Constructing method of model of lacking of Langerhans cells of small rat skin
A construction method and mouse technology, which can be used in pharmaceutical formulations, preparations for in vivo experiments, and medical preparations containing active ingredients, etc., can solve problems such as reducing the density of epidermal Langerhans cells, achieve short cycle times, overcome The effect of long cycle and simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] 1. Experimental animals and materials:
[0017] BALB / c mice, (6-8 weeks old, SPF grade, male), were purchased from Shanghai Xipuer-Bicai Laboratory Animal Co., Ltd., animal certificate number: SCXK (Shanghai) 2003-0002.
[0018] Drug: 0.2 mg / g (0.02%) clobetasol propionate ointment (hospital preparation of Changhai Hospital Affiliated to Second Military Medical University), document number (Nanzhizi (2006) F50030).
[0019] 2. Experimental method:
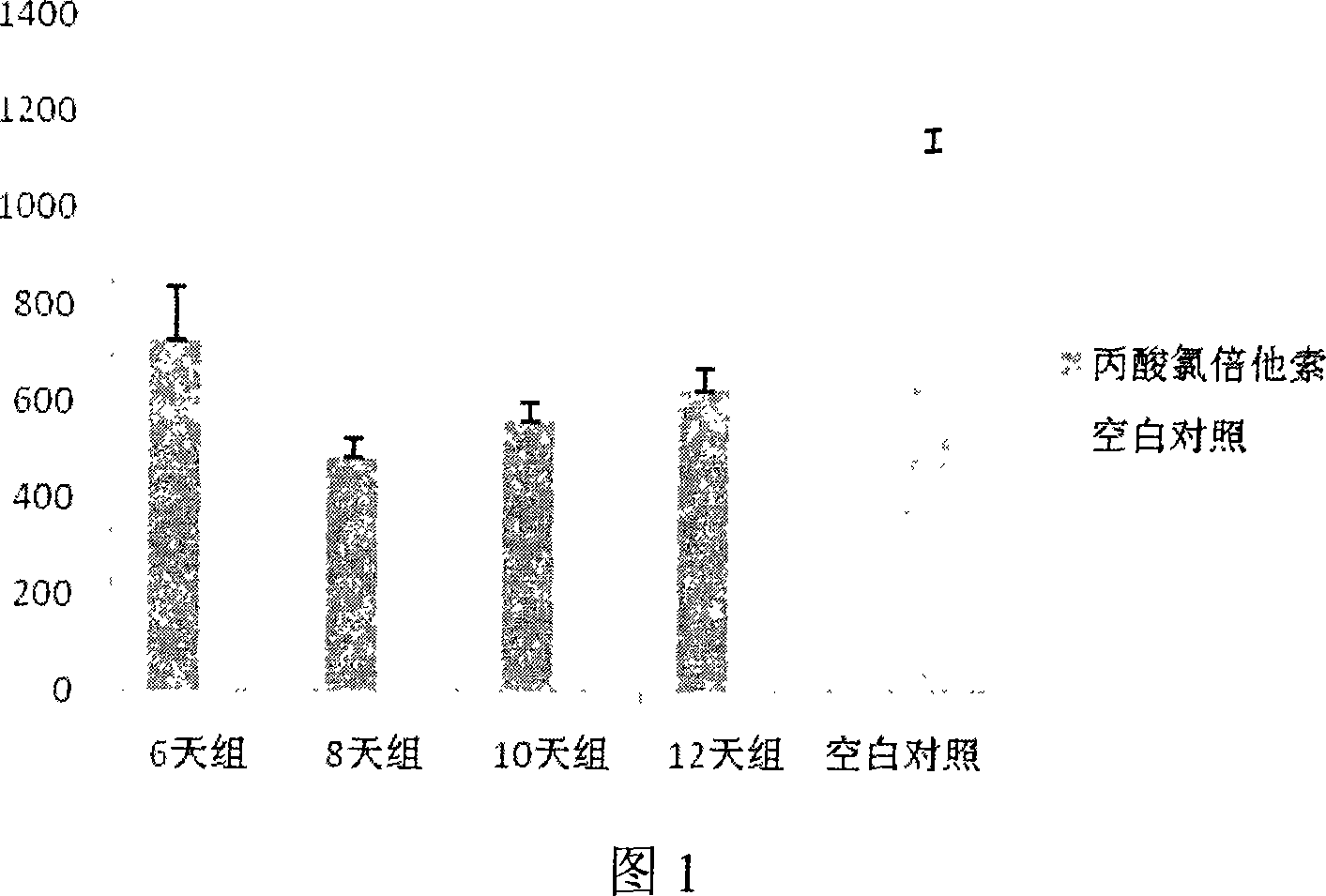
[0020] 40 BALB / c mice were randomly divided into 5 groups, including 8 normal control groups, and 4 groups of modeling components, 8 mice in each group, which were administered transdermally for 6 days, 8 days, and 10 days, respectively. and 12 days. All experimental mice were treated with hair removal on the chest and abdomen before the experiment, and then they were reared for 48-72 hours. The normal control group was fed routinely throughout the experiment, and the rest of the groups were smeared with clobetasol propio...
