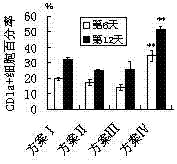
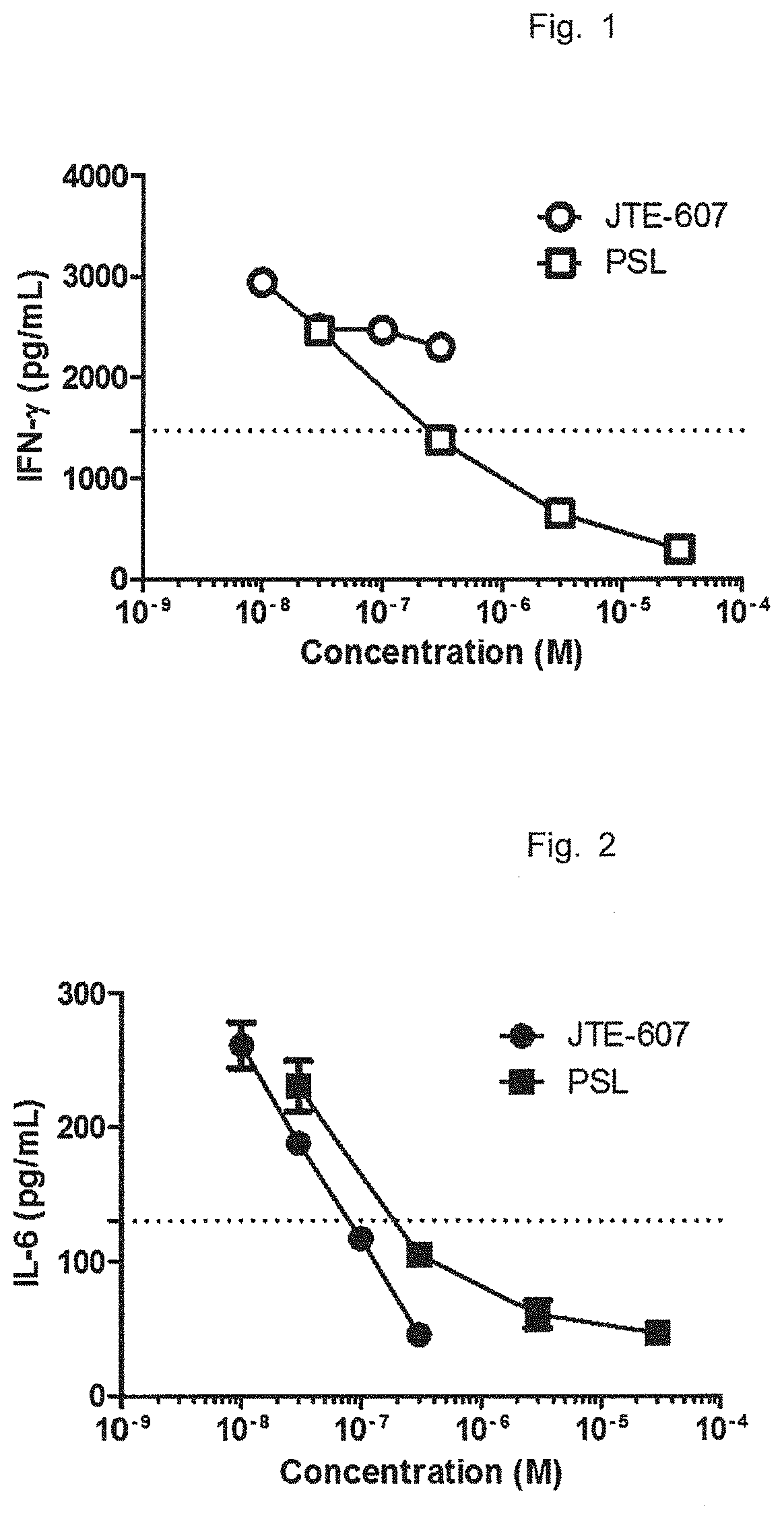
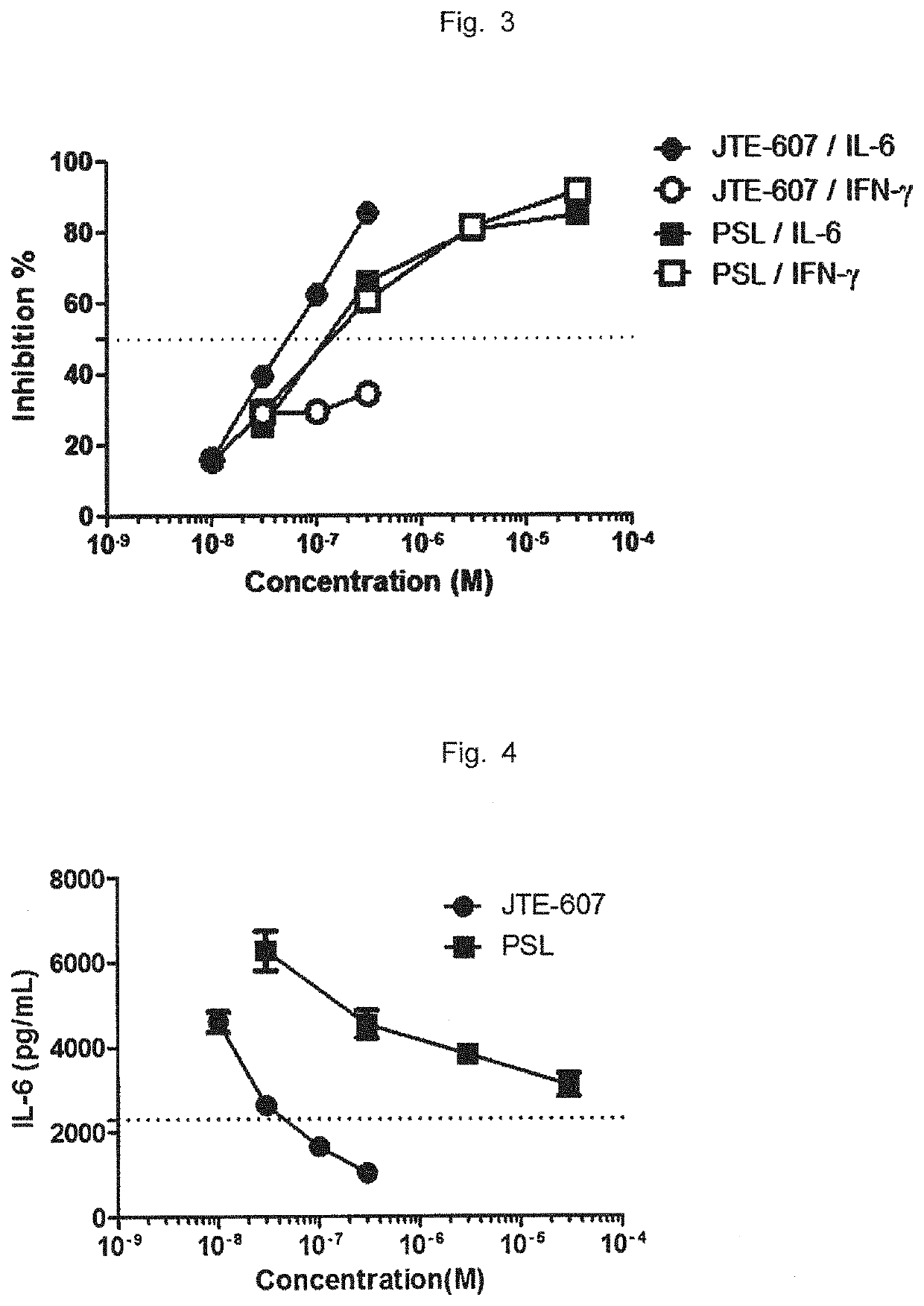
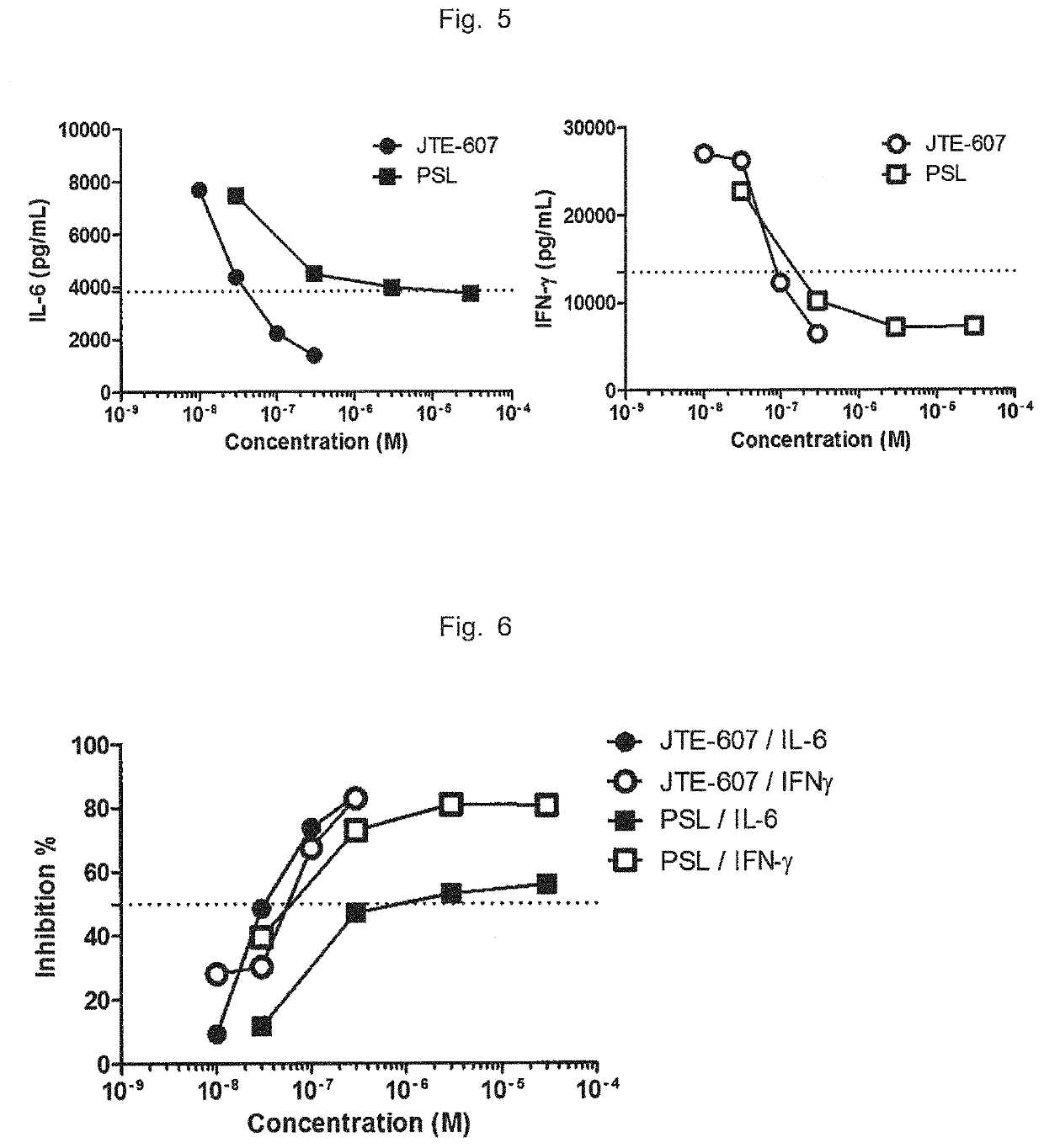
Patents
Literature
38 results about "Langerhan cell" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A Langerhans cell is a specific kind of white blood cell. Found largely in the epidermis, the outer layer of the skin, as well as in lymph nodes, Langerhans cells are an important element of the immune system.
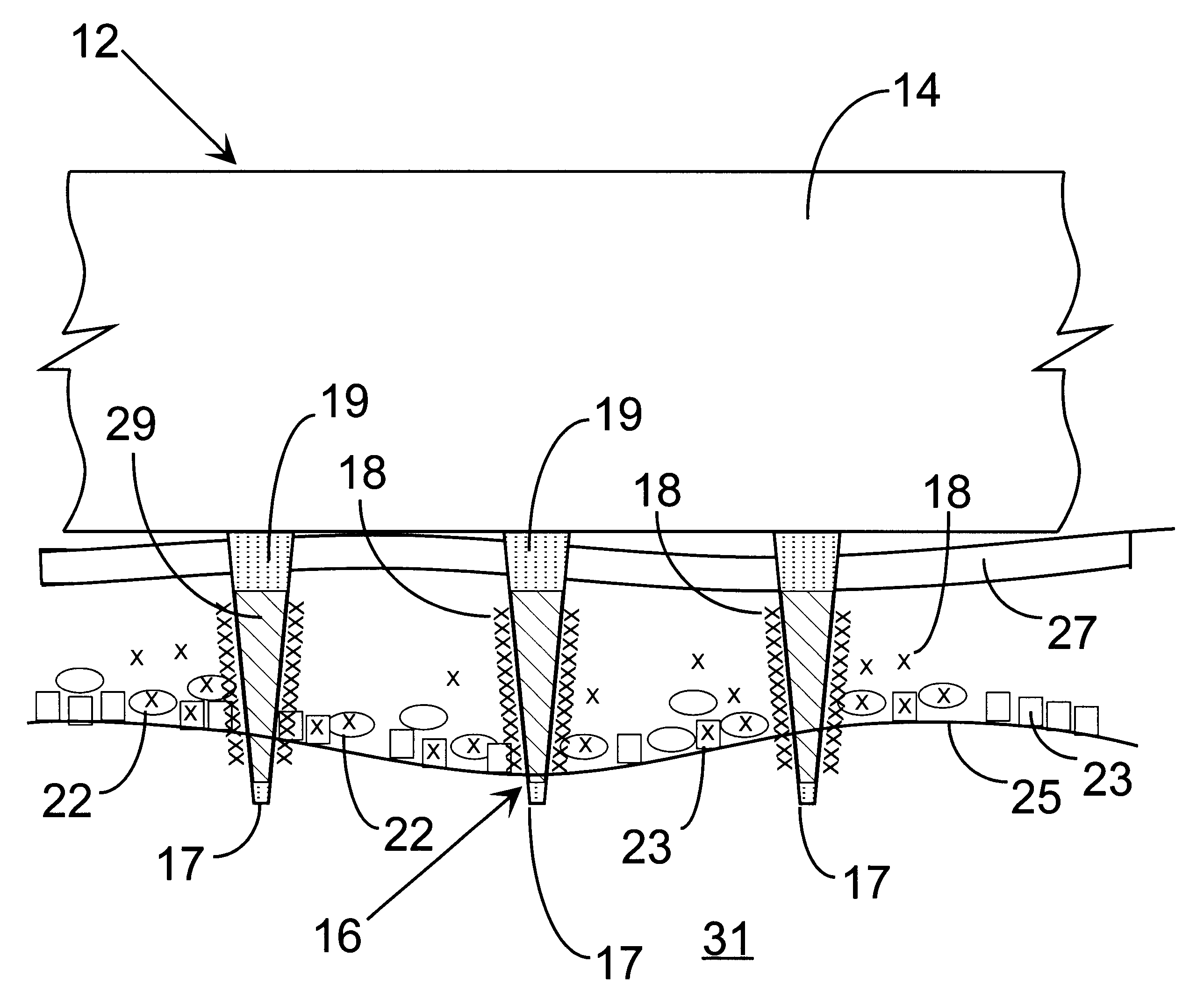
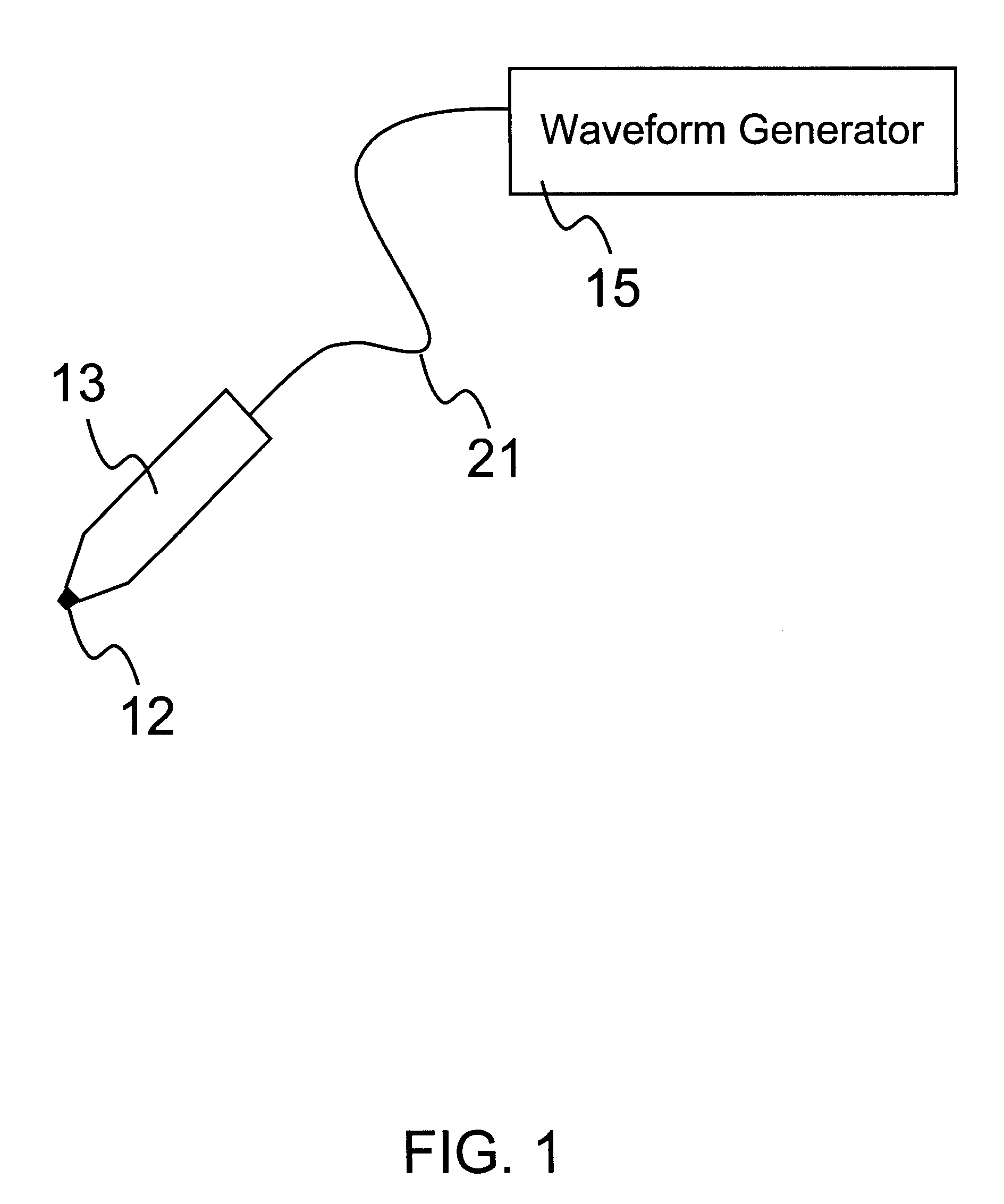
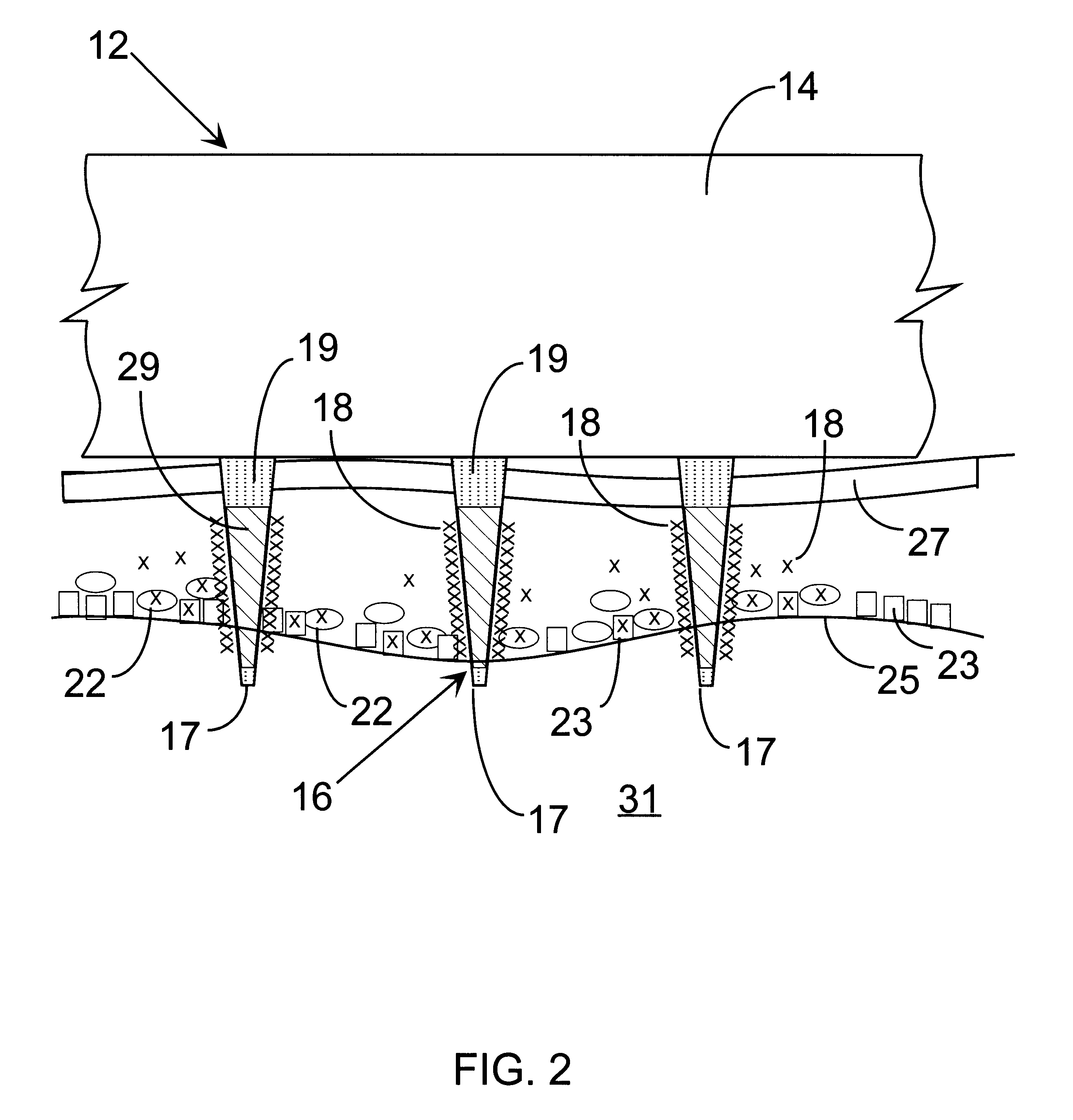
Delivery of macromolecules into cells
InactiveUS6603998B1Raise the threshold voltageReducing pulse widthBioreactor/fermenter combinationsElectrotherapyLangerhan cellA-DNA
An object of the invention is to provide a method for delivery of macromolecules into biological cells, such as Langerhans cells (22) in the epidermis (20) of a patient, which includes the steps of coating electodes (16) in an electrode assembly (12) with solid phase macromolecules to be delivered, such as a DNA, and / or RNA vaccine or a protein-based vaccine, attaching the electrode assembly (12) having the coated electrodes (16) to an electrode assembly holder (13), providing a waveform generator (15), establishing electrically conductive pathways between the electrodes (16), and the waveform generator (15), locating the electrodes (16) such that the biological cells are situated therebetween, such as by penetrating the needle electrode (16) into the epidermis (20) above the epidermal basal lamina, and providing pulse waveform from the waveform generator (15) to the electrodes (16), such that macromolecule on the electrodes (16) is driven off of the electrodes (16), and delivered into the biological cells, such as the Langerhans cells (22).
