Self-healing hydrogel capable of promoting wound healing and cancer therapy and preparation method thereof
A technology for tumor treatment and wound healing, applied in the field of biomedical materials, can solve the problems of easy infection and healing of skin wounds, poor anti-infection ability, weak ability to inhibit cancer cells, etc., and achieve poor water solubility, convenient operation, and good biocompatibility sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
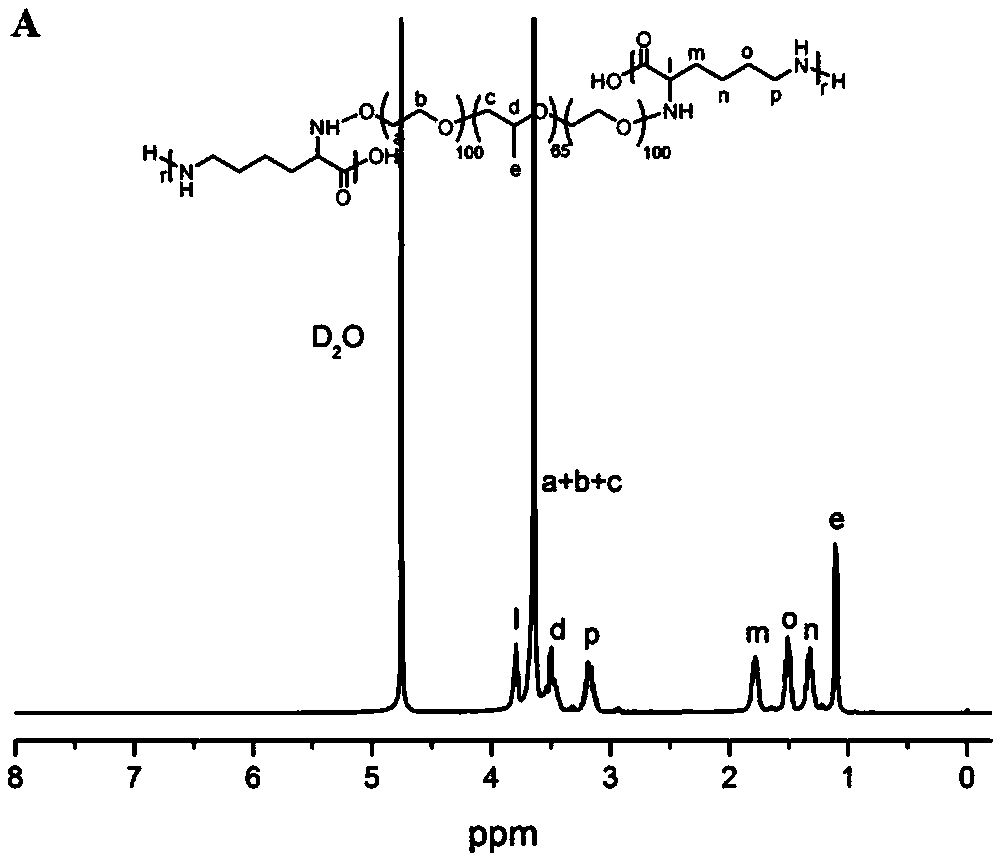
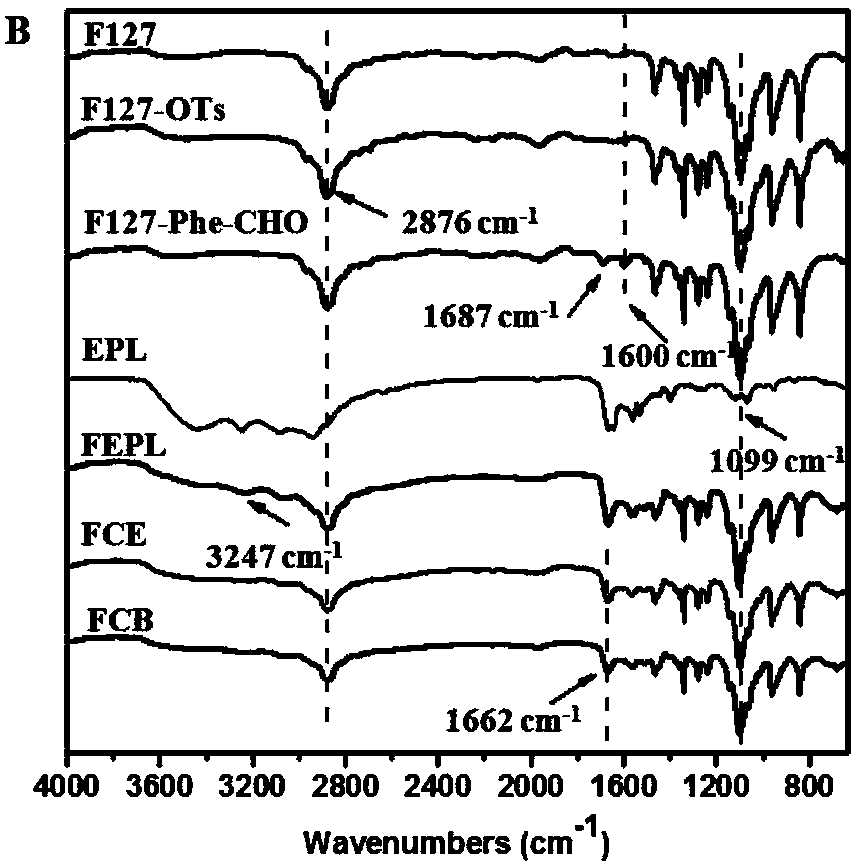
[0043](1) Preparation of Pluronic F127-p-toluenesulfonate (F127-OTs): Dissolve 12.3g F127 in 70mL anhydrous dichloromethane, then add 0.83mL triethylamine and 1.14g p-toluenesulfonyl chloride at room temperature Under reaction 24h. After the reaction, the organic phase was washed successively with dilute hydrochloric acid and saturated sodium bicarbonate solution, and extracted. Then, the organic phase was concentrated and precipitated in ether to obtain F127-OTs (yield 90%).
[0044] (2) Preparation of F127-p-hydroxybenzaldehyde (F127-Phe-CHO): Dissolve 2.5g of F127-OTs in 50mL of DMF, then add 0.11g of 4-hydroxybenzaldehyde and 0.12g of potassium carbonate (K 2 CO 3 ), reacted at 80°C for 72h. After the reaction was completed, after it was cooled to room temperature, 50 mL of water was added and extracted with dichloromethane. After the organic phase was dried over anhydrous magnesium sulfate, it was precipitated in ether to obtain F127-Phe-CHO (yield 90%).
[0045] (3)...
Embodiment 2