Embodiment 2
[0025] 1. Experimental animals and materials:
[0026] BALB / c mice, (6-8 weeks old, SPF grade, male), were purchased from Shanghai Xipuer-Bicai Laboratory Animal Co., Ltd., animal certificate number: SCXK (Shanghai) 2003-0002.
[0027] Drug: 0.2mg / g (0.02%) clobetasol propionate ointment (hospital preparation of Changhai Hospital Affiliated to Second Military Medical University), approval number is (Nanzhizi (2006) F50030).
[0028] (2wu / g) Recombinant Human Interferon α2b Cream (Recombinant Human Interferon α2bCream, Anhui Anke Bioengineering (Group) Co., Ltd.), the approval number is (Guoyao Zhunzi S20020032).
[0029] 2. Experimental method:
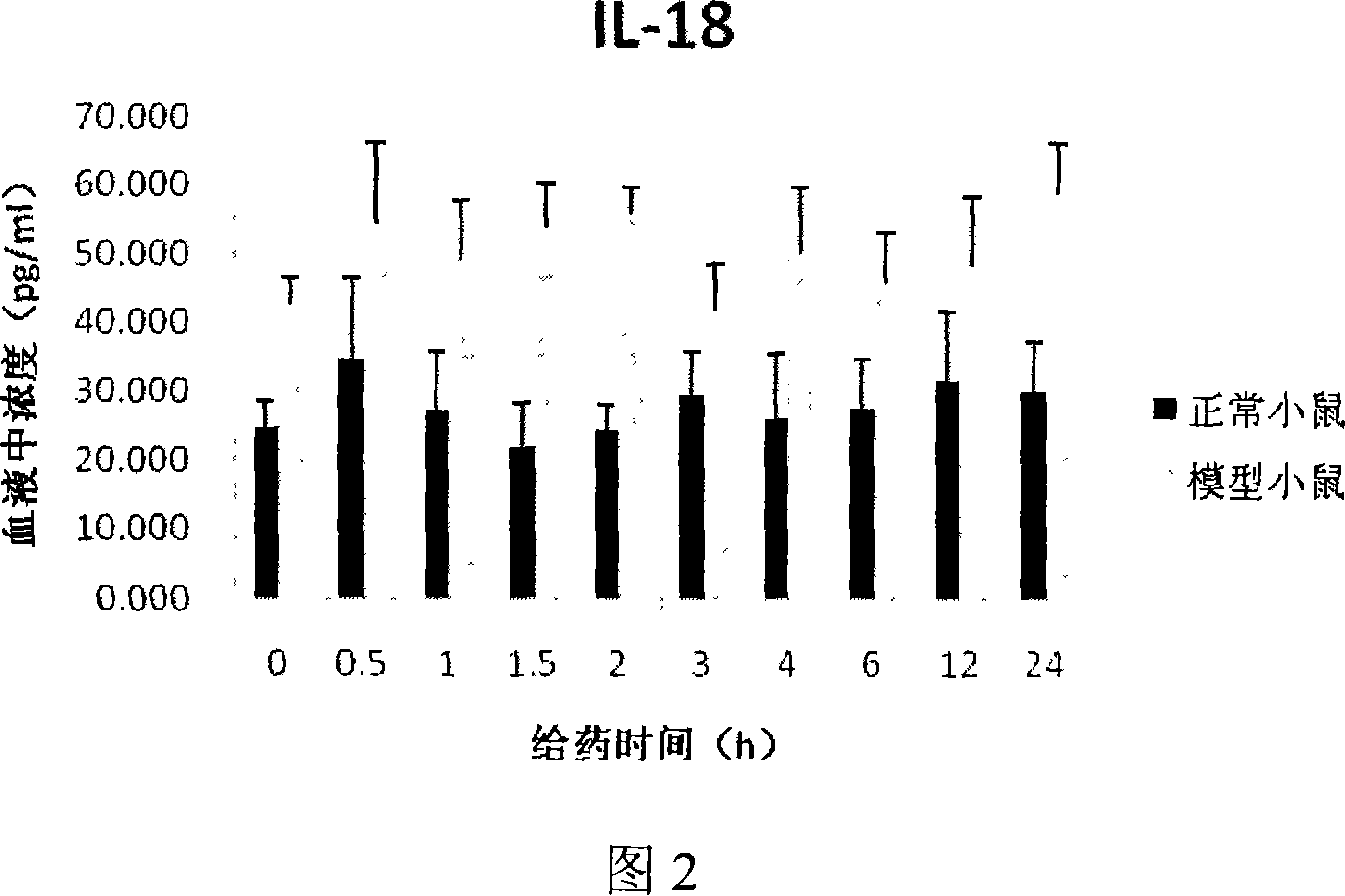
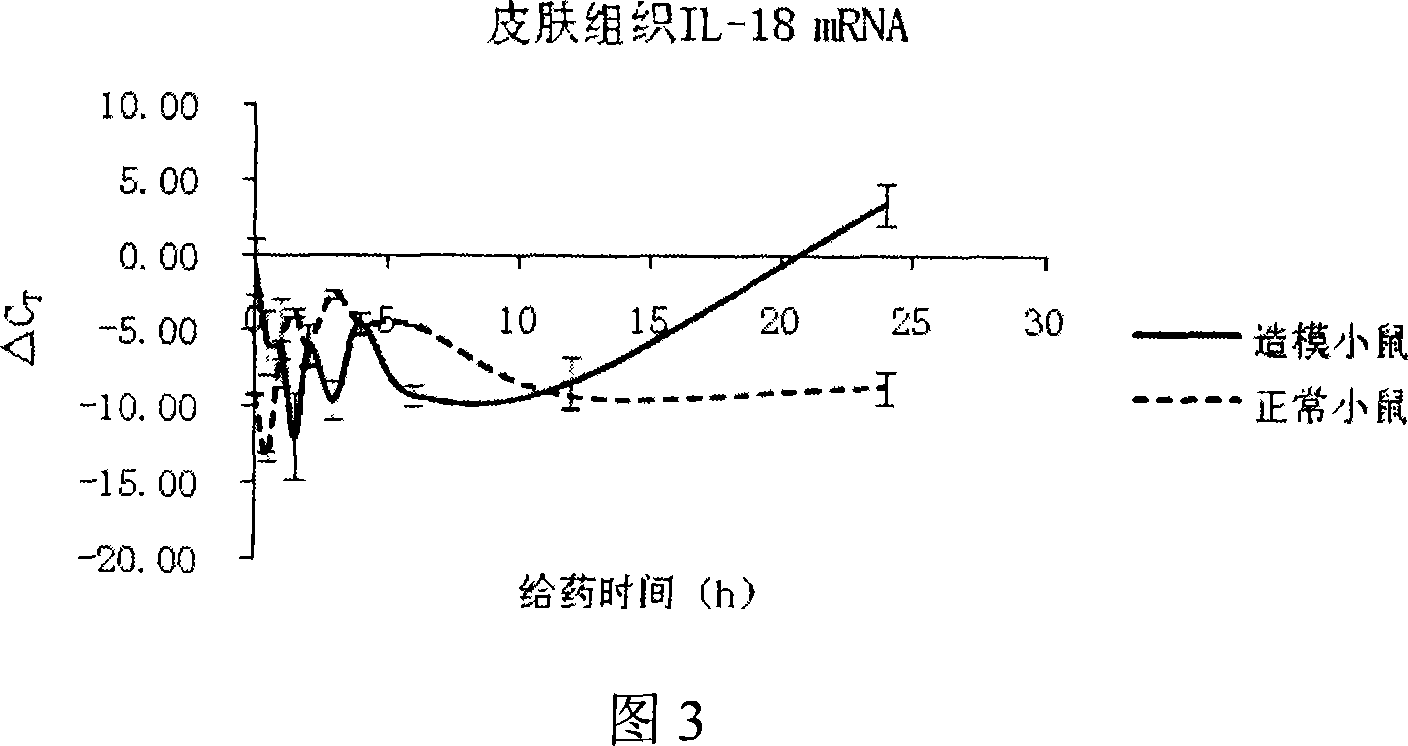
[0030]100 BALB / c mice were randomly divided into 20 groups according to body weight, 5 in each group, including 10 groups of normal mice and 10 groups of model mice, 50 in each group. All the experimental mice should be treated with hair removal on the chest and abdomen before the experiment, and then fed for 48-72 hours. 10 groups...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com