Owner:CELLECTIS SA
Adjuvant for transcutaneous immunization
A transcutaneous immunization system delivers antigen to immune cells without perforation of the skin, and induces an immune response in an animal or human. The system uses an adjuvant, preferably an ADP-ribosylating exotoxin, to induce an antigen-specific immune response (e.g., humoral and / or cellular effectors) after transcutaneous application of a formulation containing antigen and adjuvant to intact skin of the animal or human. The efficiency of immunization may be enhanced by adding hydrating agents (e.g., liposomes), penetration enhancers, or occlusive dressings to the transcutaneous delivery system. This system may allow activation of Langerhans cells in the skin, migration of the Langerhans cells to lymph nodes, and antigen presentation.
Owner:UNITED STATES OF AMERICA
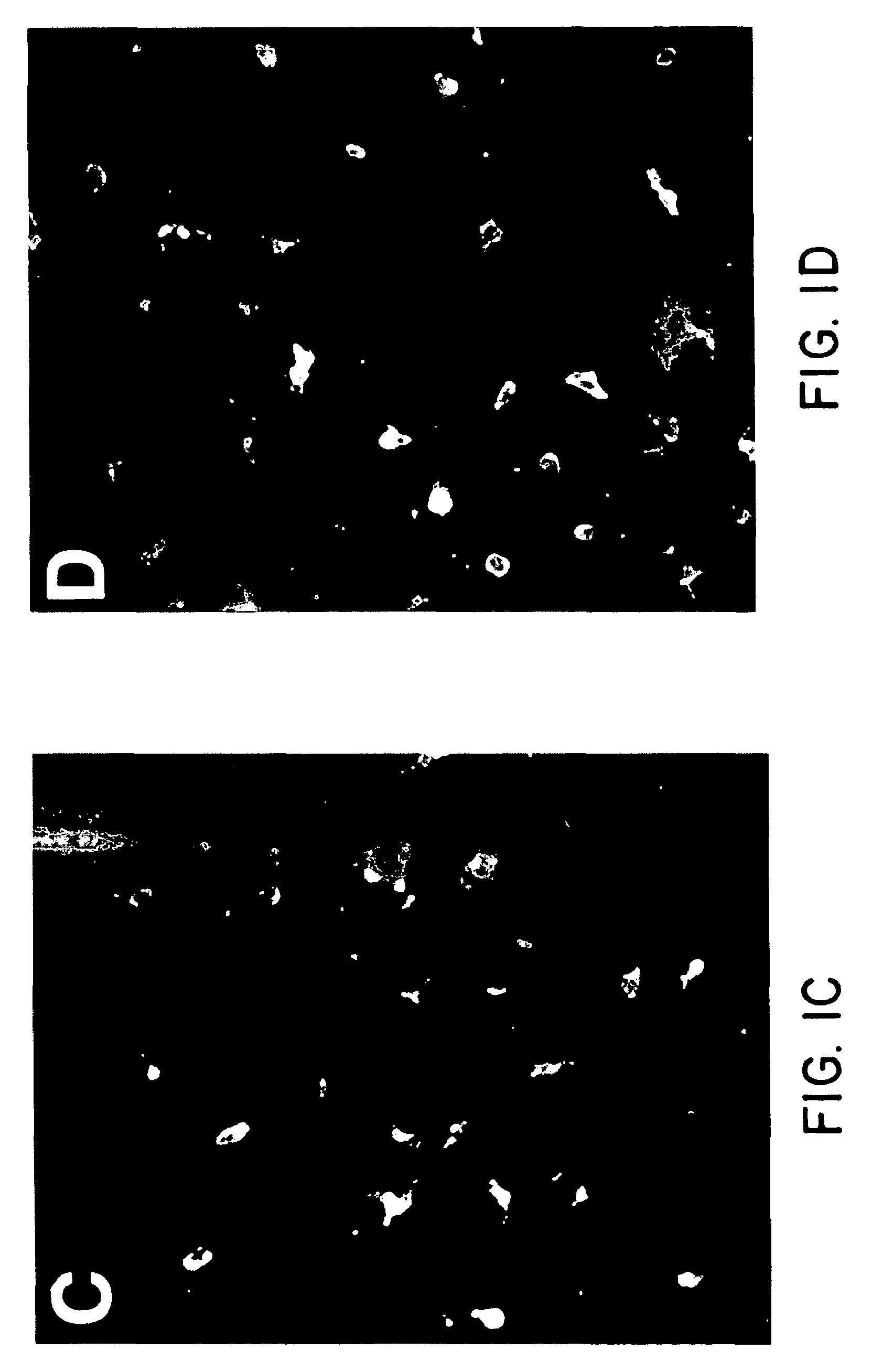
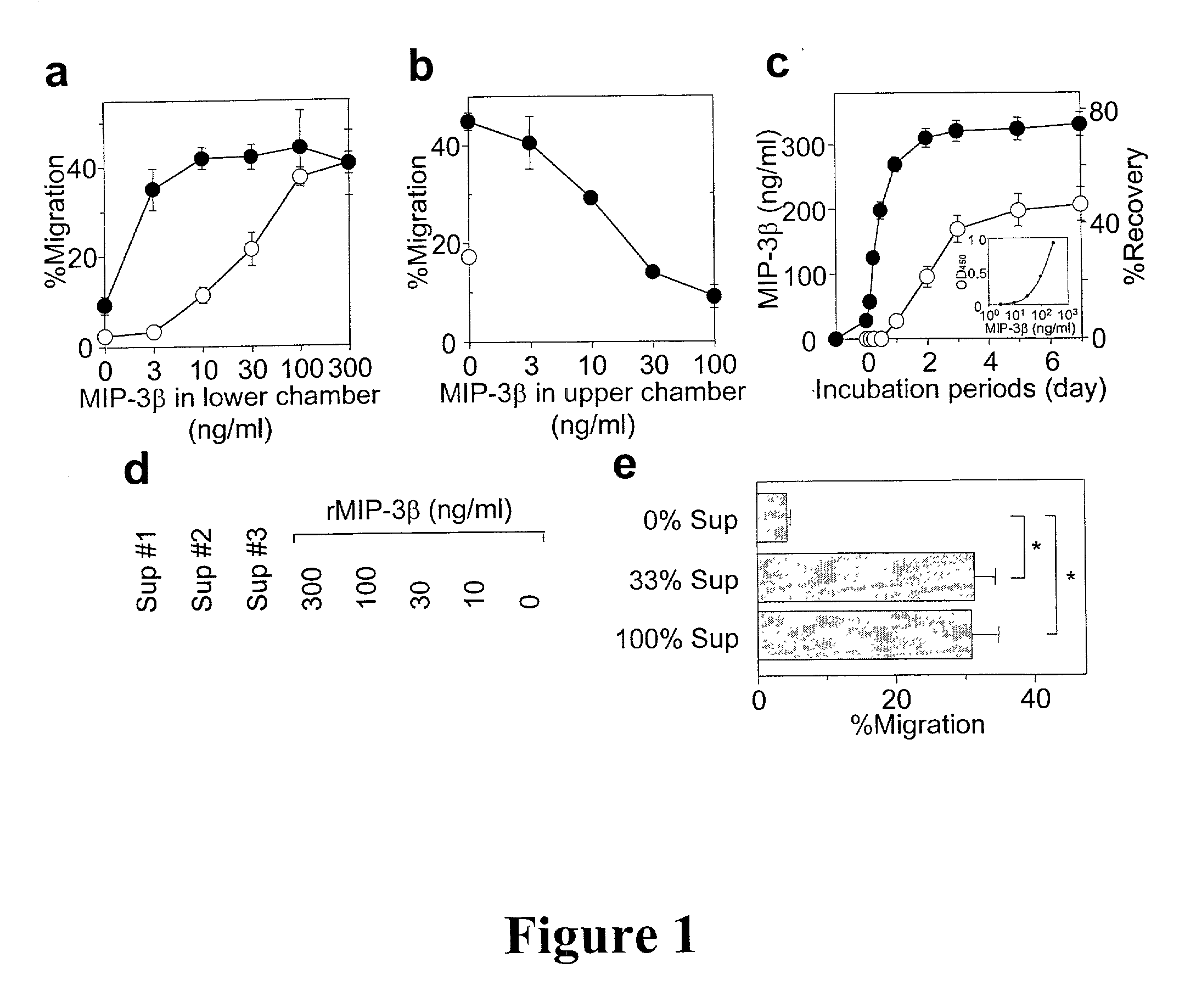
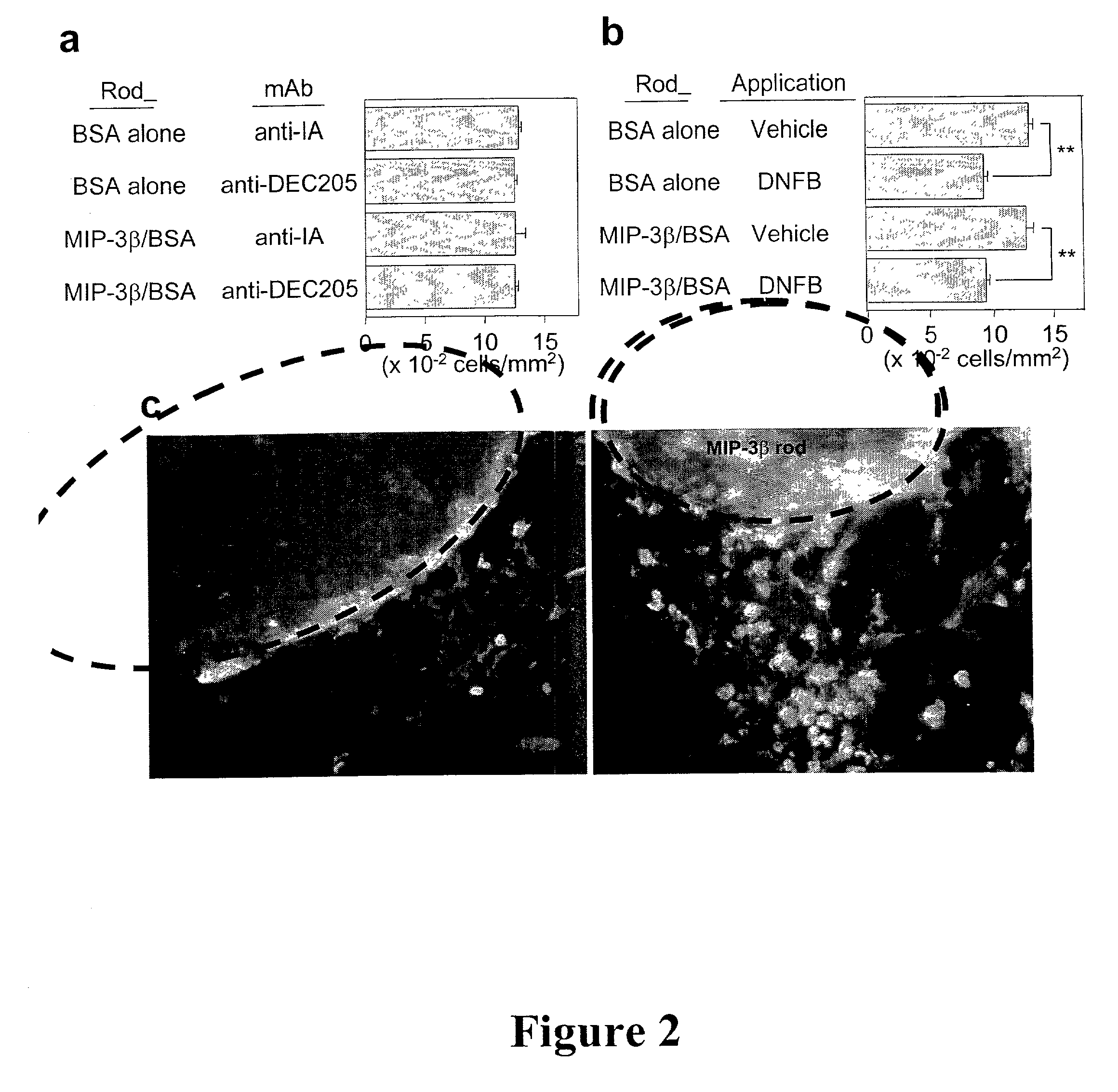
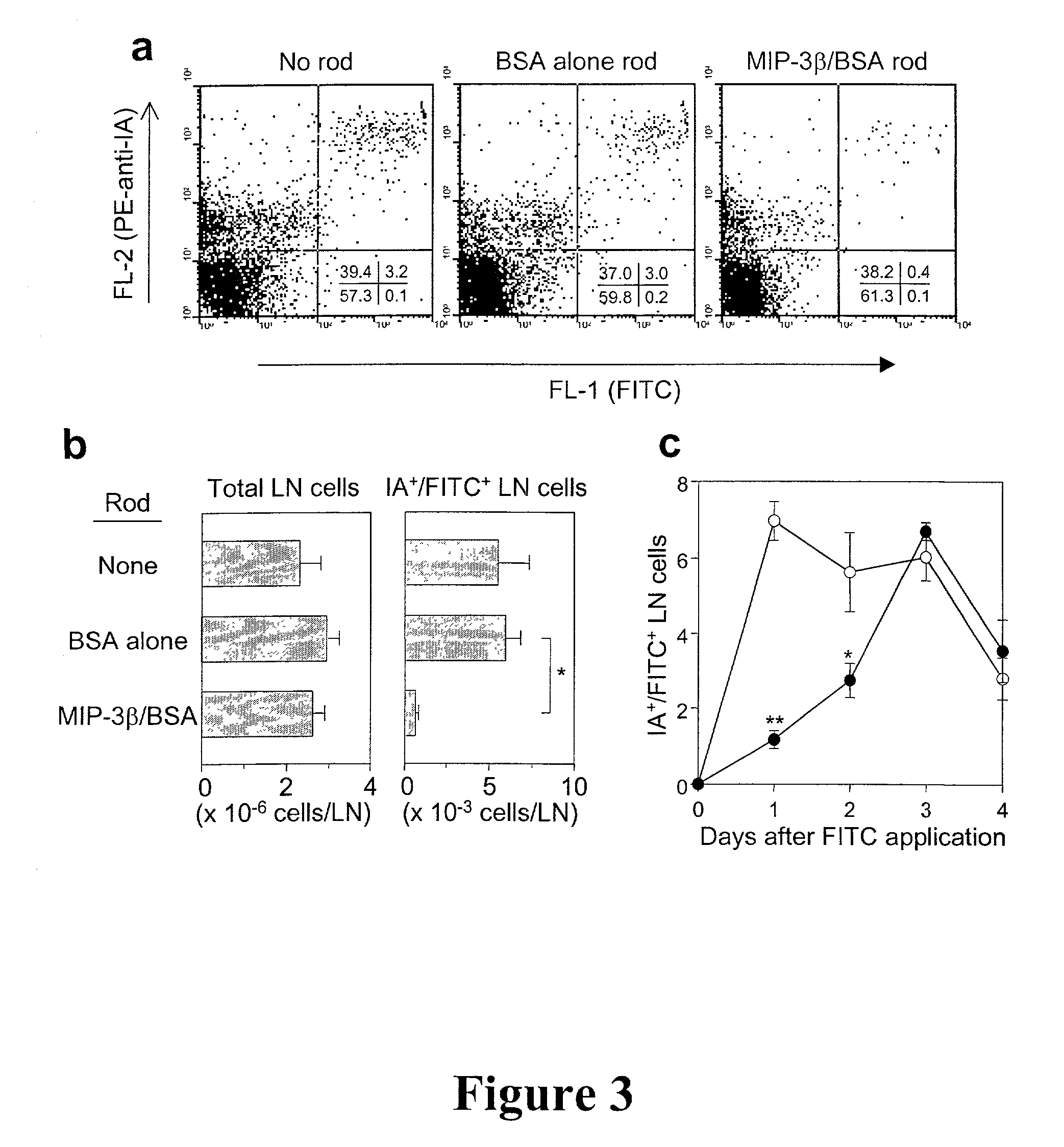
In situ langerhans cell vaccine
A method for entrapping migratory antigen presenting cells (APCs) and particularly Langerhans cells (LCs,) in vivo is provided. The method entails creating an artificial gradient of APC-attracting chemotactic factor in the homing path of APCs in vivo. Also provided is a composition for entrapping APCs and particularly, migratory LCs. In addition, a method for loading APCs in situ with antigen is provided. The method comprises entrapping APCs in vivo and subsequently loading the APCs in situ with antigen. Correspondingly, a composition for loading APCs in situ is also provided. Further provided is a method for stimulating the migration of entrapped APCs to draining lymph nodes. The ability to stimulate the migration of entrapped APCs to draining lymph nodes is useful, inter alia, for regulating an immune response in a subject. In addition, an in situ APC-based vaccine is provided which does not require any time-consuming, costly ex vivo manipulations.
Owner:US SOUTHWESTERN MEDICAL CENT
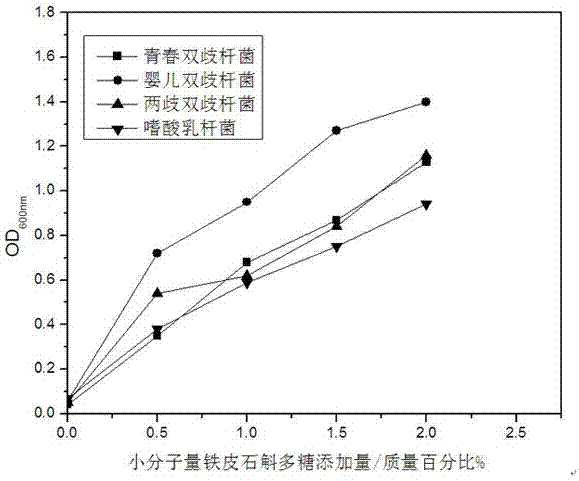
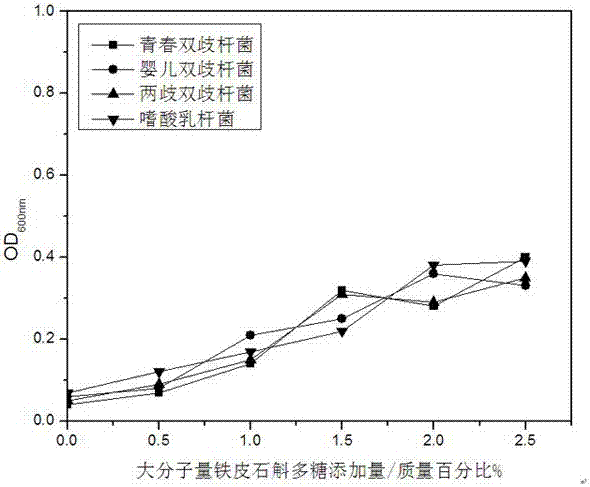
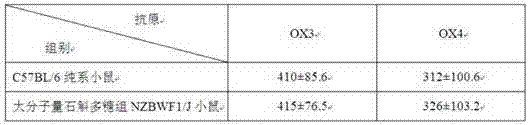
Preparation method of small molecular weight dendrobium candidum polysaccharides
ActiveCN106868072APromote proliferationMaintain healthMicroorganism based processesFermentationLangerhan cellFiltration
The invention relates to a preparation method of small molecular weight dendrobium candidum polysaccharides. The preparation method comprises following steps: A, bacterial strain activation and expanding culture are carried out, wherein under aseptic conditions, an appropriate amount of a liquid culture medium is used for preparing a fusarium avenaceum bacterium suspension; B, high pressure homogenization is repeated for 5 to 10 times using a high pressure micro jet homogenizer so as to obtain a dendrobium candidum wall-broken homogenate; C, fermentation culturing is carried out, wherein an appropriate amount of a liquid culture medium is injected into a fermentation tank, the dendrobium candidum wall-broken homogenate and the fusarium avenaceum bacterium suspension are weighed, and are delivered into the fermentation tank for fermentation so as to obtain a fermentation liquid; and D, polysaccharide purification is carried out, wherein after fermentation, active carbon is used for decolouring, an obtained product is allowed to stand for 1h for absorption, filtering is carried out so as to remove residue and obtain a filtrate, vacuum pumping filtration is carried out so as to obtain a precipitate, the precipitate is washed with ethanol for three times, and vacuum drying is carried out so as to obtain the small molecular weight dendrobium candidum polysaccharides. The average molecular weight of the small molecular weight dendrobium candidum polysaccharides is lower than 300kDa; and the small molecular weight dendrobium candidum polysaccharides are capable of promoting propagation of skin probiotics, increasing the density of langerhans cells on skin, and improving skin immunity.
