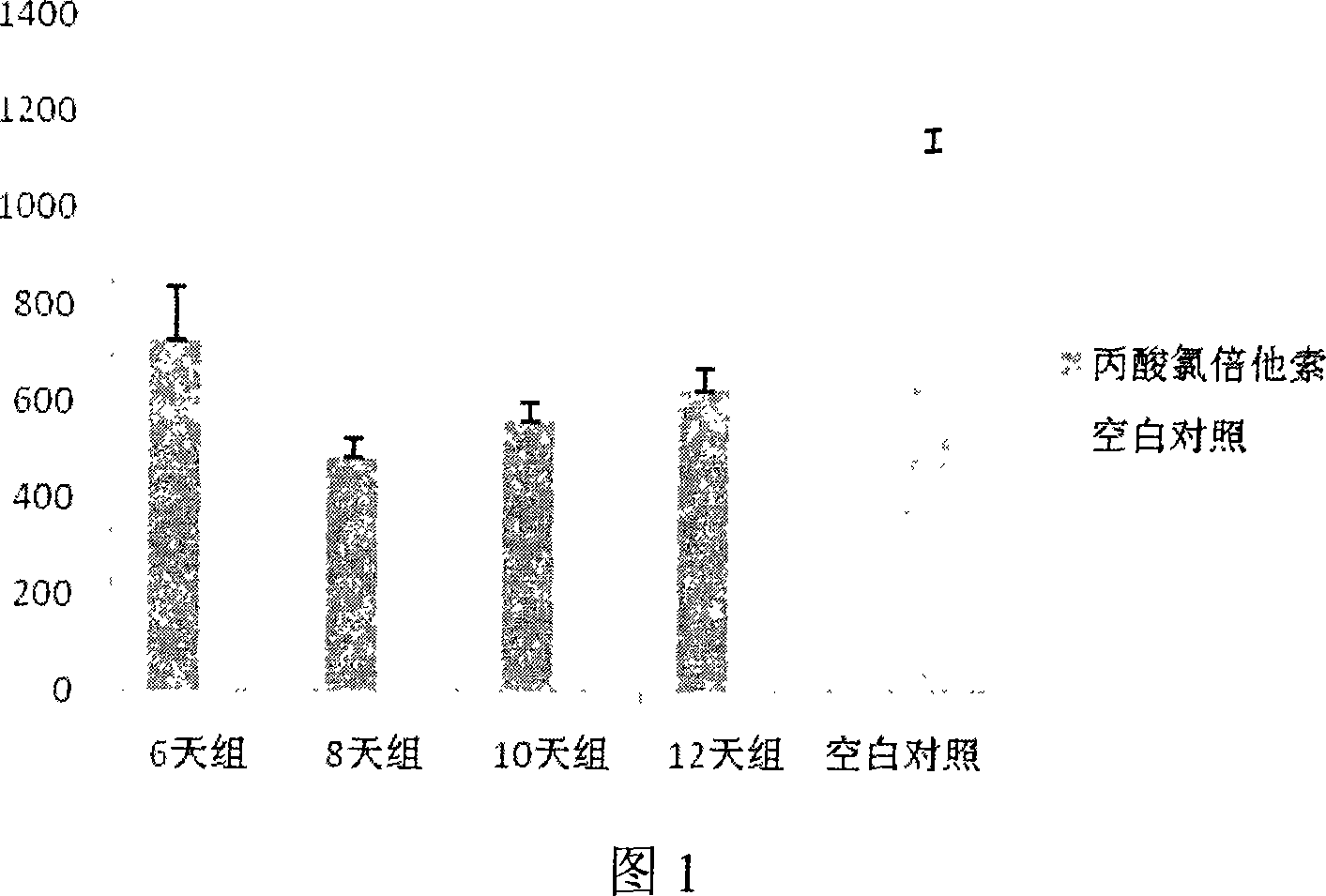
InactiveCN101032625ALong solution cycleOvercome the cycleOrganic active ingredientsIn-vivo testing preparationsLangerhan cellAbdominal skin
The present invention belongs to the field of medicine evaluating and detecting technology, and is especially process of constructing mouse skin Langerhans cell deletion model. Langerhans cell takes an important role in skin immune system. The mouse skin Langerhans cell deletion model constructing process includes adopting clobetasol propionate ointment as the model forming medicine and smearing it homogeneously onto the breast and abdominal skin of unhaired mouse. The histochemical staining and immunohistochemical experiments show that the said mouse model can well simulate the skin Langerhans cell deletion status, and may be used in screening skin immune medicine and in researching skin immune system inhibiting mechanism and medicine action mechanism. The present invention has simple process, short period, high repeatability, low cost and other advantages.