[0050] (1) Preparation of Pluronic F127-p-toluenesulfonate (F127-OTs): Dissolve 12.3g F127 in 70mL anhydrous dichloromethane, then add 0.83mL triethylamine and 1.14g p-toluenesulfonyl chloride at room temperature Under reaction 24h. After the reaction, the organic phase was washed successively with dilute hydrochloric acid and saturated sodium bicarbonate solution, and extracted. Then, the organic phase was concentrated and precipitated in ether to obtain F127-OTs (yield 90%).
[0051] (2) Preparation of F127-p-hydroxybenzaldehyde (F127-Phe-CHO): Dissolve 2.5g of F127-OTs in 50mL of DMF, then add 0.11g of 4-hydroxybenzaldehyde and 0.12g of potassium carbonate (K 2 CO 3 ), reacted at 80°C for 72h. After the reaction was completed, after it was cooled to room temperature, 50 mL of water was added and extracted with dichloromethane. After the organic phase was dried over anhydrous magnesium sulfate, it was precipitated in ether to obtain F127-Phe-CHO (yield 90%).
[0052] (3...
Embodiment 3
[0057] (1) Preparation of P123-p-toluenesulfonate (P123-OTs): Dissolve 5.8g P123 in 70mL anhydrous dichloromethane, then add 0.56mL triethylamine and 0.76g p-toluenesulfonyl chloride at room temperature Reaction 24h. After the reaction, the organic phase was washed successively with dilute hydrochloric acid and saturated sodium bicarbonate solution, and extracted. Then, the organic phase was concentrated and precipitated in ether to obtain P123-OTs (yield 87%).
[0058] (2) Preparation of P123-p-hydroxybenzaldehyde (P123-Phe-CHO): Dissolve 2 g of P123-OTs in 40 mL of DMF, then add 0.1 g of 4-hydroxybenzaldehyde and 0.11 g of potassium carbonate (K 2 CO 3 ), reacted at 80°C for 72h. After the reaction was completed, after it was cooled to room temperature, 40 mL of water was added and extracted with dichloromethane. After the organic phase was dried over anhydrous magnesium sulfate, it was precipitated in ether to obtain P123-Phe-CHO (yield 91%).
[0059] (3) Preparation o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com