Owner:PROYA COSMETICS
In vitro production of dendritic cells from CD14+ monocytes
InactiveUS20050008623A1High feasibilityHigh degree of reproducibilityBiocideCosmetic preparationsLangerhan cellMonolayer
The invention relates to the use of CD14+ monocytes for the production of dendritic cells. The invention comprises the use of CD14+ monocytes isolated from peripheral circulating blood for obtaining, by differentiation, at least one mixed population of Langerhans cells and interstitial dendritic cells, both Langerhans cells and interstitial dendritic cells being preconditioned and undifferentiated, and / or differentiated and immature, and / or mature, and / or interdigitated. The invention comprises their use in suspension, monolayer and three-dimensional cell and tissue models. The invention comprises the use of these cells and of these models as study models for the assessment of immunotoxicity / immunotolerance, for the development of cosmetic and pharmaceutical active principles and for the development and implementation of methods of cell and tissue therapy.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +1
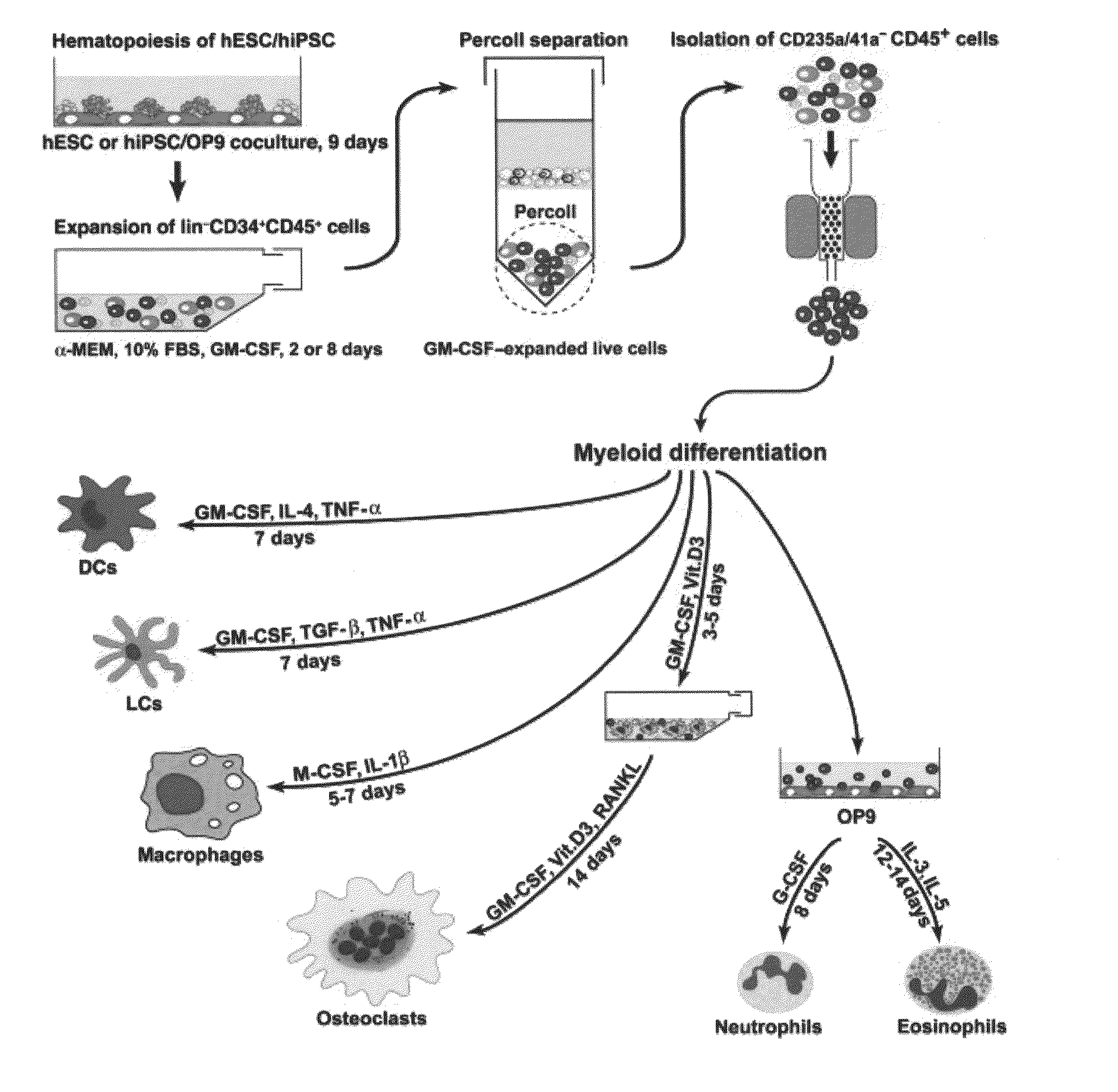
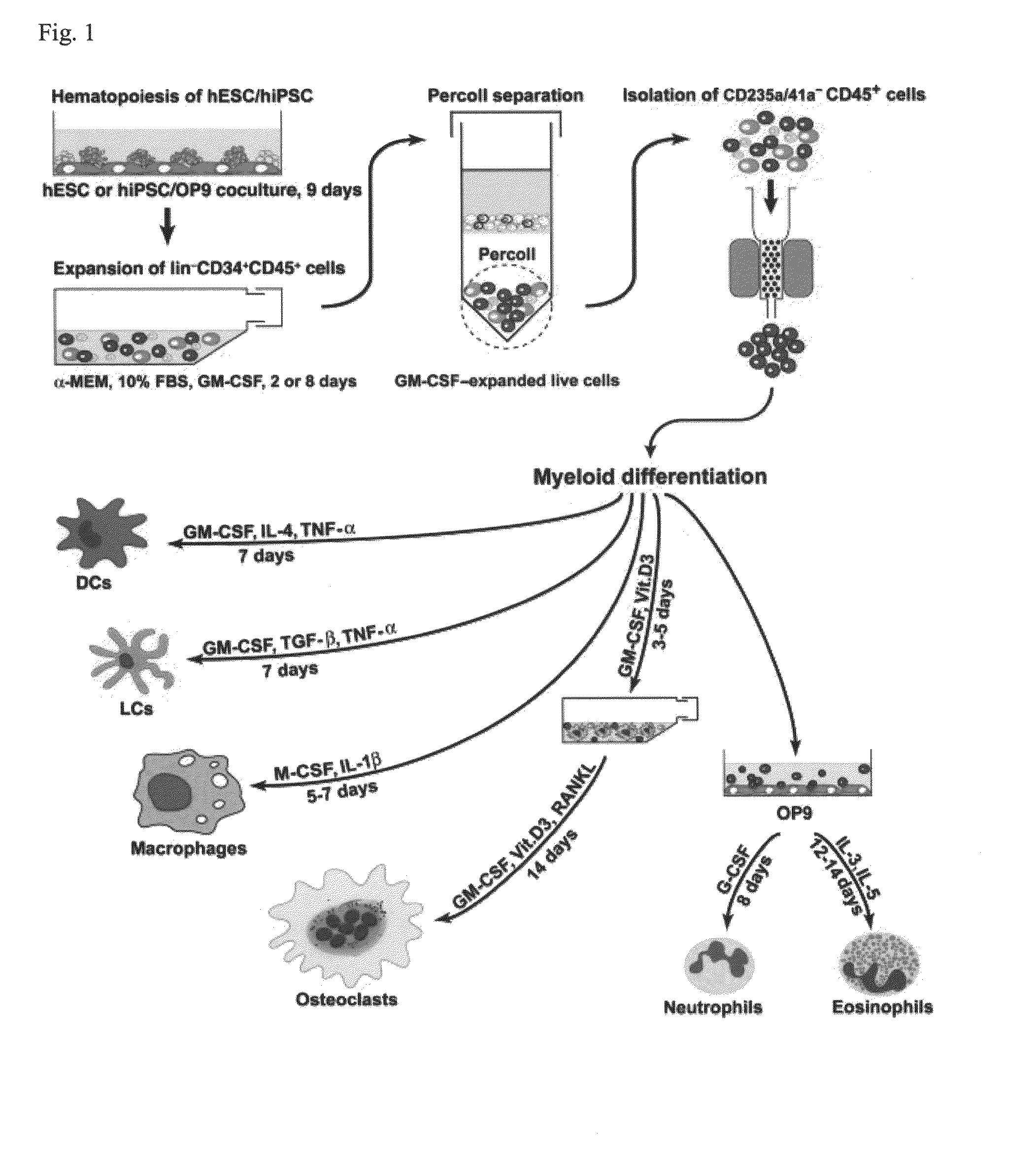
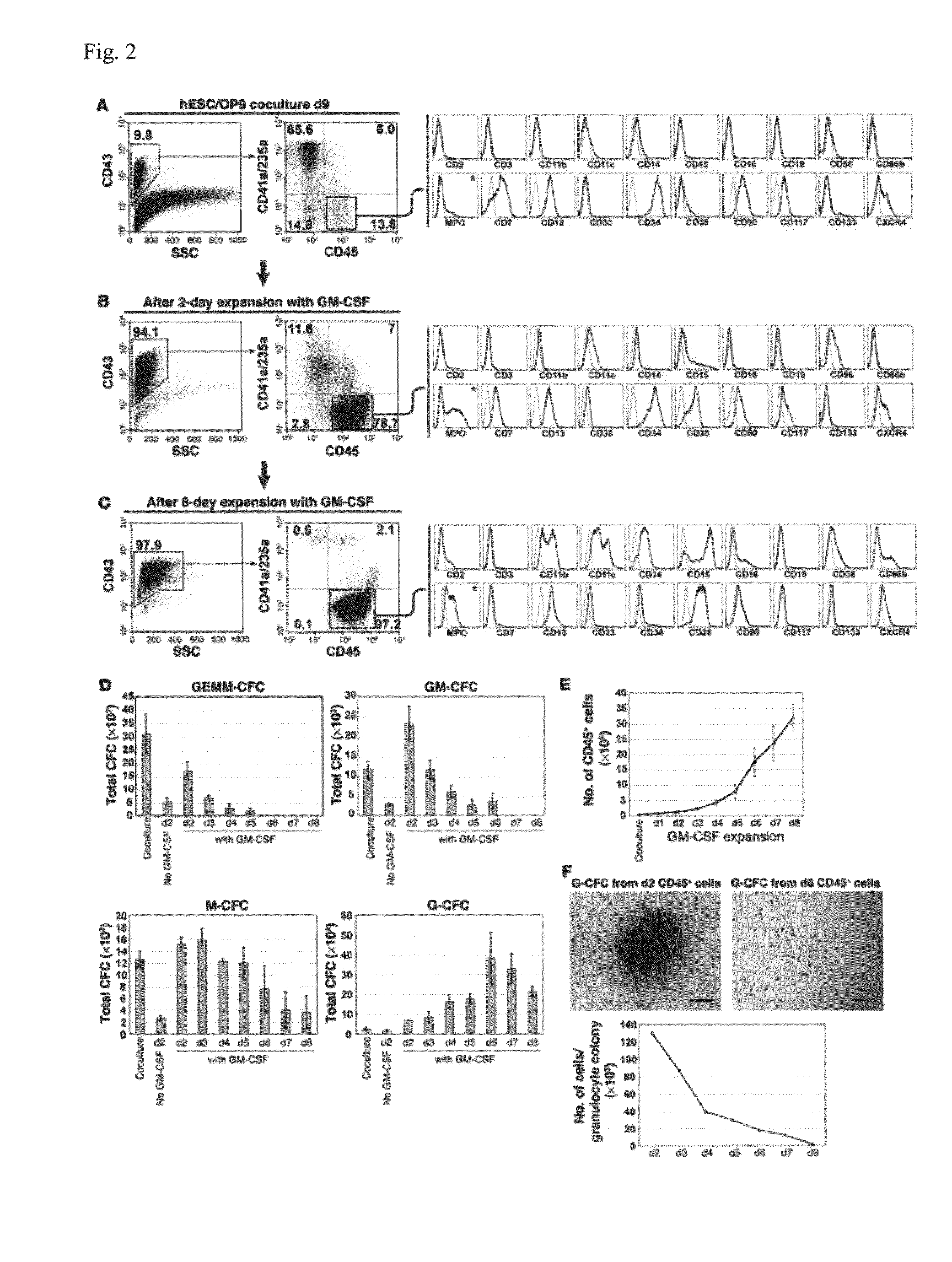
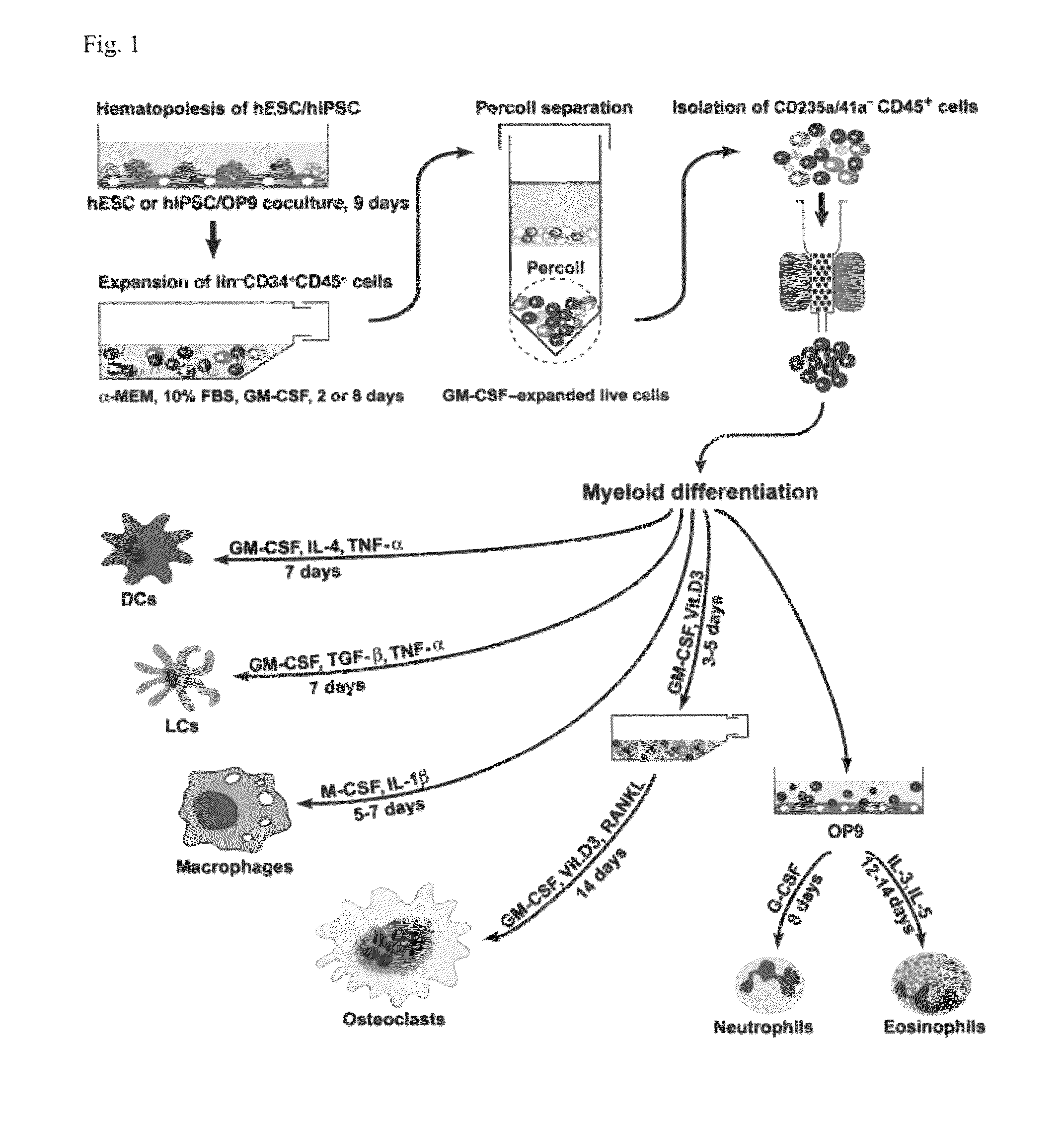
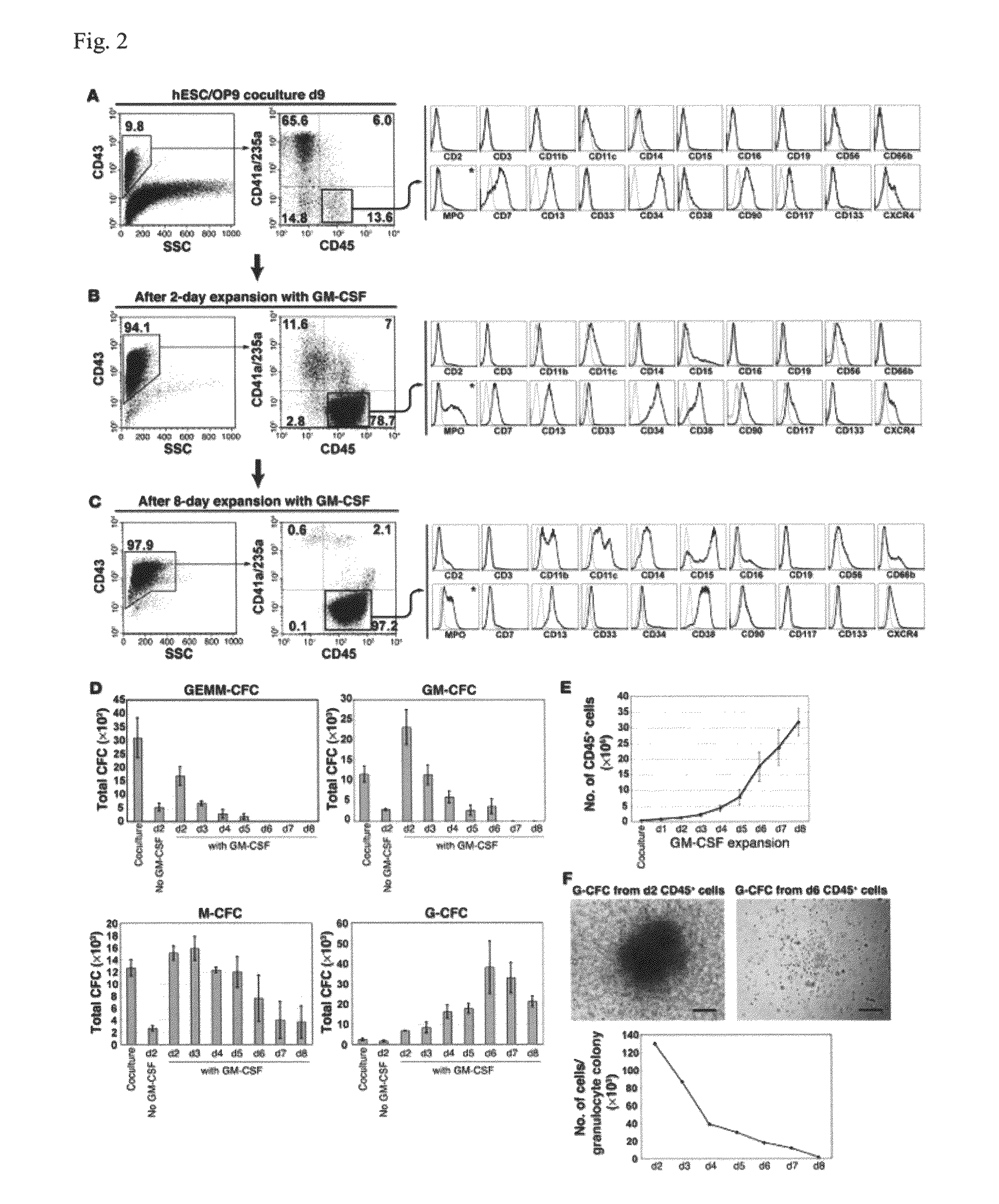
Generation of mature myelomonocytic cells through expansion and differentiation of pluripotent stem cell-derived lin-CD34+CD43+CD45+progenitors
ActiveUS20100081199A1Generate efficientlyCulture processSkeletal/connective tissue cellsLangerhan cellInduced pluripotent stem cell
Owner:WISCONSIN ALUMNI RES FOUND
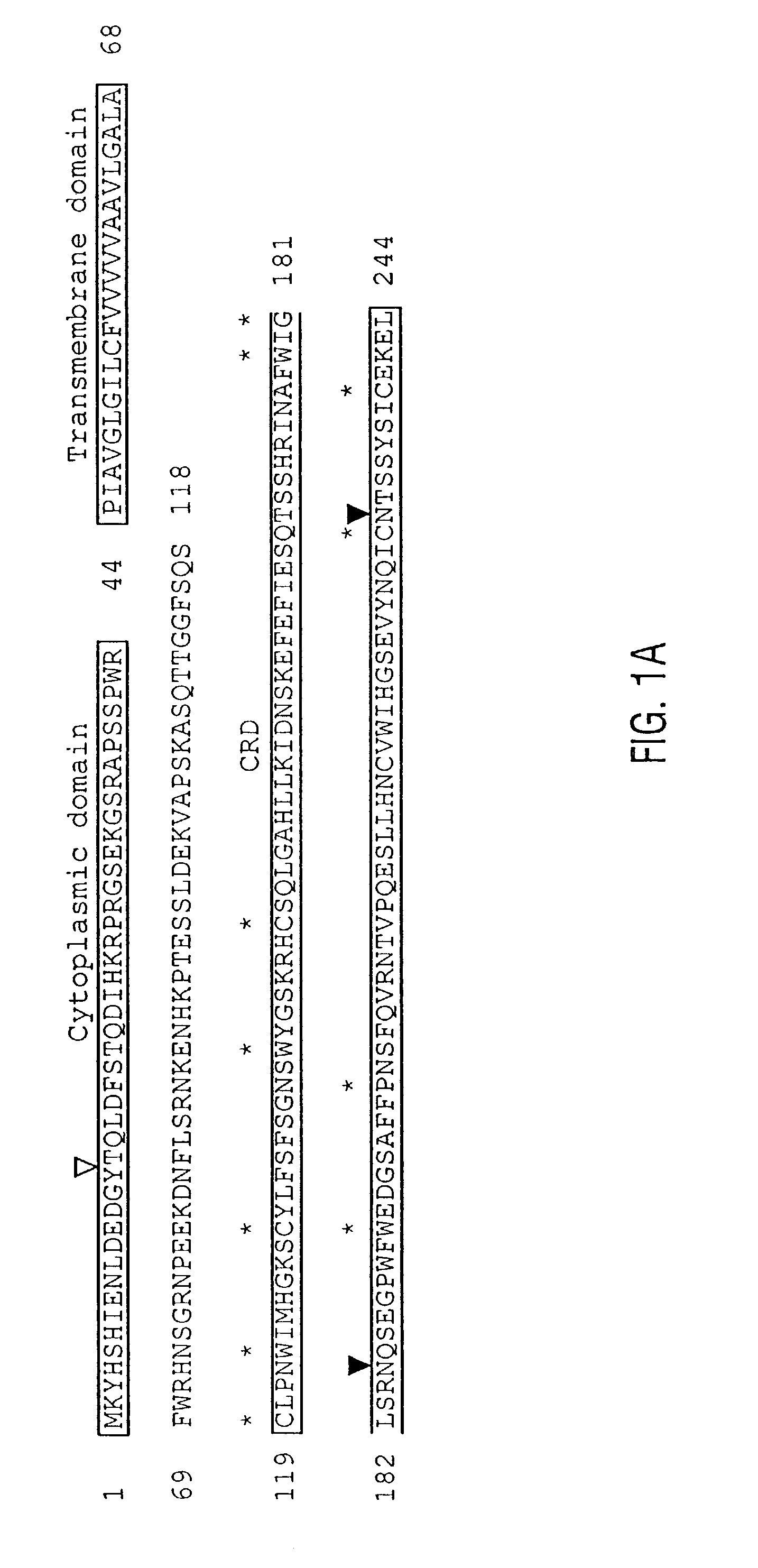
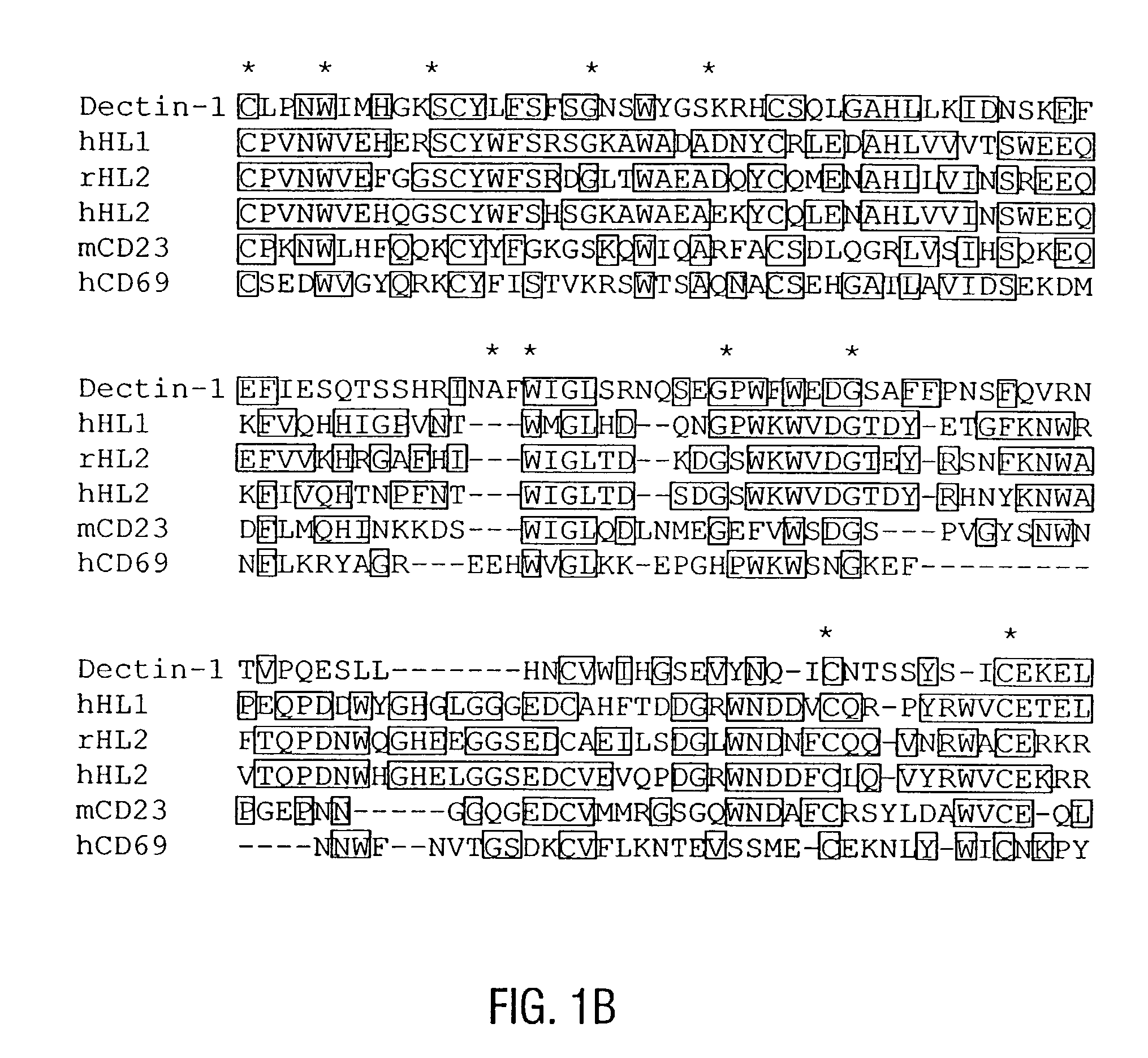
Unique dendritic cell-associated C-type lectins, dectin-1 and dectin-2; compositions and uses thereof
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
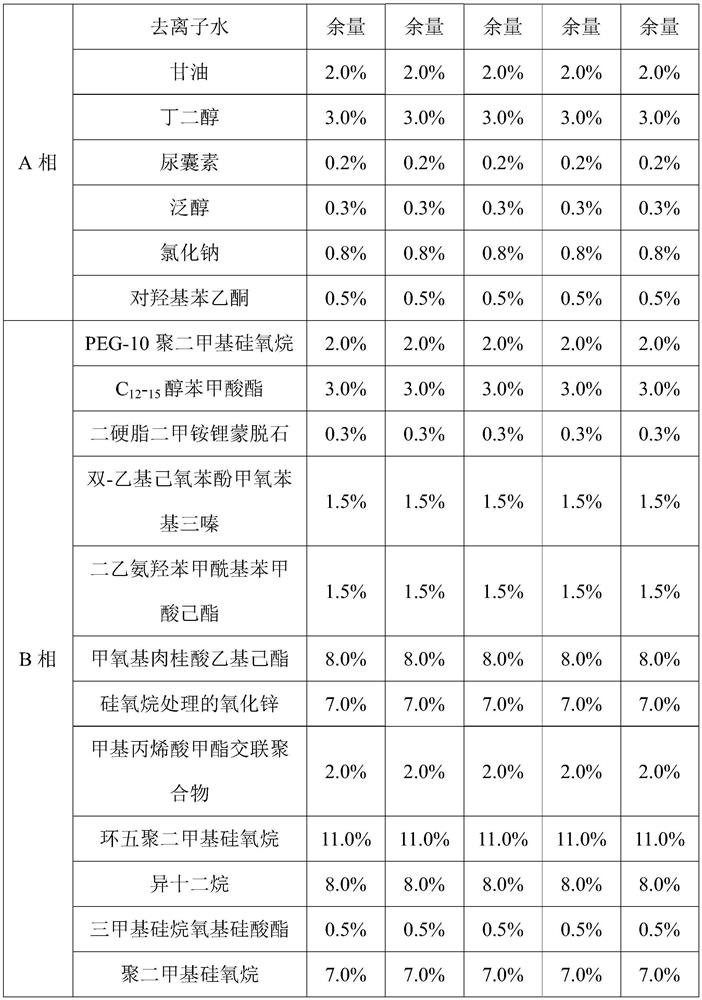
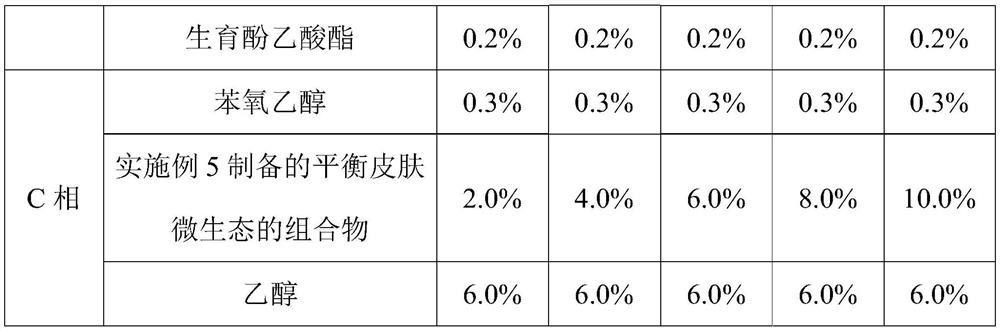
Composition for balancing skin micro-ecology as well as preparation method and application thereof
PendingCN112451431AIncrease endogenous water contentEnhanced barrier functionCosmetic preparationsAntipyreticBiotechnologyLangerhan cell
The invention relates to a composition for balancing skin micro-ecology as well as a preparation method and application thereof. The composition for balancing the skin micro-ecology comprises a saccharomycetes ferment filtrate, a bifida ferment lysate, a lactobacillus ferment, trehalose and a saccharide isomeride. According to the composition disclosed by the invention, all the components are matched with one another and have a synergistic effect, so that the composition for balancing the skin micro-ecology can preserve moisture, improve the skin barrier function, promote the proliferation ofskin beneficial bacteria, inhibit harmful bacteria, repair Langerhans cells damaged by ultraviolet rays and enhance the ultraviolet immune function of the skin. A sunscreen lotion containing the composition for balancing the skin micro-ecology can effectively preserve moisture and repair ultraviolet injury, can reduce inflammatory spots, improve skin flaws and enhance skin gloss, and has a wide development prospect in the field of cosmetics.