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Construction method of model for detecting cell differentiation functional
InactiveCN1884491APromote growth and differentiationPromote differentiationMicrobiological testing/measurementSkeletal/connective tissue cellsBone Marrow Stromal CellCementoblastoma
The invention relates to a lay down method for model used in detecting cell differentiation function and the method includes the following steps: (1) preparing heelpiece, enamel matrix protein (EMPs) and myeloid stroma cell; (2) covering the heelsurface of the said heelpiece with EMPs; (3) inoculating the myeloid stroma cell on the heelpiece surface obtained by step (2); (4) placing the heelpiece obtained by step (3) into the politef film pocket; (5) placing the heelpiece covered by the pocket under naked mouse skin. The advantages of this invention include connecting the cell culture in vitro and growth in vivo, insulating the impact of naked mouse cell with screen film and inspecting the impact of EMPs to the marrow stroma cell differentiation, which consequently can provide effective research model for studying EMPs application mechanism. At the 8th week of the experiment, different histological structures are formed by different processing groups and the cellular cementum samples are formed on the heelsurface trained by EMPs, which indicates that the EMPs can improve the marrow stroma cell differentiation to the cementoblastoma and also indicates that the research model is successful.
Owner:SHANGHAI NINTH PEOPLES HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
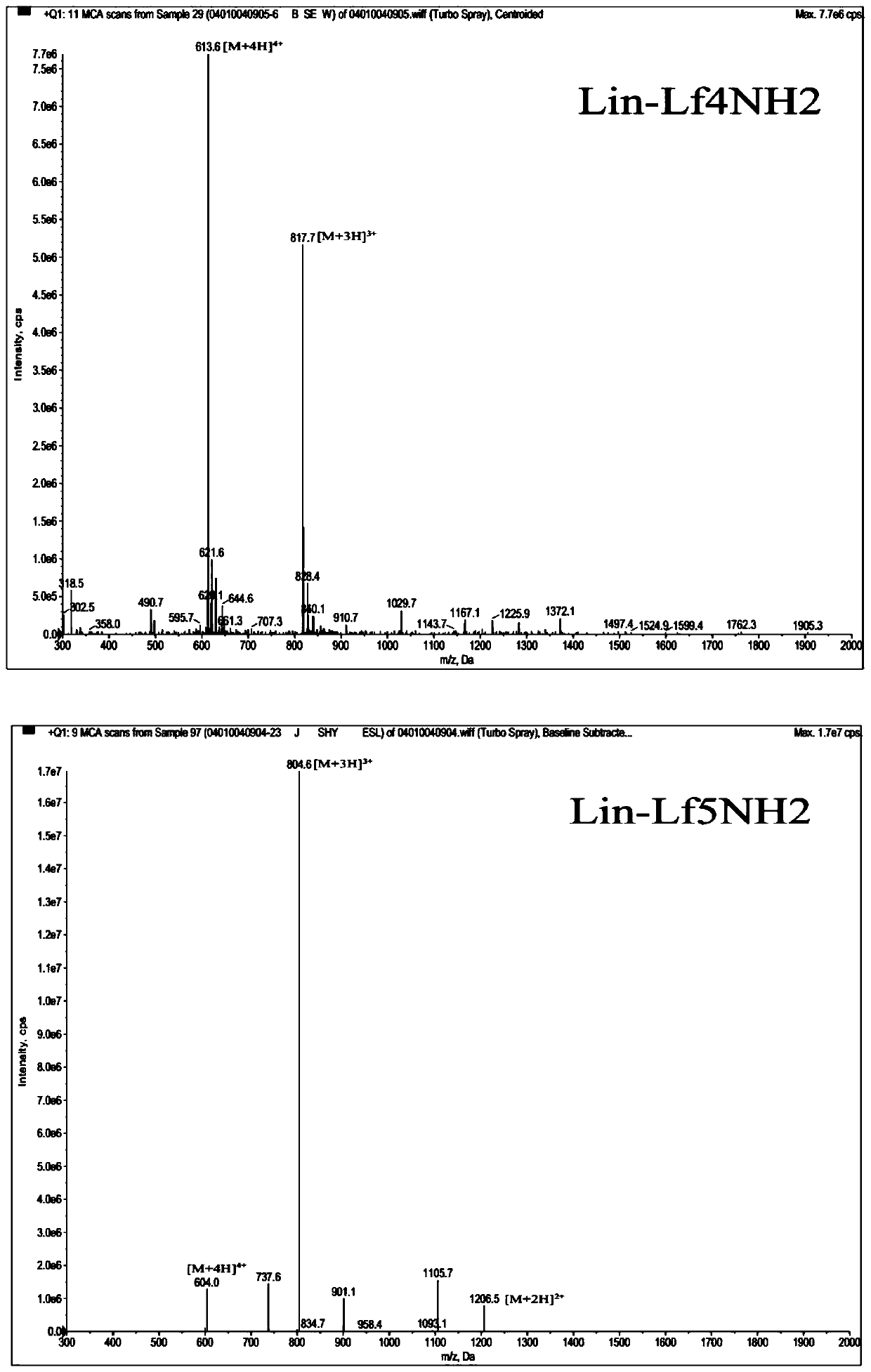
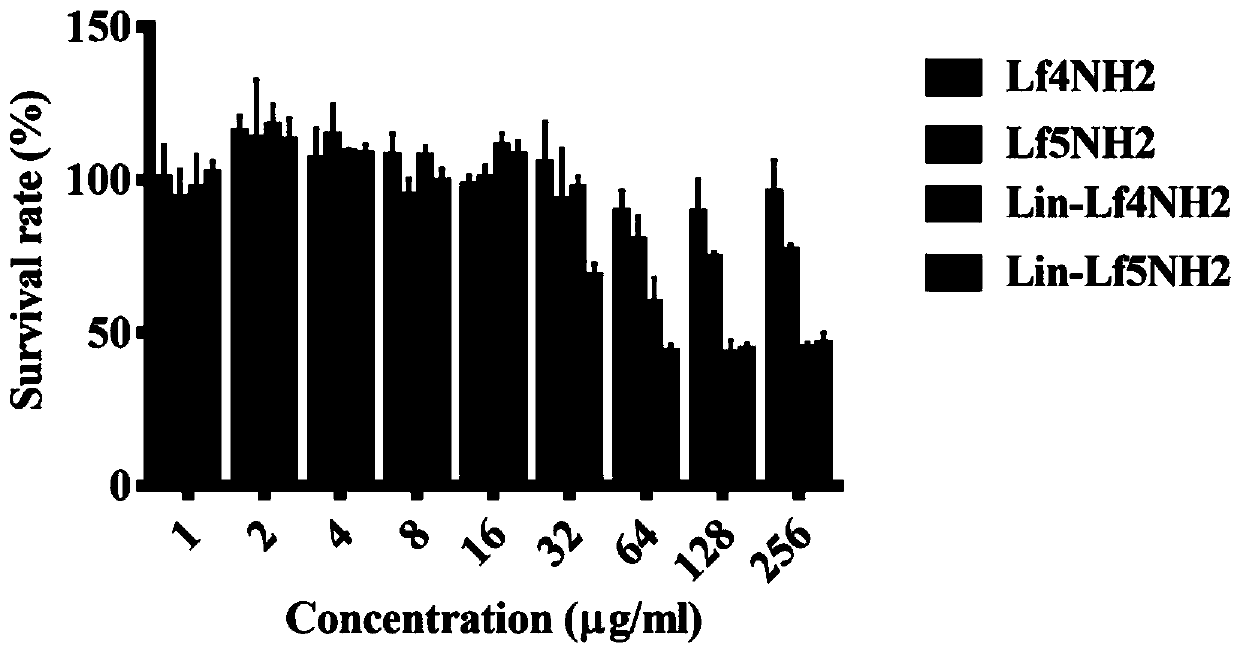
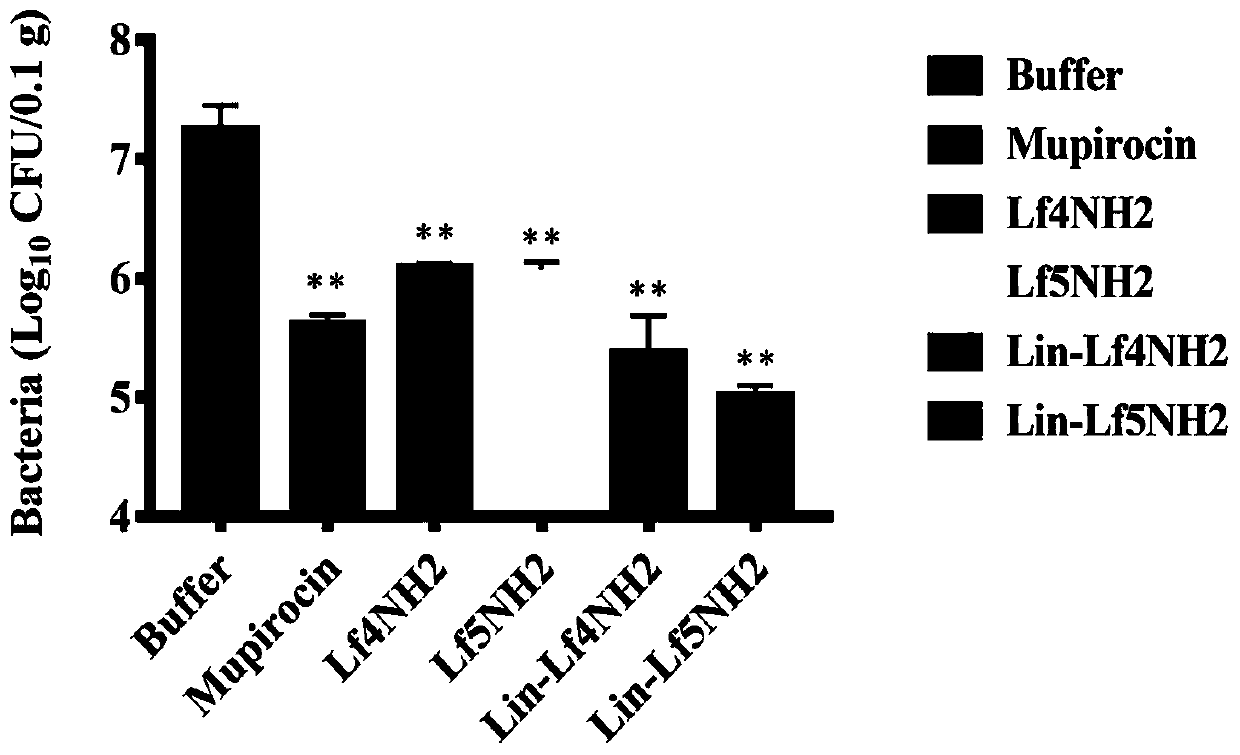
Lipopeptides Lin-Lf4NH2 and Lin-Lf5NH2 and application thereof
ActiveCN111574619AReduce bacterial loadAvoid damageAntibacterial agentsPeptide/protein ingredientsTherapeutic effectTissue damage
The invention provides novel lipopeptides Lin-Lf4NH2 and Lin-Lf5NH2 and application thereof. The lipopeptides Lin-Lf4NH 2 / Lin-Lf 5NH2 are obtained by coupling linoleic acid to the N end of an antibacterial peptide LfcinB4 / LfcinB5 and performing amidation modification on the C end of the antibacterial peptide LfcinB4 / LfcinB 5. Experiments show that the lipopeptides Lin-Lf4NH2 and Lin-Lf5NH2 havea good inhibiting effect on gram-positive bacteria and gram-negative bacteria, have a smaller MIC (minimal inhibitory concentration) value and good thermal stability than a parent peptide, can remarkably reduce the skin bacteria carrying amount and relieve skin tissue injury in a mouse skin abscess model test, have a better treatment effect than Lf4NH2 and Lf5NH2, are small-molecule lipopeptideswith high application value, can be used for preparing novel antibacterial and anti-infection medicines and the like, and have a wide application prospect.