Owner:GUANGZHOU HUANYA COSMETIC SCI & TECH CO LTD
Generation of mature myelomonocytic cells through expansion and differentiation of pluripotent stem cell-derived lin-CD34+CD43+CD45+progenitors
ActiveUS8846395B2Generate efficientlyCulture processSkeletal/connective tissue cellsPluripotential stem cellLangerhan cell
Owner:WISCONSIN ALUMNI RES FOUND
Influenza Vaccination
InactiveUS20090004222A1Improve immunityImproved heterosubtypic immunitySsRNA viruses negative-senseViral antigen ingredientsLangerhan cellIntramuscular injection
Influenza viruses have traditionally been administered by intramuscular injection. The invention is based on the idea of using alternative routes of delivery for influenza vaccines, more specifically routes that do not require as large a dose of antigen. Delivery of influenza antigen to the Langerhans cells is the route of choice according to the invention. This route has been found to be particularly useful for vaccinating patients who are naive to influenza virus (i.e. have not previously mounted an immune response to an influenza virus), which means that it is advantageous for immunising young children.
Owner:NOVARTIS VACCINES & DIAGNOSTICS INC
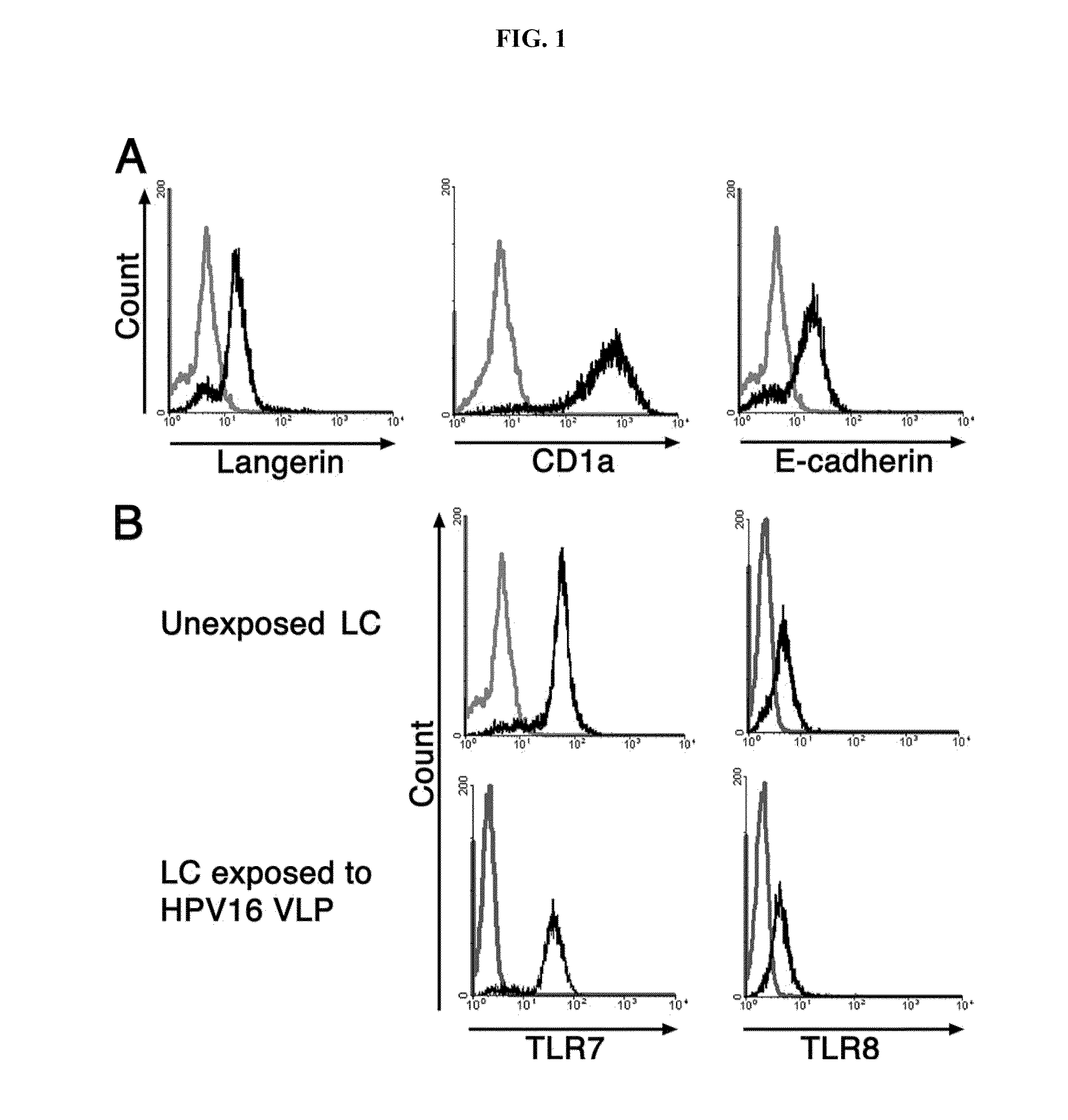
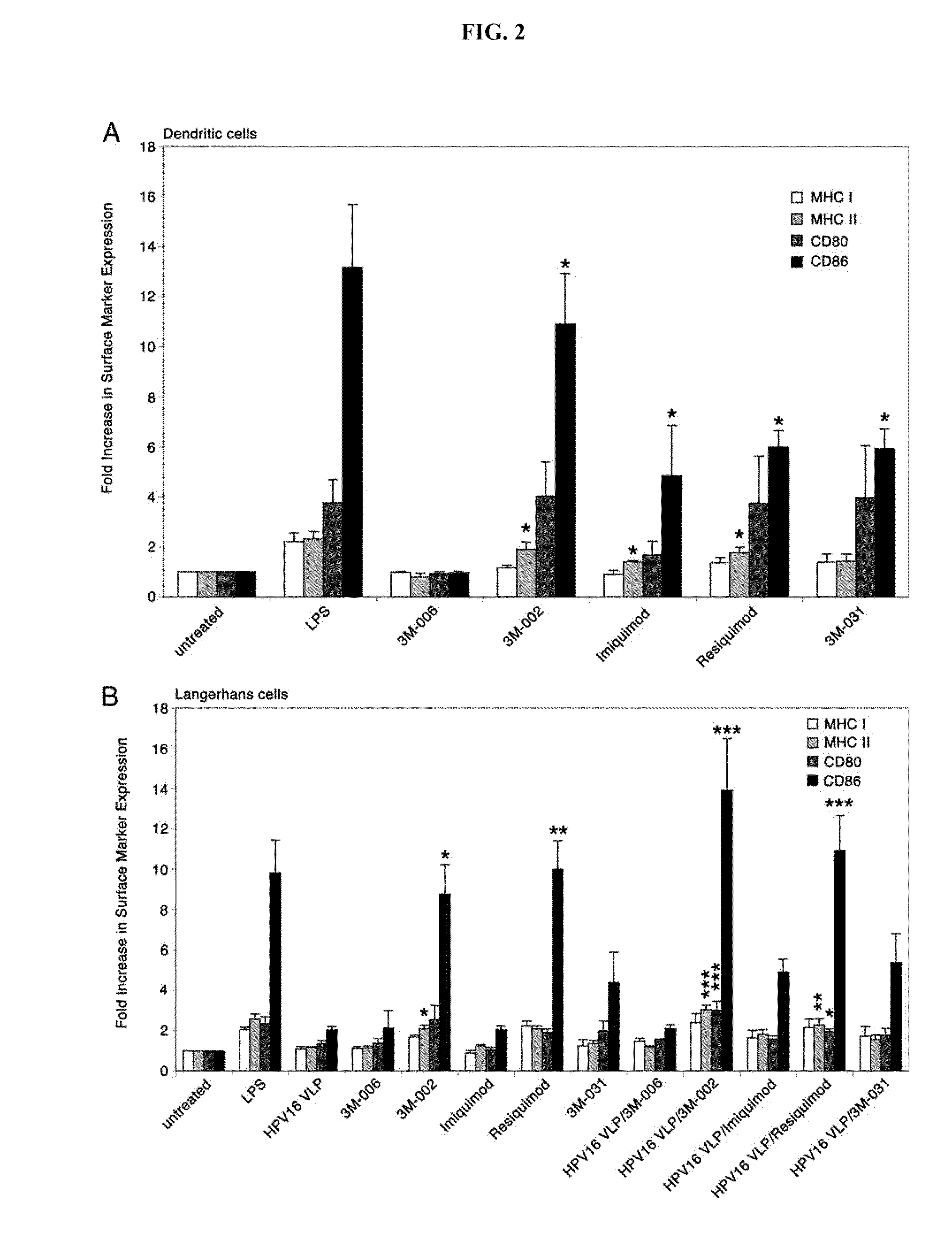
Methods and Compositions of Toll-Like Receptor (TLR) Agonists
InactiveUS20110077263A1Avoid inductionReduce likelihood of developmentBiocideMicrobiological testing/measurementLangerhan cellHuman papillomavirus
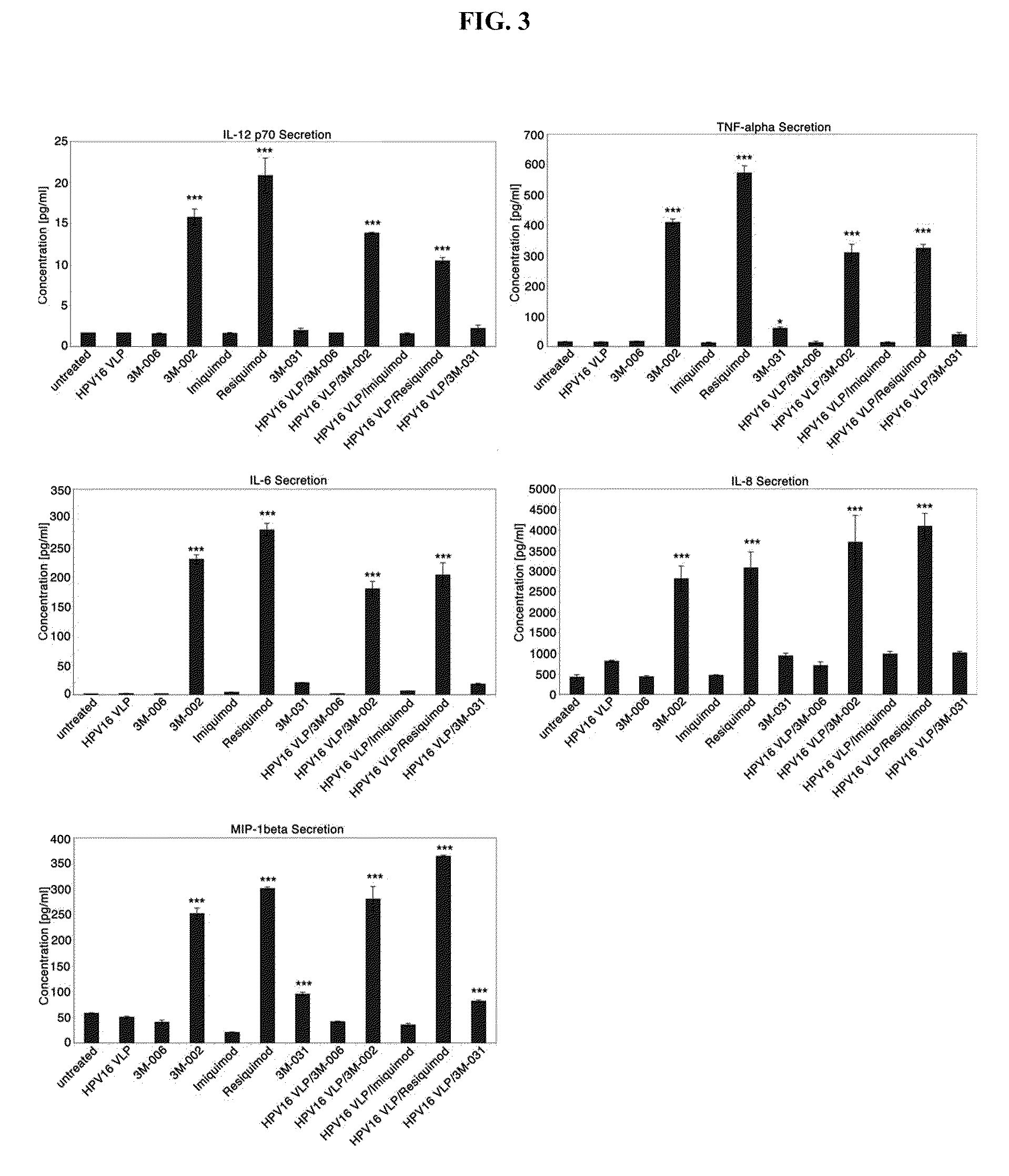
There is provided a method of activating a Langerhans cell (LC) exposed to a human papillomavirus (HPV) to induce a HPV-specific immune response, by administering to a subject an effective amount of a toll-like receptor (TLR) agonist, thereby activating the LC exposed to the HPV to induce the HPV-specific immune response.