Owner:FEED RESEARCH INSTITUTE CHINESE ACADEMY OF AGRICULTURAL SCIENCES
Preparation method and application of orthosiphon aristatus extract
InactiveCN105902612AImprove epidermal hyperplasiaCosmetic preparationsToilet preparationsMouse skinPhotoaging
The invention belongs to the field of medical technology and discloses a preparation method of an orthosiphon aristatus extract and application thereof in preventing and treating skin aging, and particularly relates to a treatment and protection effect of orthosiphon aristatus on the photoaging of ultraviolet-induced mouse skin. The result indicates that the orthosiphon aristatus can effectively improve the bad conditions such as skin thickening, dryness, loose skin, thick and deep wrinkles and leather-like appearance caused by UV. Meanwhile, the histopathological result indicates that the orthosiphon aristatus can obviously improve the degenerative changes of skin structure caused by UV and maintain normal distribution of collagen fiber and elastic fiber. Moreover, the orthosiphon aristatus can remarkably inhibit the oxidative stress of skin by reducing the lipid peroxidation and increasing the antioxidant enzyme activity, enhance the skin immunity and improve the function of lymphoid organ by inhibiting excessive inflammatory factors, and down-regulate the level of MMP-1 and MMP-3 of photoaging mouse. Experimental result shows that the orthosiphon aristatus can be clinically used for preventing and treating skin aging and can be applied to household chemicals.
Owner:刘肖娣 +1
Medicine for treating psoriasis
The invention discloses a medicine for treating psoriasis and belongs to the field of natural medicines. A series of experiments show that the sulforaphene relieves epidermal hyperplasia and inflammatory response of a psoriasis-like mouse skin lesion part induced by imiquimod; excessive proliferation of human keratinocytes is inhibited, and cell apoptosis is promoted; the symptoms of the psoriasis are relieved, and an obvious treatment effect on the psoriasis is achieved. The sulforaphene is separated from brassica plants including but not limited to radish, broccoli, cabbage, leaf mustard and horseradish, the components are natural and nontoxic, the psoriasis treatment effect is remarkable, the psoriasis is not prone to relapse after healing, and the sulforaphene has wide application prospects in preparation of the medicine for treating psoriasis.
Owner:BEIJING UNIV OF CHEM TECH
Non-diagnostic evaluation method after mouse back psoriasis model construction
PendingCN114708975ALarge and complete lesionsLong period of onsetMedical simulationAnimal husbandryStatistical analysisSkin damage
The invention provides a non-diagnostic evaluation method for a mouse back psoriasis model after construction, which comprises the following steps: S1, determining the percentage of a mouse skin lesion area in a drug application area skin lesion area, and scoring by 0-2 scores according to a skin lesion area proportion B, 0% = 0 score, 1%-30% = 1 score, and 31%-100% = 2 score; s2, determining an erythema score E, wherein the E is divided into 0-2 scores according to the color depth of the erythema; s3, a scale score D is determined, scoring is carried out according to the scale covering skin damage area, the scale covering with no scale is equal to 0 score, the scale covering with 30% of skin damage is equal to 1 score, the scale covering with 60% of skin damage is equal to 2 score, and the scale covering with 90% or above of skin damage is equal to 3 score; s4, determining a plaque score I, performing scoring by 0-3 scores according to the size of the plaque, and if no is equal to 0 score, determining the average diameter of the plaque as lt; 2 mm is equal to 1 minute, the average diameter of the plaque is 2-4 mm and is equal to 2 minutes, and the average diameter of the plaque is gt; 4 mm is equal to 3 minutes; s5, the skin lesion severity S is determined, S = B + E + D + I, the mouse back skin psoriasis inflammation severity is more accurately described and quantitatively compared according to the S score, and intuitive and accurate statistical analysis is facilitated.