Owner:UNIV OF SOUTHERN CALIFORNIA
Diagnosis and treatment of inflammation and hyperactive immune conditions
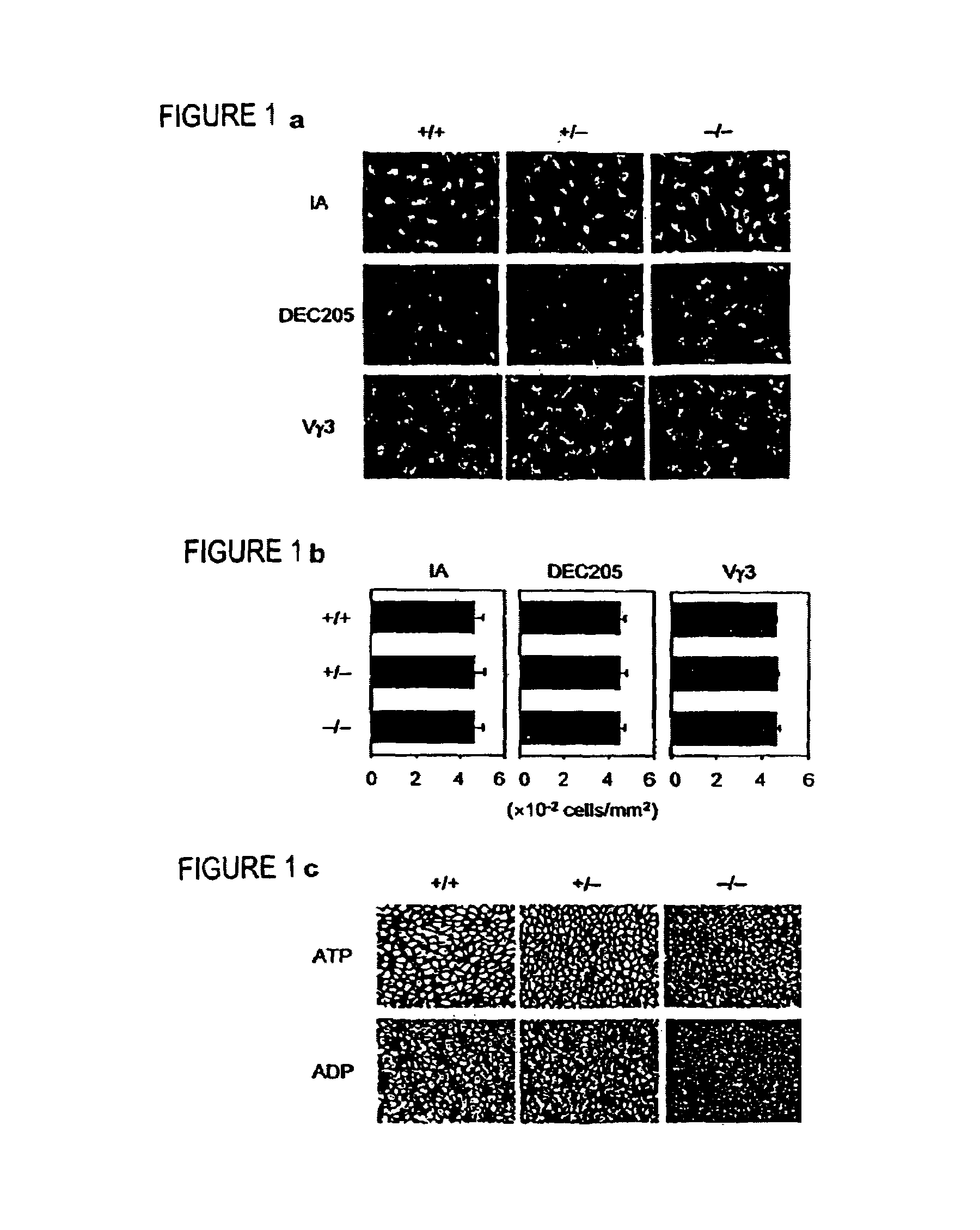
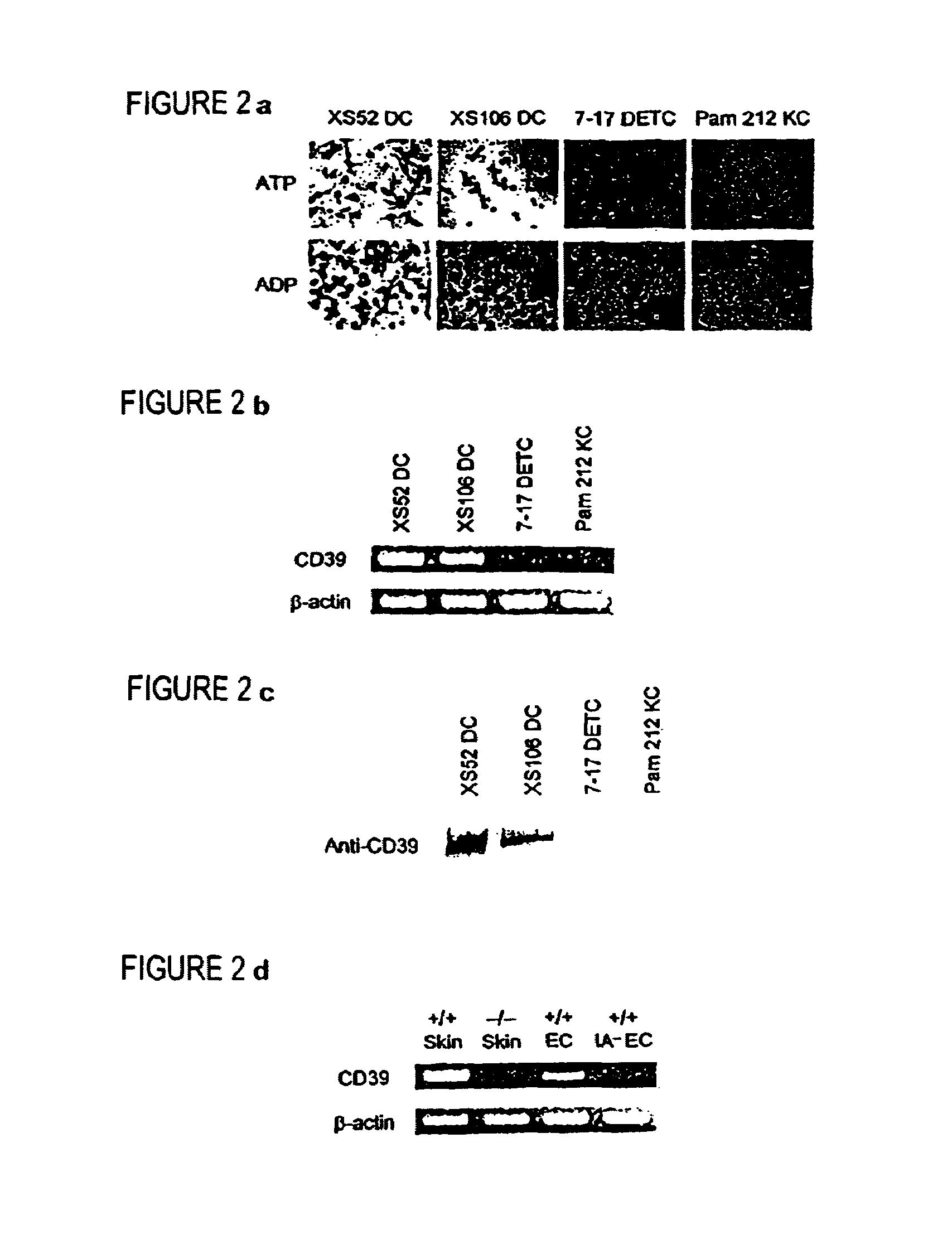
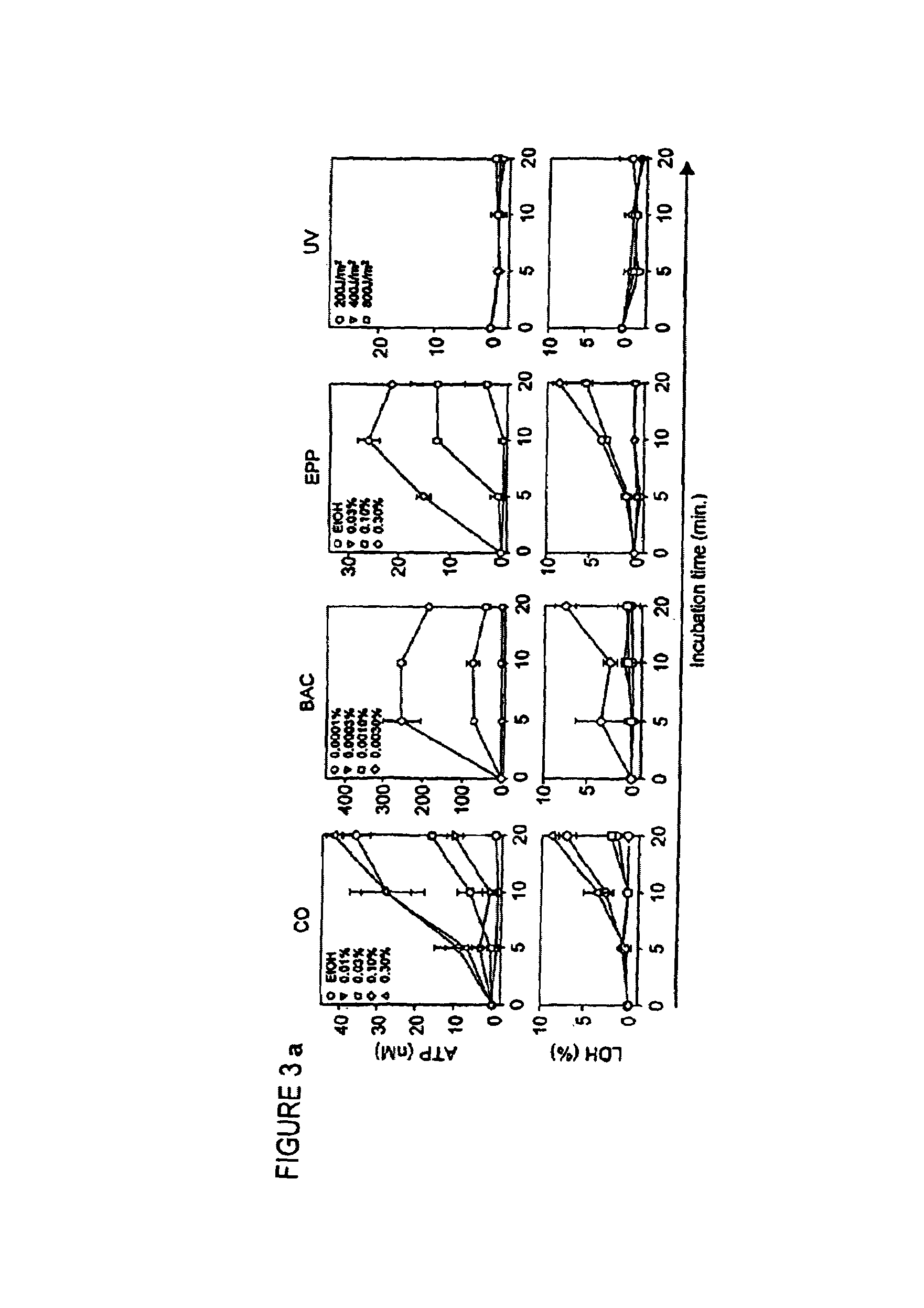
Ecto-NTPDase function on Langerhans cells is demonstrated to counteract the nucleotide inflammatory response caused by certain types of chemical irritants. The present invention takes advantage of this observation by, first, providing methods for screening of chemicals for irritant potential based on their ability to induce nucleotide release from keratinocytes. Second, methods are provided for the prevention and treatment of inflammation using NTPDase protein or gene therapy. And third, there also are provided methods for screening candidate compounds for NTPDase modulatory activity, thereby identifying possible pro- and anti-inflammatory agents. Additionally, the role of NTPDases and P2 receptors in hyperactive immune conditions such as autoimmune diseases and allergic reactions such as allergic contact dermatitis has been demonstrated. Therefore, the invention also provides methods for the prevention and treatment of hyperactive immune conditions by using NTPDase inhibitors and / or P2 receptor inhibitors. Further provided are methods for screening candidate compounds for modulatory activity of NTPDase-mediated immune conditions, thereby identifying other possible immunotherapeutic agents.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Diabetes autoantibody immunoblotting reagent kit
InactiveCN101042399AMaterial analysis by observing effect on chemical indicatorLangerhan cellDisease
This invention discloses one diabetes disease self antigen print agent case, whose islets of Langerhans cell extracts mixture protein composed of Langerhas element, glutamic acid decarboxylase and Langerhas cell; using protein print technique through SDS-polyacrylamide gel electrophoresis ranked by molecule size then into print film; its film bar comprises molecule of each Langerhans self antigen element to be put into reaction tank and serum; if it has antigen, then processing antigen combination and then adding enzyme cross and development agent in the zone; comparing with standard to judge whether serum has the antigen etc.
Owner:SHENZHEN BLOT BIOTECH
Method of immunotherapy for treament of human papillomavirus infection
ActiveUS9333238B2Avoid developmentAntibacterial agentsOrganic active ingredientsLangerhan cellHuman papilloma virus infection
A method of treating human papillomavirus (HPV), by administering a therapeutically effective amount of a primary cell-derived biologic to a patient infected with HPV, and inducing an immune response to HPV. A method of overcoming HPV-induced immune suppression of Langerhans cells (LC), by administering a therapeutically effective amount of a primary cell-derived biologic to a patient infected with HPV, and activating LC. A method of increasing LC migration towards lymph nodes, by administering a therapeutically effective amount of a primary cell-derived biologic to a patient infected with HPV, activating LC, and inducing LC migration towards lymph nodes. A method of generating immunity against HPV, by administering an effective amount of a primary cell derived biologic to a patient infected with HPV, generating immunity against HPV, and preventing new lesions from developing.
Owner:BROOKLYN IMMUNOTHERAPEUTICS LLC
Rapid one-step generation of antigen loaded dendritic cell vaccine from precursors
InactiveUS20060239962A1Peptide/protein ingredientsSnake antigen ingredientsLangerhan cellSurface marker
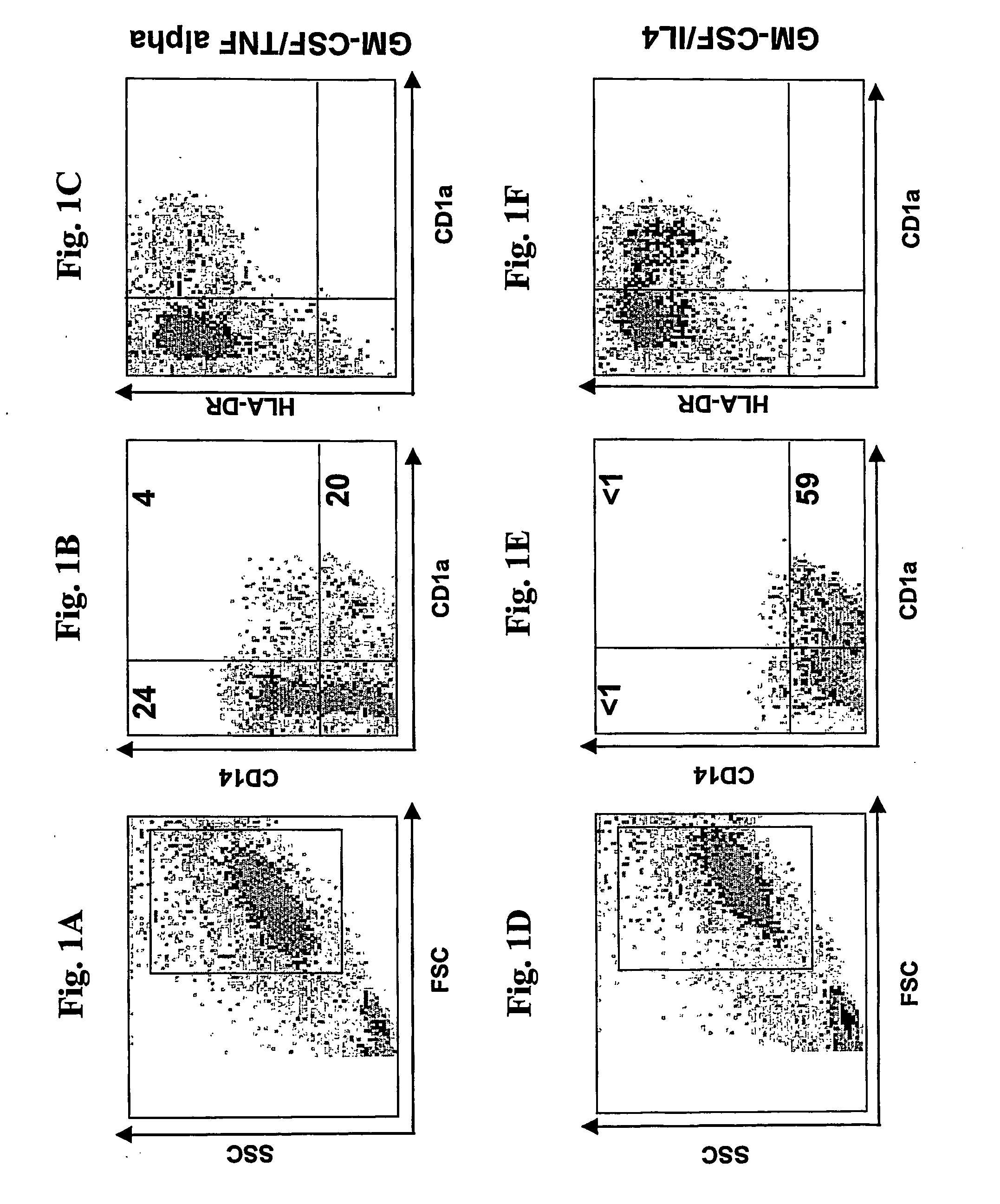
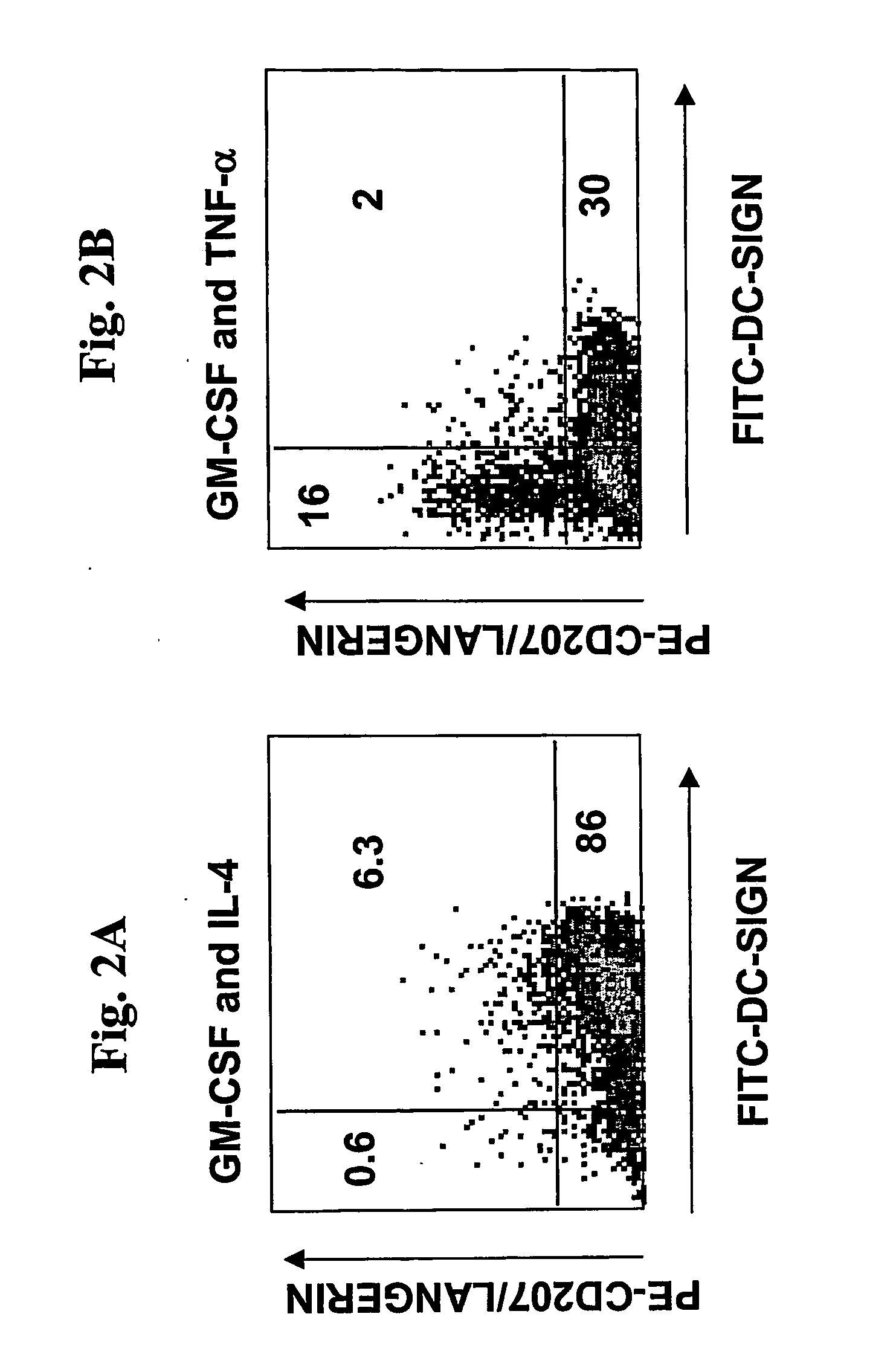
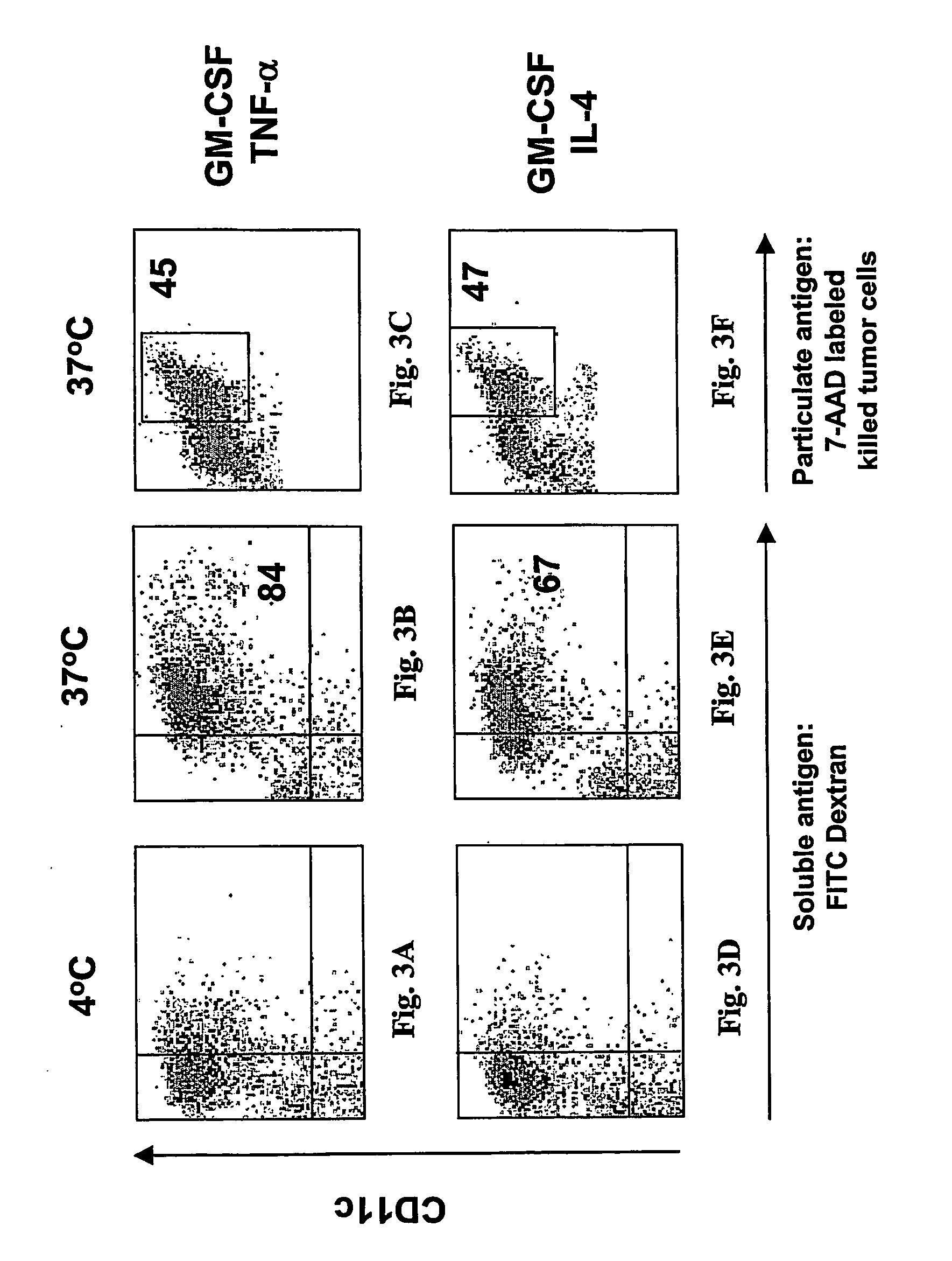
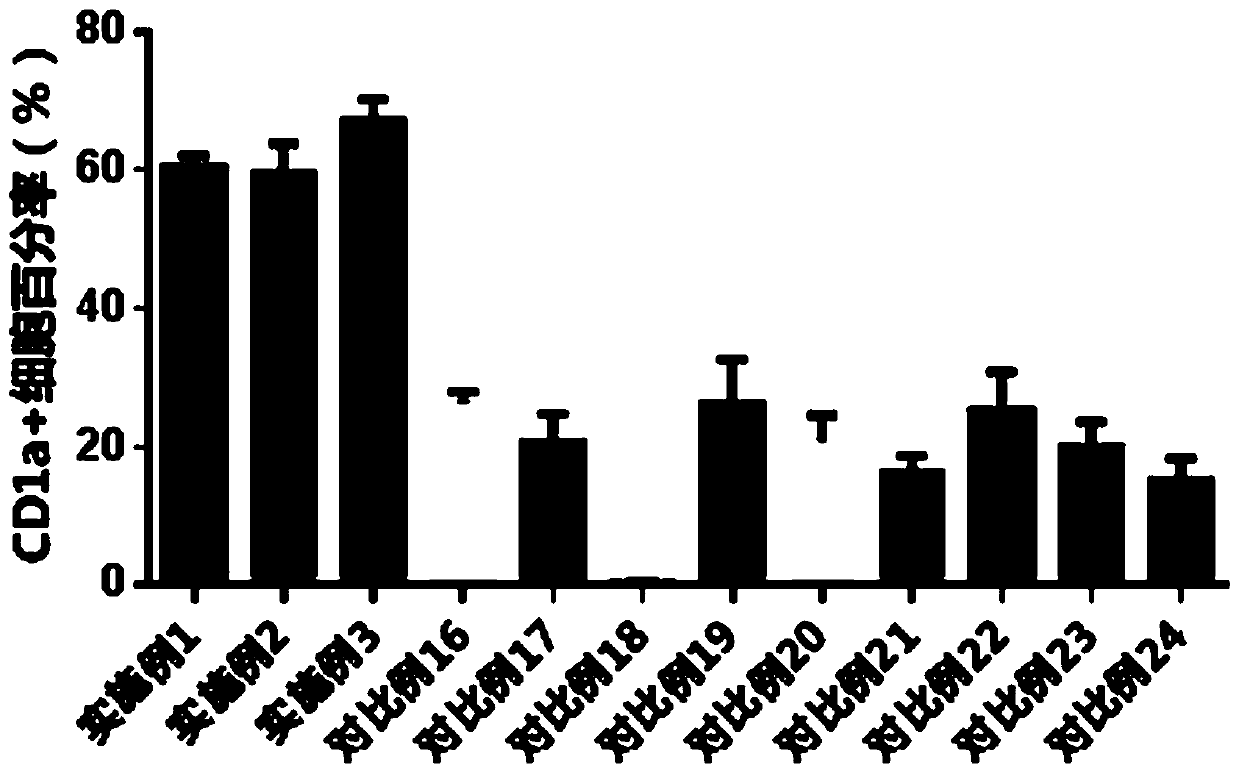
A one-step method for producing antigen loaded antigen-presenting cells from monocytes ex vivo has been found which comprises contacting the monocytes with a composition comprising an activator such as TNF alpha preferably in combination with at least one growth factor such as GM-CSF and at least one soluble or particulate antigen. According to the methods of the present invention, antigen-loaded dendritic cell vaccines can be generated within as little as three (3) days. In another method of the present invention, antigen loaded antigen-presenting cells are produced from monocytes ex vivo by contacting the monocytes with TNF alpha and granulocyte-macrophage colony stimulating factor at one time point to form antigen-presenting cells and then contacting antigen-presenting cells with soluble or particulate antigenic material antigen-presenting cells, wherein the antigen loaded antigen-presenting cells are produced in less than four days. The present invention also includes a vaccine which comprises monocyte-derived antigen loaded antigen-presenting cells, wherein the antigenpresenting cells are composed of two or more subsets selected from the group consisting of Langerhans cells with surface markers (CD 1 a+CD207+); interstitial dendritic cells with surface markers (CD 1a+CD207−); double negative dendritic cells with surface markers 20 (CD 1 a−CD 14−); and dendritic cells with surface markers (CD 14+CD 1 a−CD209+).
Owner:BAYLOR RES INST
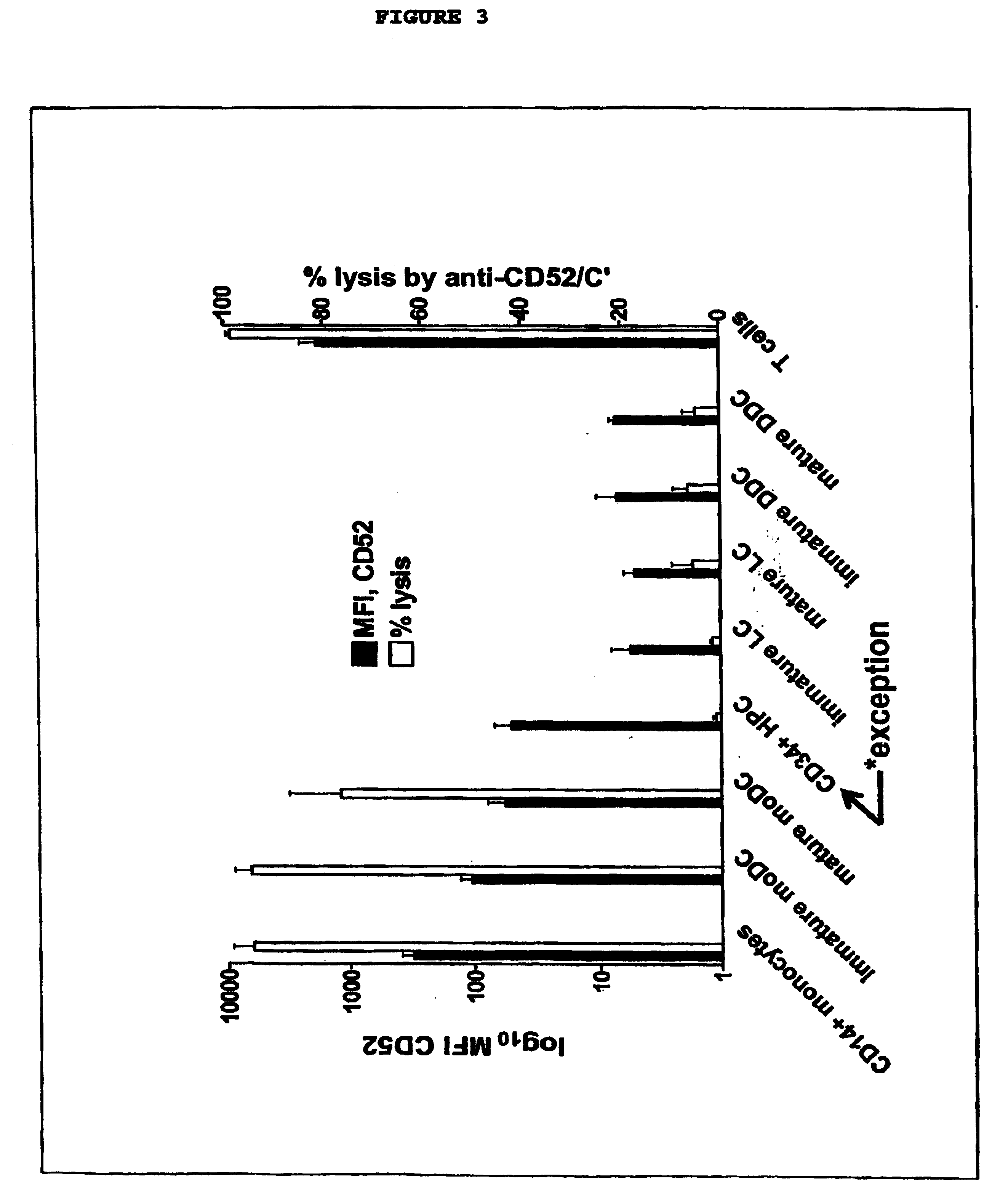
Selective elimination of cd52and uses thereof
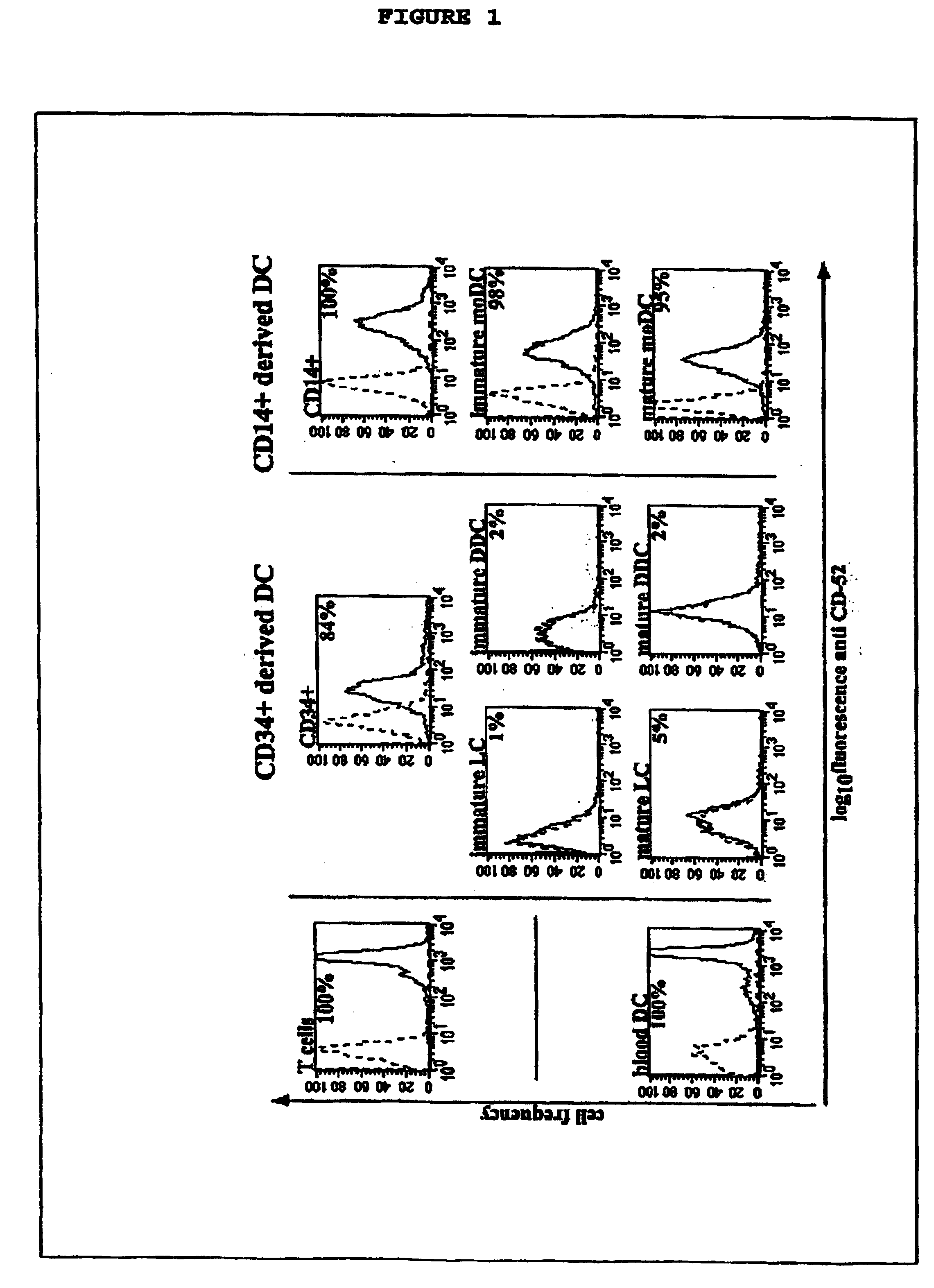
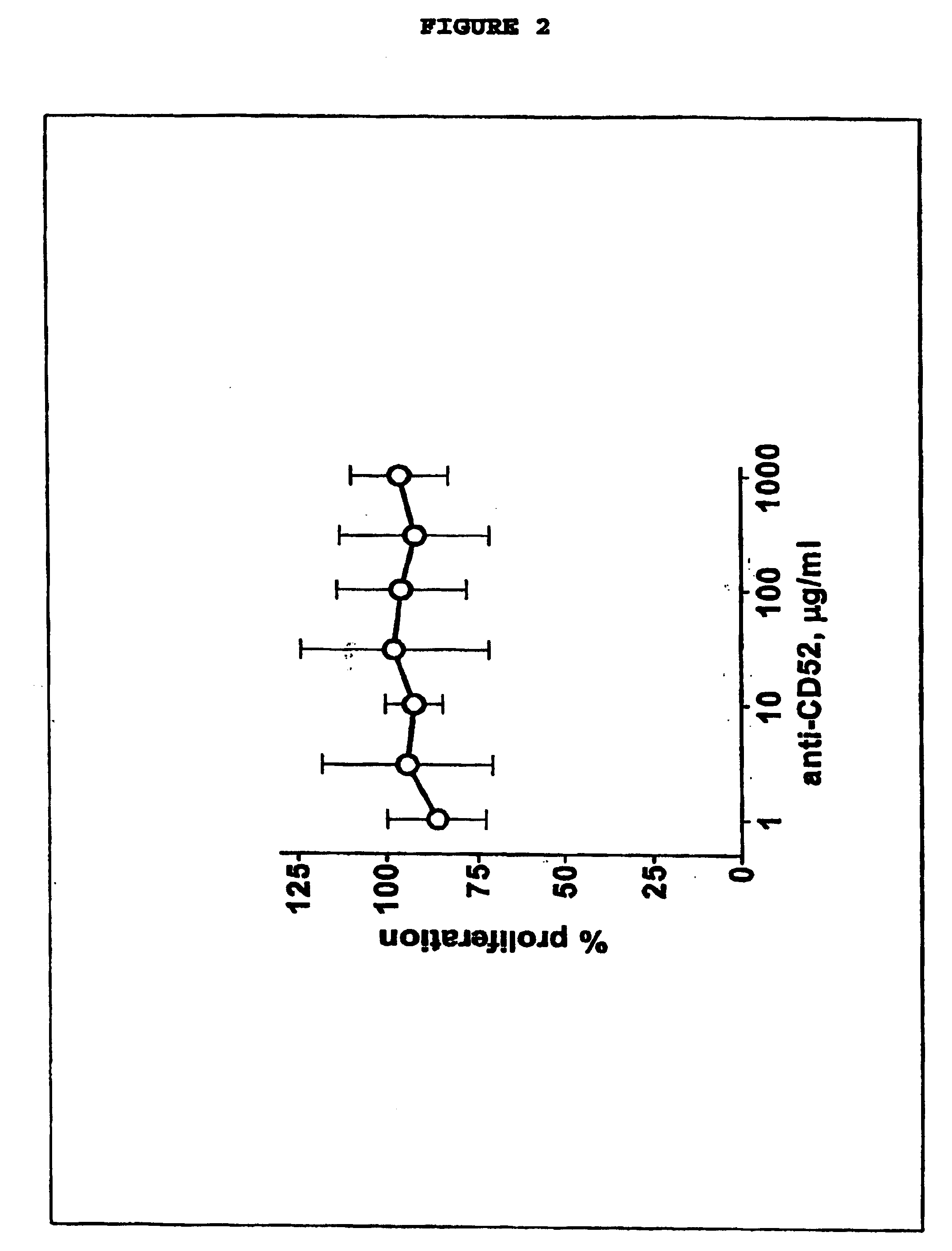
InactiveUS20050026854A1Genetic material ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsLangerhan cellInterstitial Dendritic Cells
This invention provides an agent capable of destruction of CD52+ cells, including CD52+ dendritic cells, without affecting CD52 negative dendritic cells. CD52 negative dendritic cells include hut are not limited to Langerhans cells (LCs) or dermal-interstitial dendritic cells (DDC-IDCs). This invention also provides an agent capable or selective elimination of CD52+ dendritic cells. This invention further provides an agent capable of protection of CD52 negative dendritic cells as well as a composition comprising the above described agent, a pharmaceutical composition comprising an effective amount of the above agent, and a pharmaceutically acceptable carrier. Finally, this invention provides various uses of the above agent and the above compositions.
Owner:SLOAN KETTERING INST FOR CANCER RES
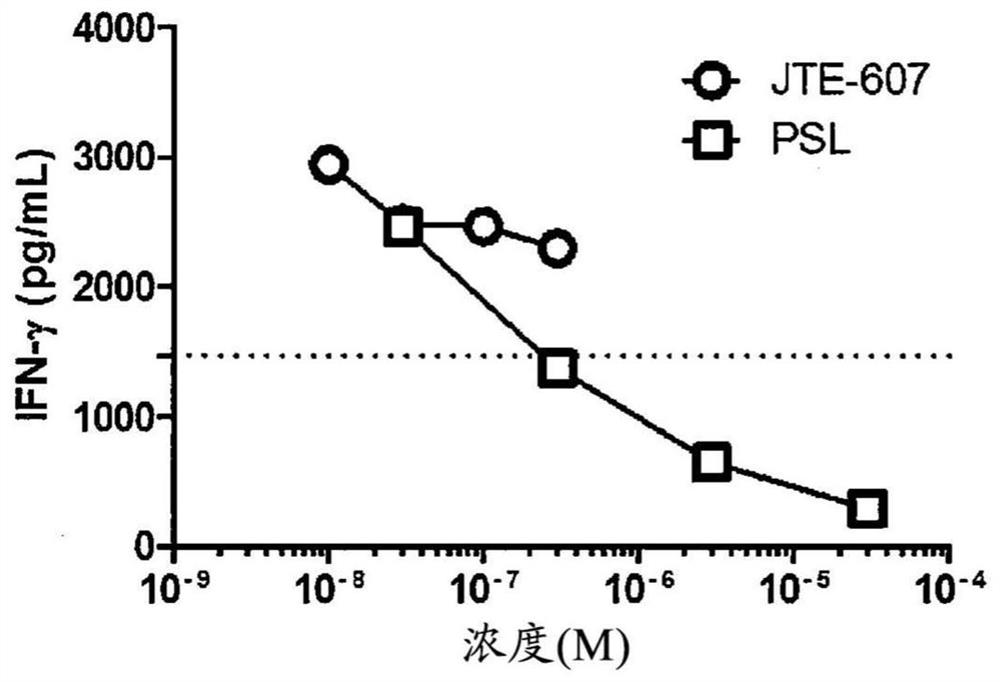
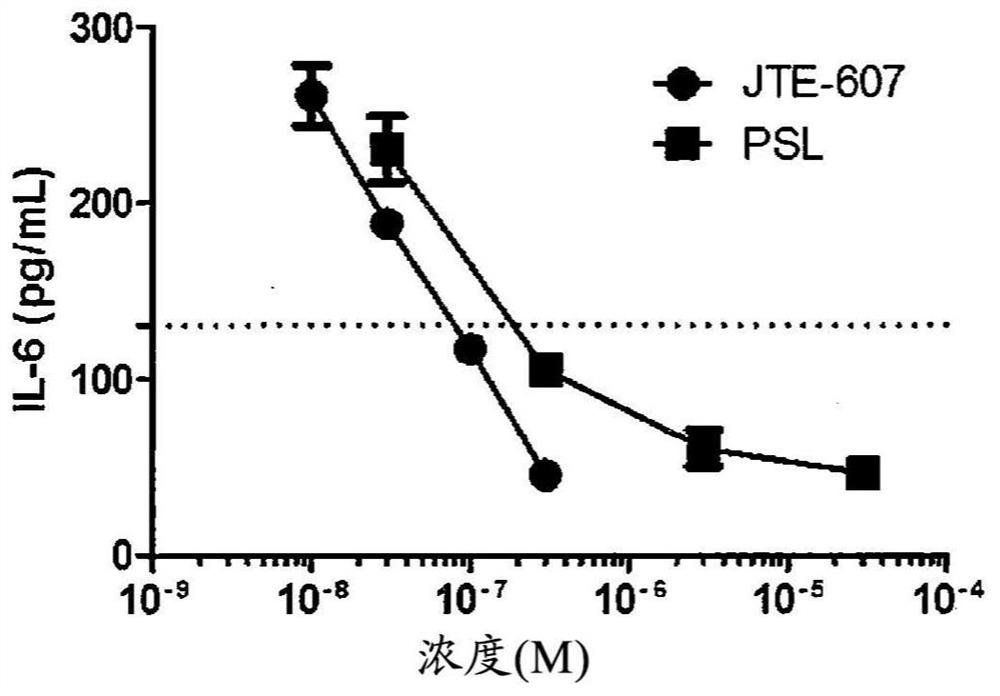
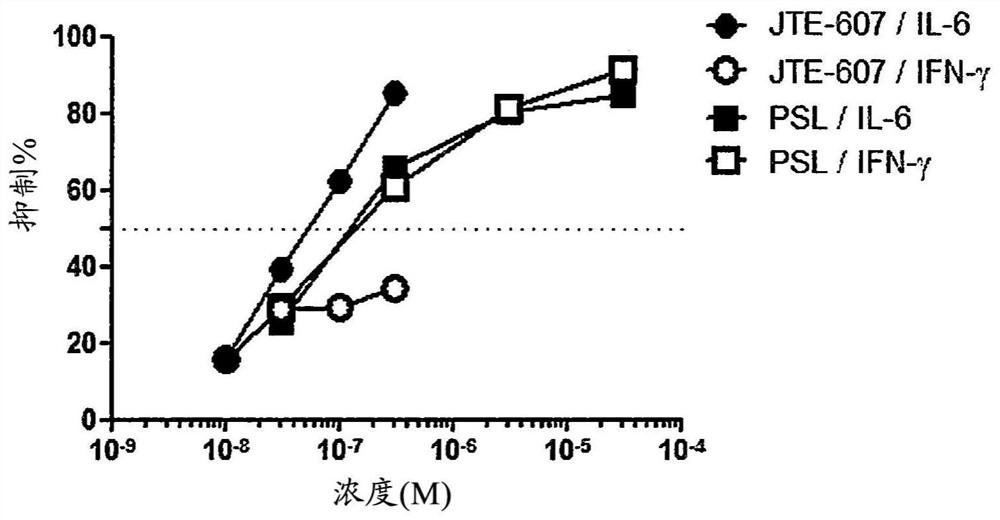
Agent for improving cytokine release syndrome, etc.
PendingCN112105362AInhibition releasePossibility of enhanced effectOrganic active ingredientsAntipyreticLangerhan cellMacrophage activation syndrome
To provide a drug for cytokine release syndrome, autoimmune-disease-related side effects, macrophage activation syndrome, hemophagocytic lymphohistiocytosis, or Langerhans cell histiocytosis. A drug for at least one selected from the group consisting of cytokine release syndrome, autoimmune-disease-related side effects, macrophage activation syndrome, hemophagocytic lymphohistiocytosis, and Langerhans cell histiocytosis, the drug containing a compound represented by formula I or a pharmacologically acceptable salt thereof.