Owner:CHINA THREE GORGES UNIV
Medicine with intervention effect on skin photoaging caused by UVA, and animal model
ActiveCN111514134AAbility to prevent photoagingUnderstand the mechanism of actionEpidermal cells/skin cellsArtificial cell constructsMouse skinMethanol
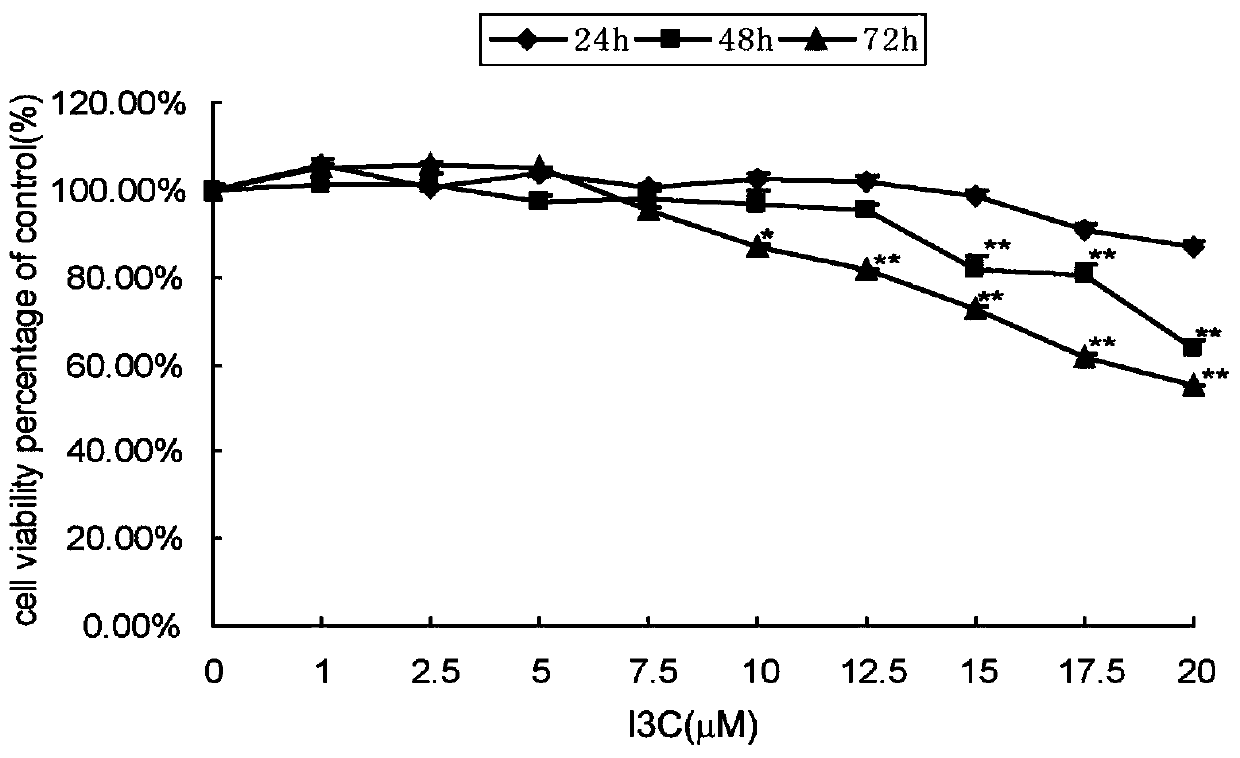
The invention belongs to the technical field of pharmaceutical preparations, and discloses a medicine with an intervention effect on skin photoaging caused by UVA, and an animal model. The medicine isindole-3-methanol, and the maximum inactive dose on HaCaT cells is 10 mu mol / L. HaCaT cells in a logarithmic phase are taken to be inoculated into a 6-hole culture plate according to 2 *105 piece / hole to be cultured in a cell culture box until the cell fusion degree is 70-80%; processing is carried out as follows: a blank control group is covered with a lightproof black paper sheet, the irradiation dose of a UVA model group is 1 J / cm2, an I3C treatment group is treated with 10 [mu] mol / L I3C while UVA irradiating is carried out, and two parallel samples are set for each dose; and the change of the number and morphology of the cells are observed after the irradiation is finished. According to the medicine, the lesions of mouse skin epidermal cells and fiber tissues caused by excessive UVAirradiation can be improved, such that the formation of skin photoaging can be delayed.
Owner:GUANGZHOU CENT FOR DISEASE CONTROL & PREVENTION (GUANGZHOU HYGIENE INSPECTION CENT GUANGZHOU CENT FOR FOOD SAFETY RISK SURVEILLANCE & ASSESSMENT INST OF PUBLIC HEALTH OF GUANGZHOU MEDICAL UNIV) +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com