Owner:TORII PHARMA
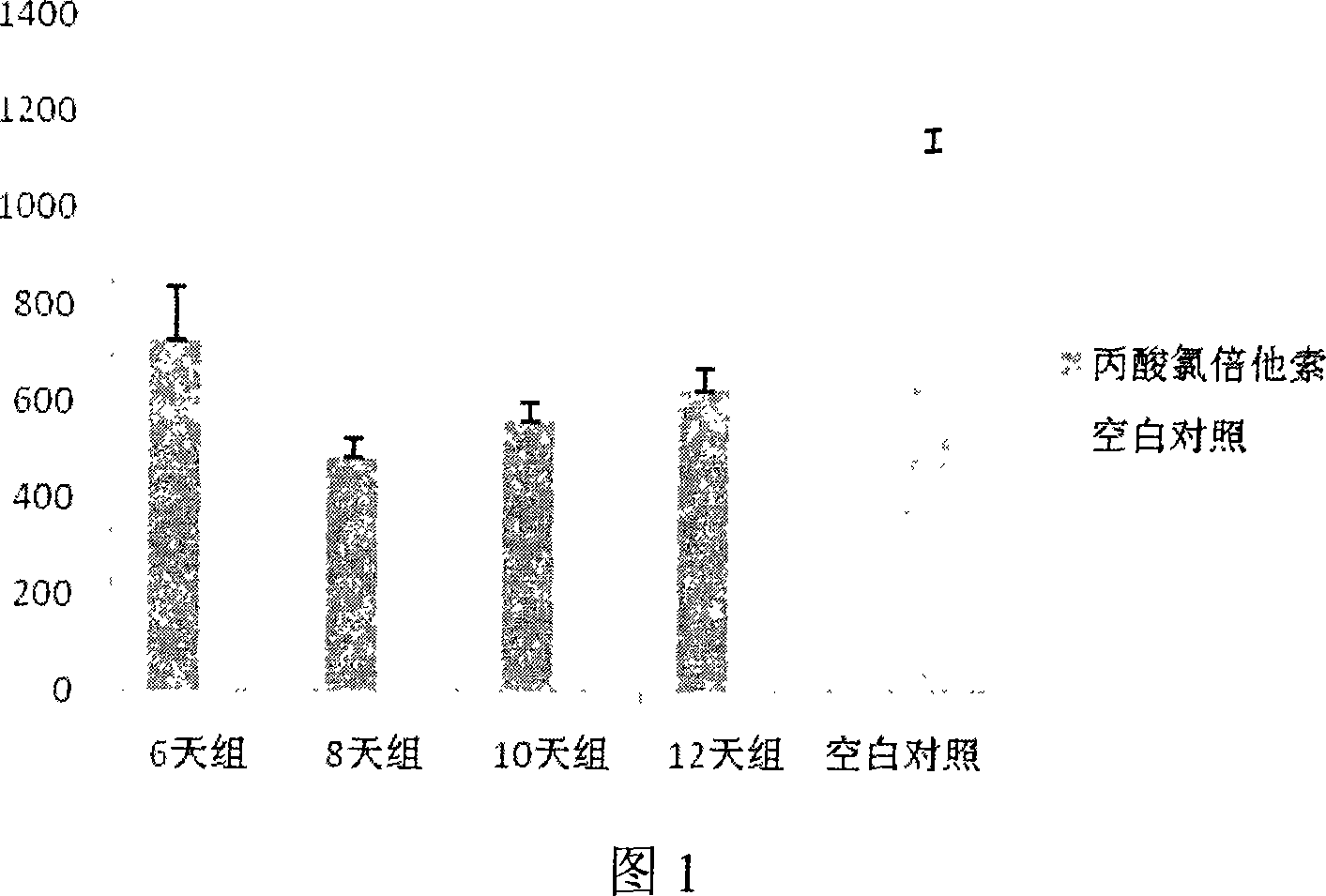
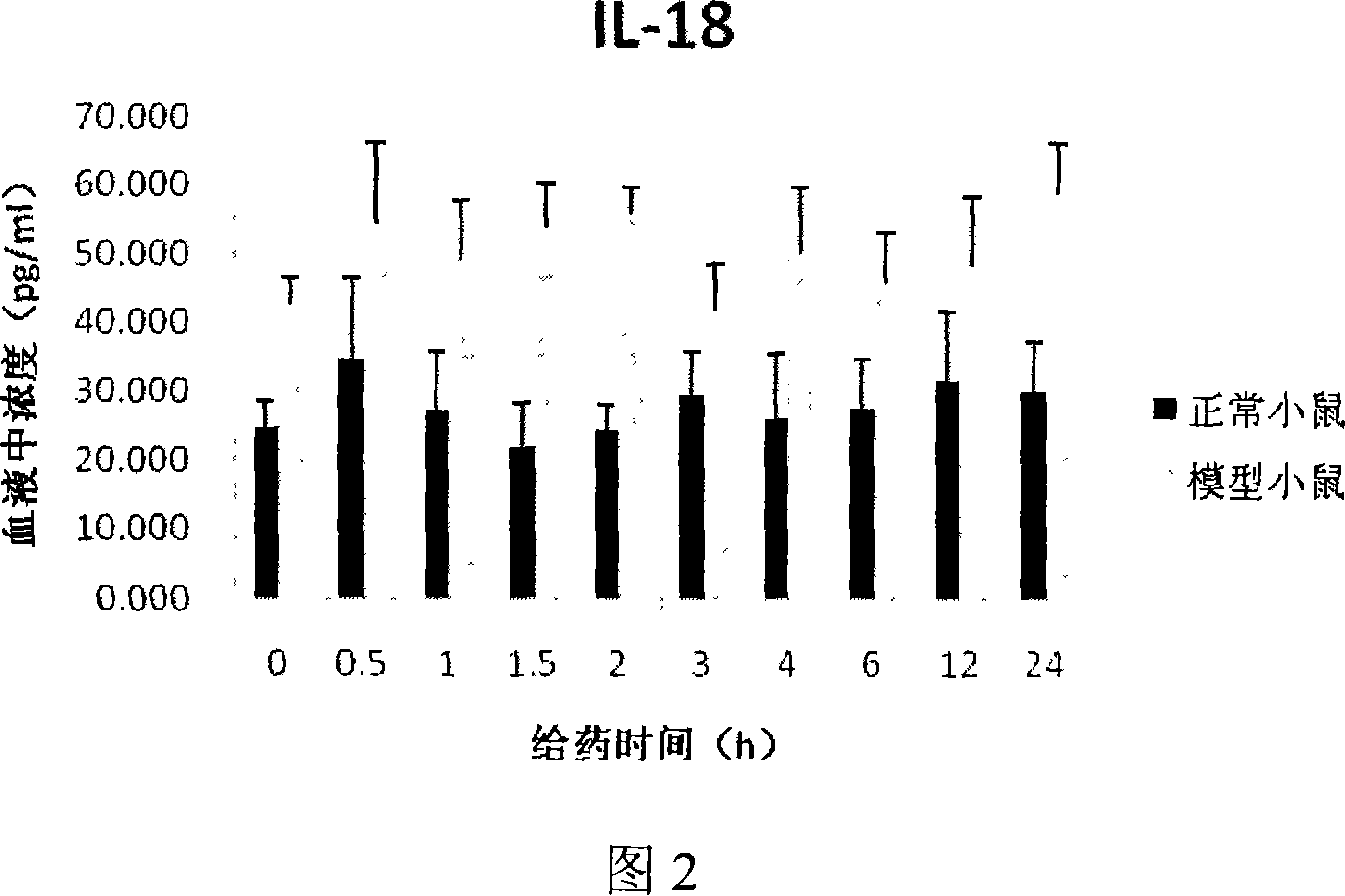
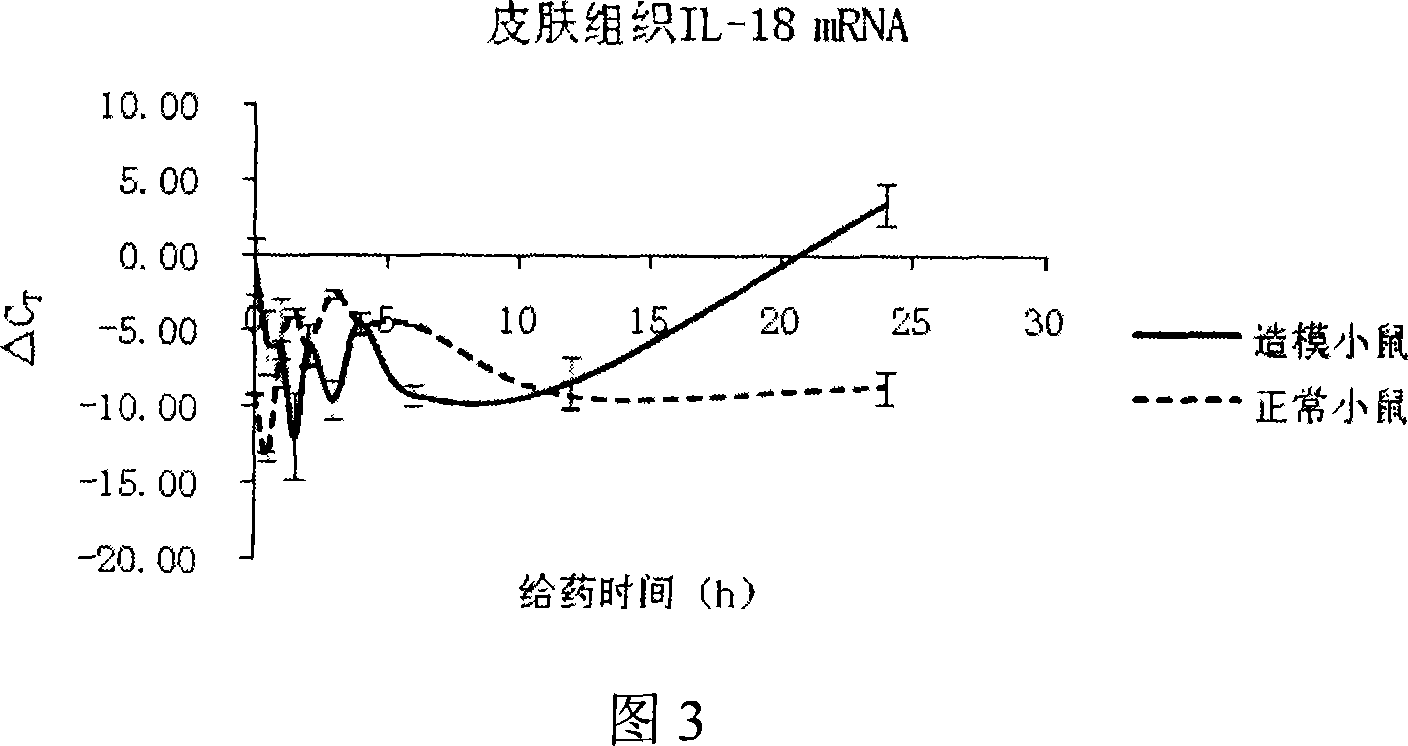
Constructing method of model of lacking of Langerhans cells of small rat skin
InactiveCN101032625ALong solution cycleOvercome the cycleOrganic active ingredientsIn-vivo testing preparationsLangerhan cellAbdominal skin
The present invention belongs to the field of medicine evaluating and detecting technology, and is especially process of constructing mouse skin Langerhans cell deletion model. Langerhans cell takes an important role in skin immune system. The mouse skin Langerhans cell deletion model constructing process includes adopting clobetasol propionate ointment as the model forming medicine and smearing it homogeneously onto the breast and abdominal skin of unhaired mouse. The histochemical staining and immunohistochemical experiments show that the said mouse model can well simulate the skin Langerhans cell deletion status, and may be used in screening skin immune medicine and in researching skin immune system inhibiting mechanism and medicine action mechanism. The present invention has simple process, short period, high repeatability, low cost and other advantages.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
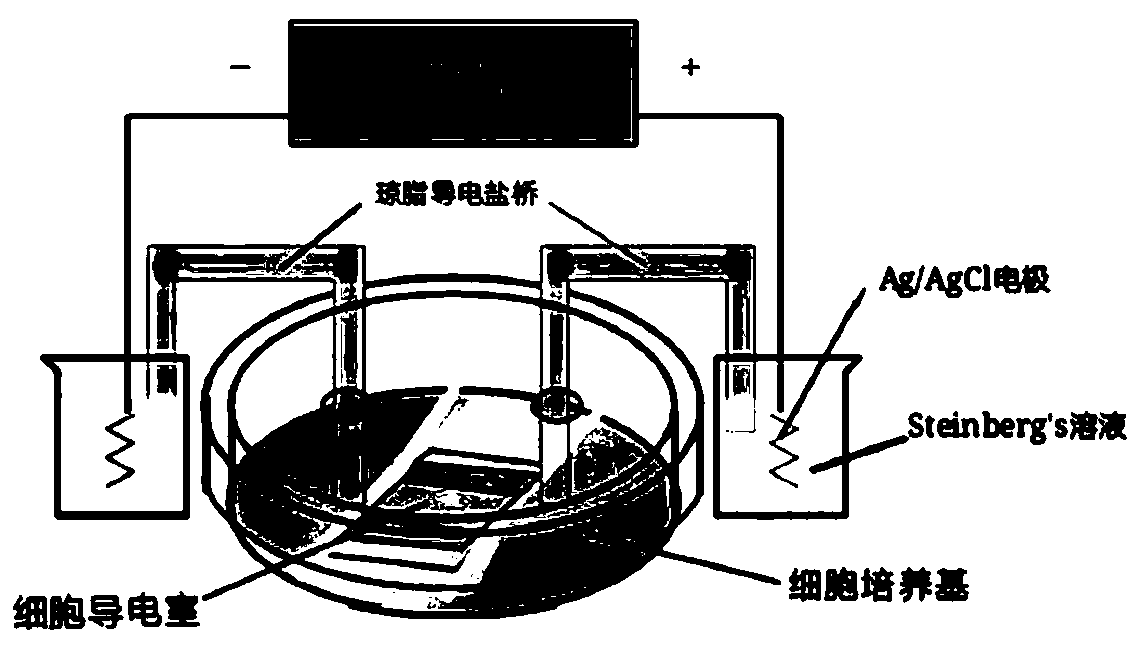
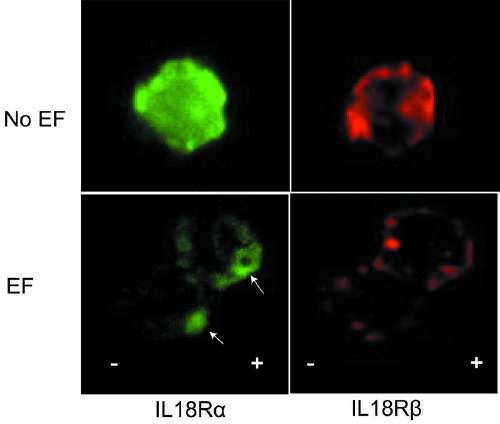
Method for regulating and controlling body immunoreaction through regulation of skin DCs (dendritic cells)/ LCs (Epidermal Langerhans cells) by electric signal to be presented to T cell, and purpose of method
PendingCN111172151AImprove securityEasy to manufactureElectrical/wave energy microorganism treatmentImmunological disordersLangerhan cellImmunomodulating Agent
The invention discloses a method for regulating and controlling body immunoreaction through the regulation of skin DCs (dendritic cells) / LCs (Epidermal Langerhans cells) by an electric signal to be presented to a T cell. The method comprises the following steps of: cells or skin tissues are put between an electrode A and an electrode B; a direct current power supply is connected between the electrode A and the electrode B; an electric field is formed between the electrode A and the electrode B; current enough to guide and control DCs / LCs cell migration and localization is given; and therefore, the skin DCs / LCs can be regulated through the electric signal to be presented to the T cell to regulate and control the body immunoreaction. The method disclosed by the invention has potential tobe used for preparing a novel vaccine adjuvant, or the method disclosed by the invention and other adjuvants are jointly used for regulating immunoreaction. The method can be used for targeting specific immunomodulators and accurate cancer treatment under a situation that the method is not induced and distributed by a chemical tendency, and common clinical side effects are reduced. Compared with existing common immunomodulators, the method disclosed by the invention has the advantages of a wide adjustable range and low cost, and is favorable for lightening the economic burdens of patients andthe society, and an operation condition of the method can be easily optimized.
Owner:宋冰 +1
Adjuvant for transcutaneous immunization
A transcutaneous immunization system delivers antigen to immune cells without perforation of the skin, and induces an immune response in an animal or human. The system uses an adjuvant, preferably an ADP-ribosylating exotoxin, to induce an antigen-specific immune response (e.g., humoral and / or cellular effectors) after transcutaneous application of a formulation containing antigen and adjuvant to intact skin of the animal or human. The efficiency of immunization may be enhanced by adding hydrating agents (e.g., liposomes), penetration enhancers, or occlusive dressings to the transcutaneous delivery system. This system may allow activation of Langerhans cells in the skin, migration of the Langerhans cells to lymph nodes, and antigen presentation.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Multi-effect youth-retaining gel containing fermentation filtrate of Ganoderma lucidum and preparation method thereof
InactiveCN109172434AIncrease elasticityProtect healthCosmetic preparationsHair removalLangerhan cellWhitening Agents
The invention discloses a multi-effect youth-retaining gel containing fermentation filtrate of Ganoderma lucidum and a preparation method thereof, belonging to the facial care field, comprising the following raw materials in parts by weight: Ganoderma fermentation filtrate 0.1-1 part, polyol 1-10 parts, moisturizer 1-10 parts, thickener 0.2-2 parts, PH value regulator 0.2-2 part, antiallergic relaxant 0.2-3 parts, skin conditioner 0.3-3 part, whitening agent 0.5-5 part, preservative 0.1-1 part, essence 0.05-0.1 part, essence solubilizer 0.1-0.4 part, and 100 parts of deionized water. A Langerhans cell in human skin is activated by adde active ingredients of natural green Ganoderma lucidum and yeast extract into the multi-effect youth-retaining gel containing Ganoderma lucidum fermentationfiltrate, and the Langerhans cell in human skin is immunologically cascaded to enhance immunity and repair functions.
Owner:DOCTOR PLANT GUANGDONG BIOTECHNOLOGY CO LTD
Unique dendritic cell-associated C-type lectins, dectin-1 and dectin-2; compositions and uses thereof
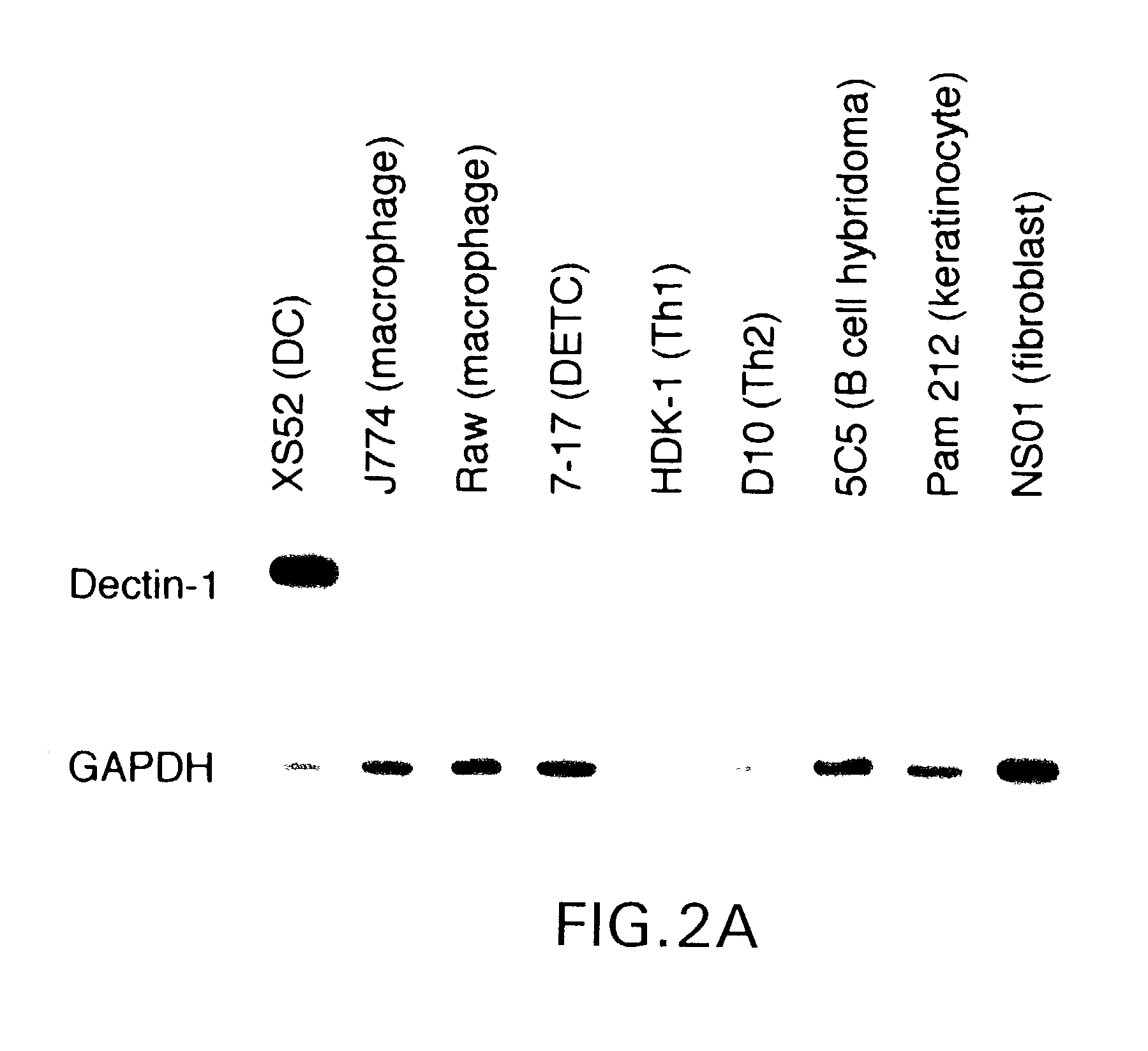
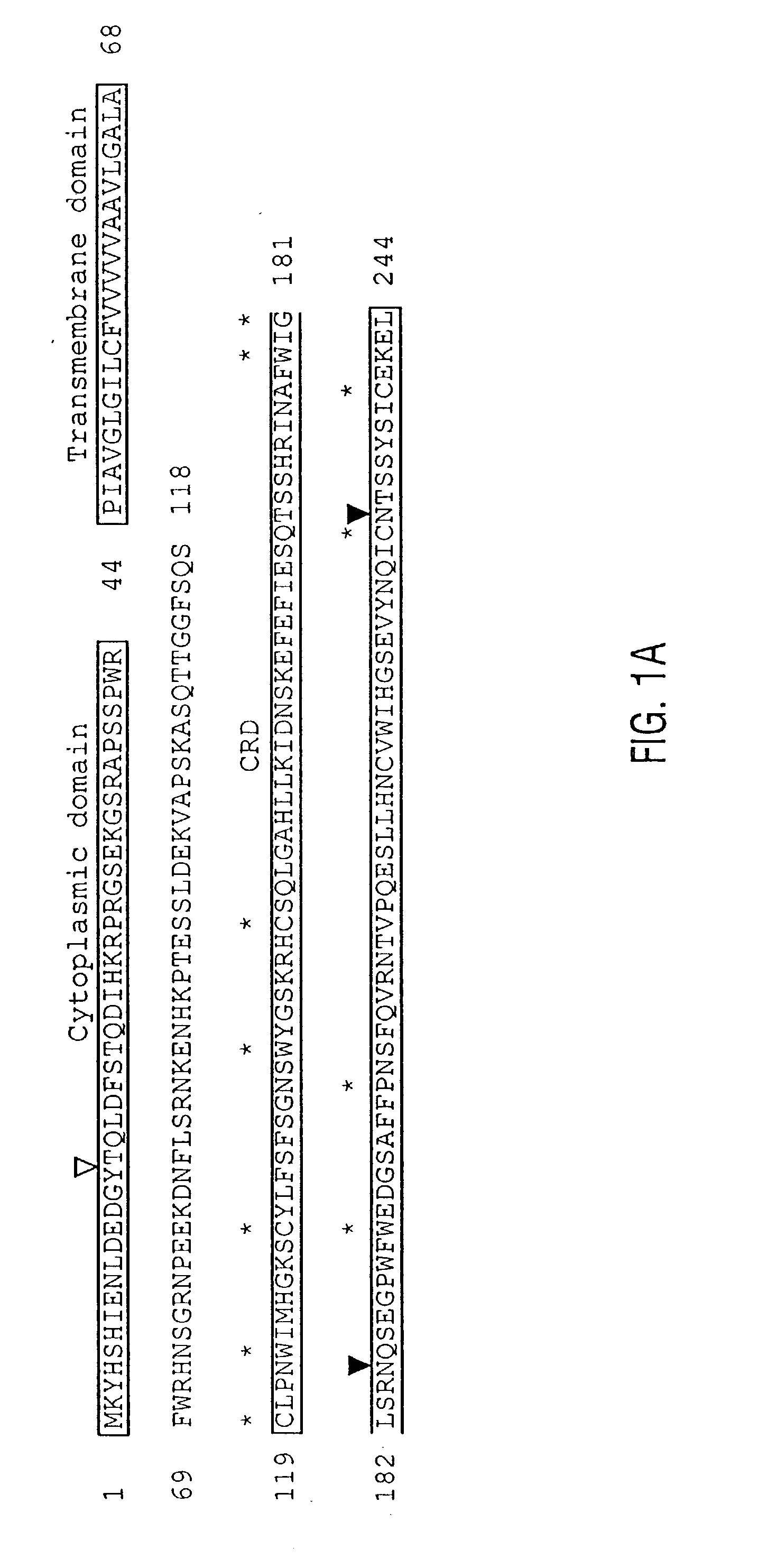
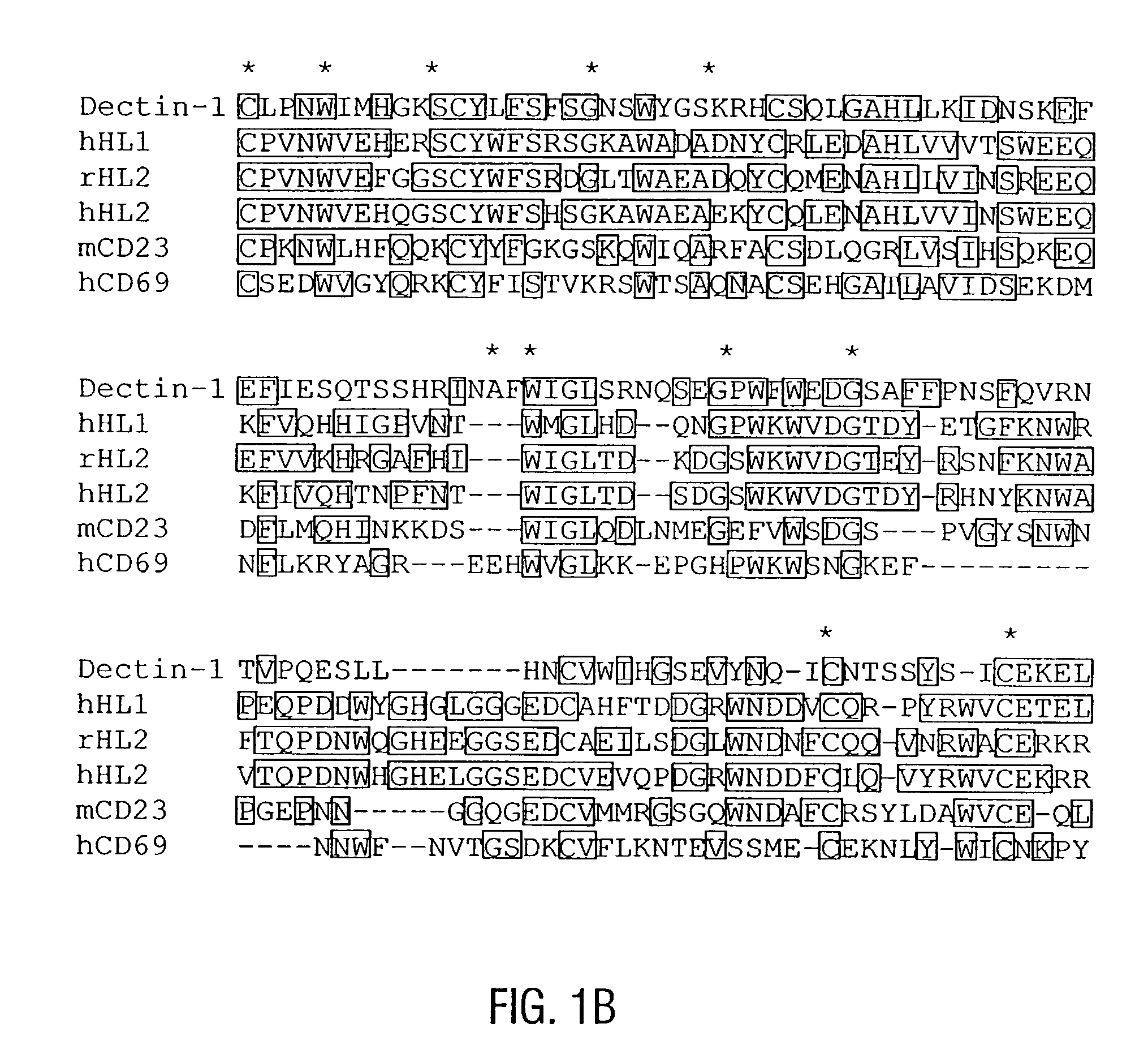
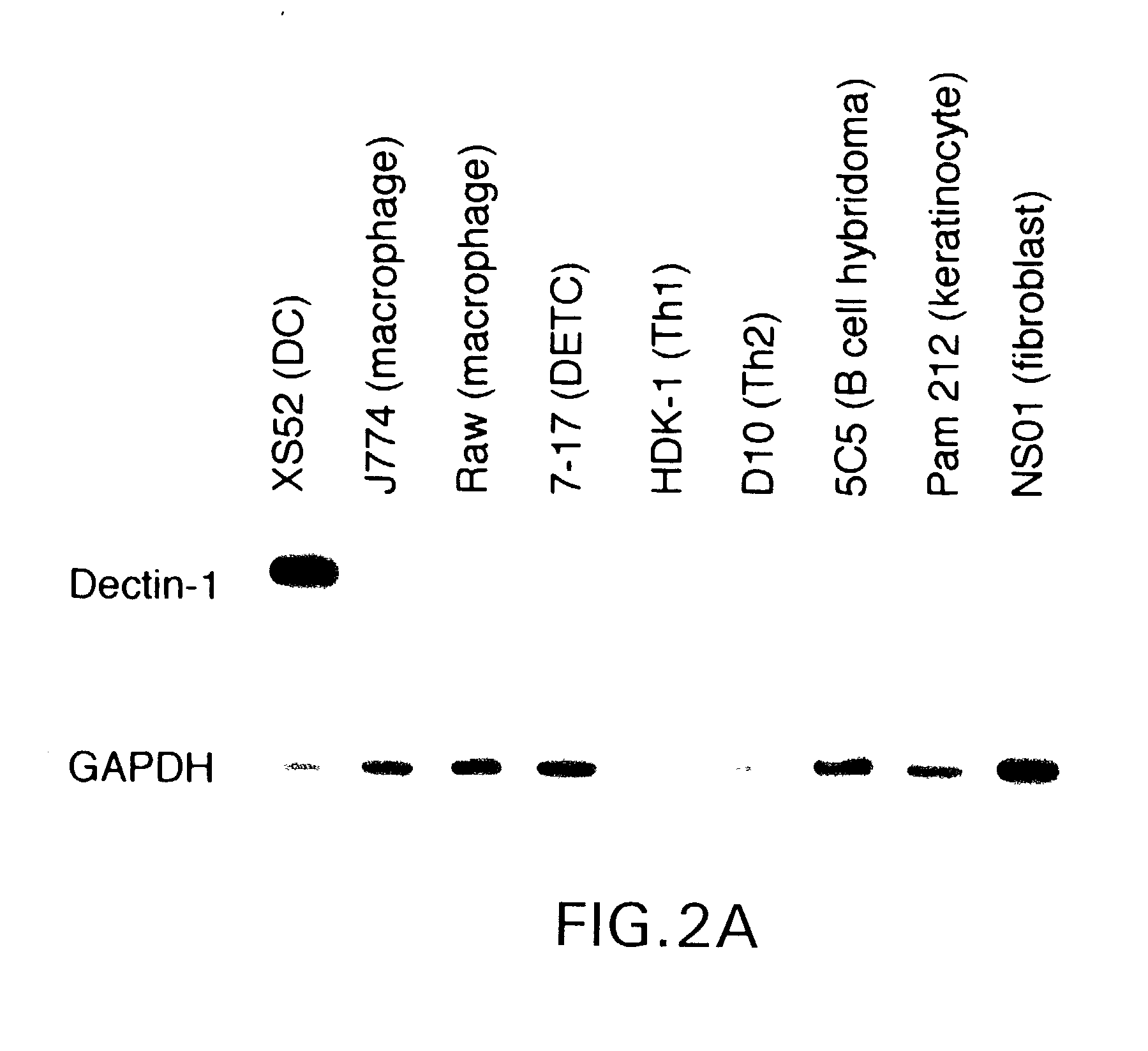
Novel genes expressed selectively by long-term dendritic cell (DC) lines (XS series) from murine epidermis which retain important features of resident epidermal Langerhans cells (LC) are provided. These genes encode distinct type II membrane-integrated polypeptides, each consisting of a cytoplasmic domain, a transmembrane domain, an extracellular connecting domain, and a C-terminal extracellular domain that exhibits significant homology to the carbohydrate recognition domains (CRD) of C-type lectins. Expression of both genes is highly restricted to cells of DC lineage (including epidermal LC). Thus, these genes encode new, DC-specific members of the C-type lectin family, now termed “DC-associated C-type lectin-1 and -2” (dectin-1 and dectin-2). Two isoforms of the dectin-1 molecule and five isoforms of the dectin-2 molecule have also been identified. The invention further provides His-tagged fusion proteins comprising 6× histidine and the extracellular domain of dectin-1 or dectin-2. Also provided are antibodies raised to synthetic peptides designed from the dectin-1 sequence or to the His-tagged fusion proteins described.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Method for producing langerhans cells or interstitial dendritic cells or both from cd14+ monocytes
InactiveUS20100297765A1Increase the number ofHigh yieldEpidermal cells/skin cellsArtificial cell constructsLangerhan cellInterstitial Dendritic Cells
The present invention relates to a method for preparing Langerhans cells or interstitial dendritic cells or both from CD14+ monocytes stemming from the peripheral circulatory blood of a living being, wherein the method comprises differentiation of CD 14+ monocytes into either Langerhans cells, interstitial dendritic cells, or into both types of cells by placing the CD14+ monocytes in the presence of a cell environment comprising epithelial cells and / or mesenchymatous cells.The present invention also relates to cell or tissue models comprising such prepared Langerhans cells and / or interstitial dendritic cells, and optionally macrophages and endothelial cells, and to the uses of such cell or tissue models.
Owner:BASF BEAUTY CARE SOLUTIONS FRANCE SAS
In vitro culture method for inducing human umbilical cord blood cells CD34+ to differentiate into Langerhans cell
InactiveCN102634483AStrong antigen presentationIncrease the number of amplificationArtificial cell constructsBlood/immune system cellsLangerhan cellTumor necrosis factor alpha
An in vitro culture method for inducing human umbilical cord blood cells CD34+ to differentiate into Langerhans cell includes culturing human umbilical cord blood cells CD34+ in a medium containing GM-CSF (granulocyte-macrophage colony stimulating factor), TNF-alpha (tumor necrosis factor-alpha), SCF (stem cell factor) and Flt3L (FMS-like tyrosine kinase 3 ligand), and differentiating the human umbilical cord blood cells CD34+ into Langerhans cells. The Langerhans cells generated are highly pure and high in yield, and have the functions of recognizing, absorbing and processing antigens and the function of activating immune response.
Owner:THE FIRST AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIVERSITY OF PLA
Ameliorative agent for cytokine release syndrome and so on
ActiveUS20210128550A1Inhibition releaseGood effectOrganic active ingredientsAntipyreticLangerhan cellMacrophage activation syndrome
[Problem to be Solved] The present invention provides a medicament for cytokine release syndrome, autoimmune-related adverse events, macrophage activation syndrome, hemophagocytic lymphohistiocytosis or Langerhans cell histiocytosis.[Means to Solve the Problem] The present invention provides a medicament for at least one selected from the group consisting of cytokine release syndrome, autoimmune-related adverse events, macrophage activation syndrome, hemophagocytic lymphohistiocytosis and Langerhans cell histiocytosis, wherein the medicament comprises a compound represented by the following formula I:or a pharmaceutically acceptable salt thereof.
Owner:TORII PHARMA
Method for producing langerhans cells or interstitial dendritic cells or both from CD14+ monocytes
InactiveUS20090081783A1Increase the number ofHigh yieldEpidermal cells/skin cellsArtificial cell constructsLangerhan cellInterstitial Dendritic Cells
The present invention relates to a method for preparing Langerhans cells or interstitial dendritic cells, or both, from CD14+ monocytes stemming from the peripheral circulatory blood of a living being, wherein the method comprises differentiation of CD14+ monocytes into either Langerhans cells, interstitial dendritic cells, or into both types of cells by placing the CD14+ monocytes in the presence of a cell environment comprising epithelial cells and / or mesenchymatous cells.The present invention also relates to cell or tissue models comprising such prepared Langerhans cells and / or interstitial dendritic cells, and optionally macrophages and endothelial cells, and to the uses of such cell or tissue models.
Owner:BASF BEAUTY CARE SOLUTIONS FRANCE SAS
Adjuvant for transcutaneous immunization
A transcutaneous immunization system delivers antigen to immune cells without perforation of the skin, and induces an immune response in an animal or human. The system uses an adjuvant, preferably an ADP-ribosylating exotoxin, to induce an antigen-specific immune response (e.g., humoral and / or cellular effectors) after transcutaneous application of a formulation containing antigen and adjuvant to intact skin of the animal or human. The efficiency of immunization may be enhanced by adding hydrating agents (e.g., liposomes), penetration enhancers, or occlusive dressings to the transcutaneous delivery system. This system may allow activation of Langerhans cells in the skin, migration of the Langerhans cells to lymph nodes, and antigen presentation.
Owner:UNITED STATES OF AMERICA
Culture medium for Langerhans precursor cells
ActiveCN111019893AIncrease acquisition rateEffective culture conditionsEpidermal cells/skin cellsArtificial cell constructsLangerhan cellIn vitro proliferation
The invention provides a culture medium for Langerhans precursor cells. The culture medium for Langerhans precursor cells comprises a basal culture medium, GM-CSF, TGF-beta, IL-1 beta, IL-3, IL-6, SCF, EPO and IL-4. According to the culture medium for Langerhans precursor cells, effective culture conditions are provided for obtaining a large number of Langerhans cells, the yield of the Langerhanscells is increased, and the problem that the quantity of seed cells is limited due to the fact that the Langerhans cells cannot be proliferated and subcultured in vitro is effectively solved.
Owner:GUANGDONG BOXI BIO TECH CO LTD
Application of dipeptide base peptidase IV effector for reducing blood sugar level of mammal
InactiveCN1269528CSimple methodOrganic chemistryPeptide/protein ingredientsLangerhan cellIntestinal structure
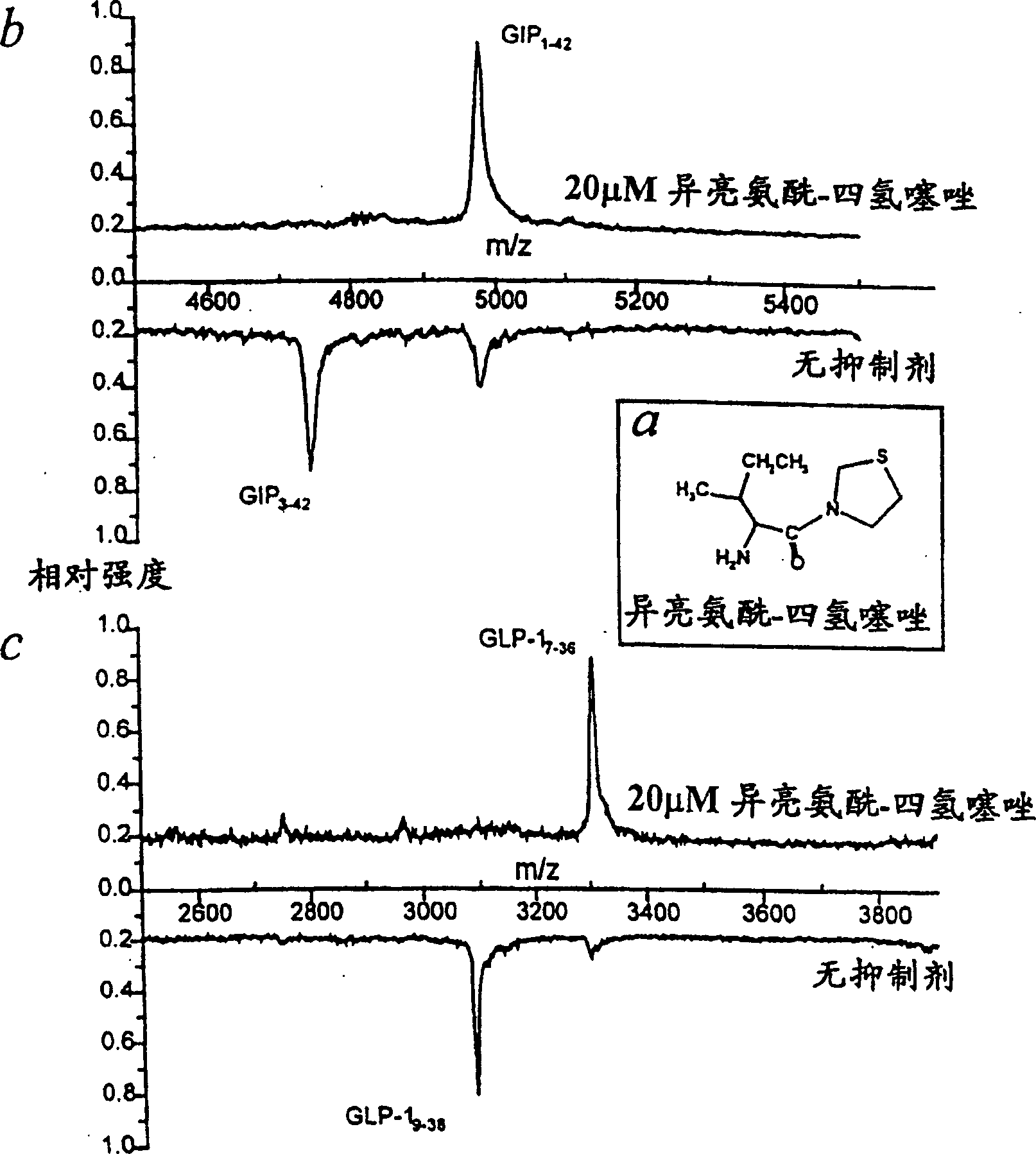
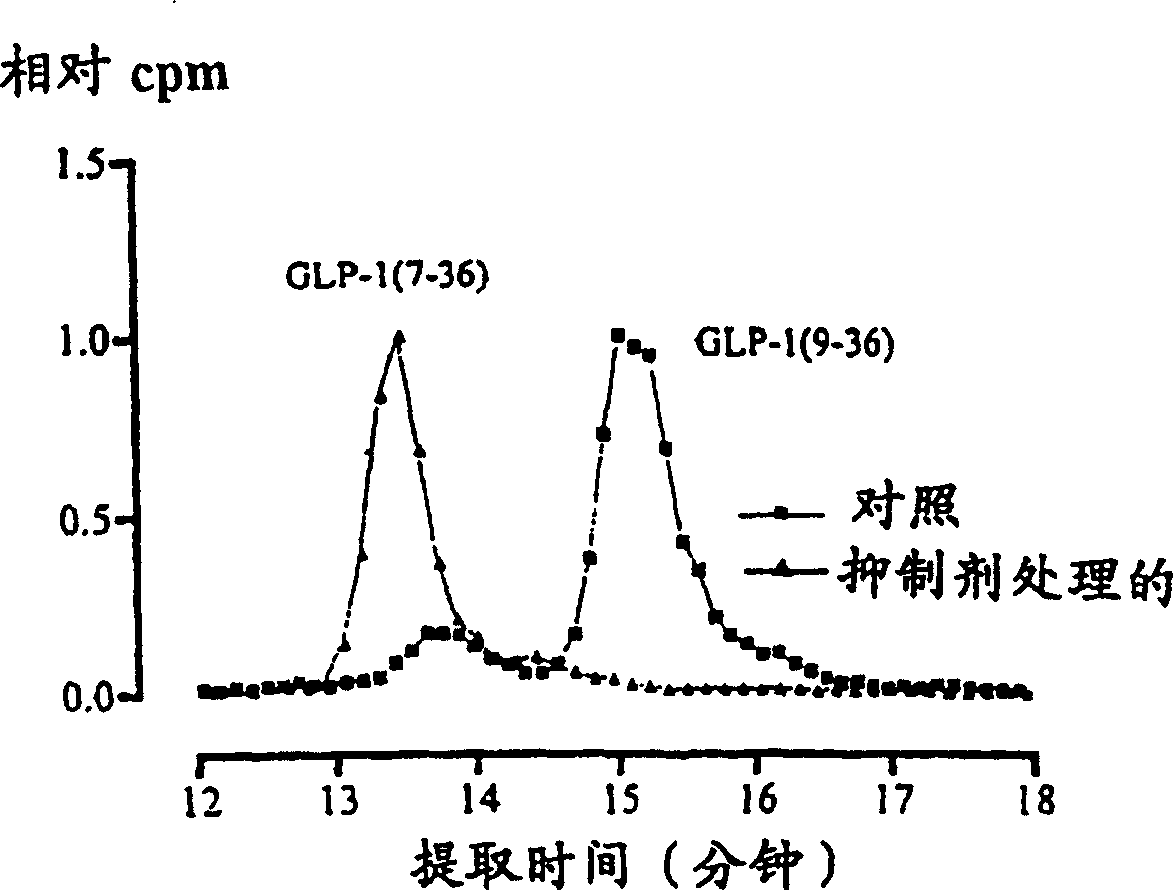
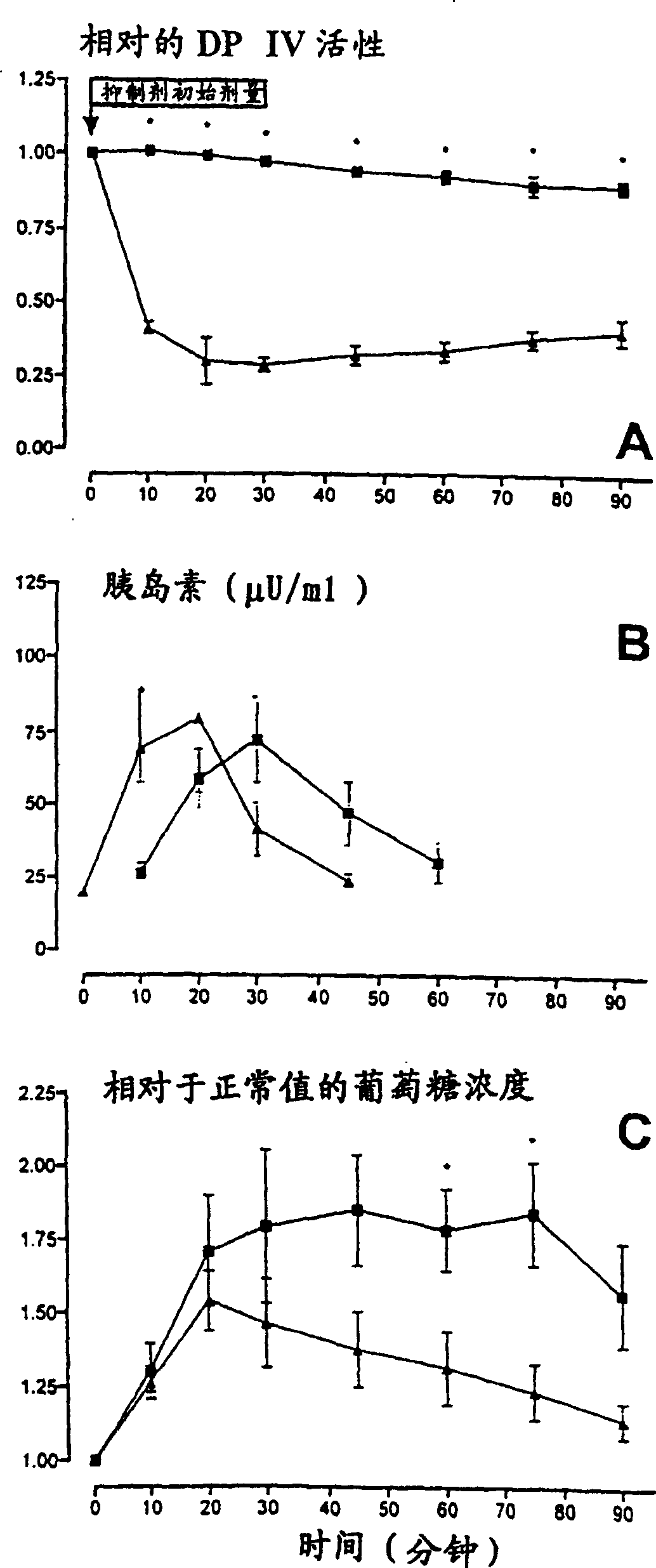
The invention relates to the use of a method in which by reducing in the blood of a mammal by administration of effectors the enzyme activity of dipeptidyl peptidase (DP IV) or enzyme activity similar to DP IV, the endogenous (or additionally exogenously administered) insulinotropic peptide gastric inhibitory polypeptide 1-42 (GIP1-42) and glucagon-like peptide amide-1 7-36 (GLP-17-36) (or similarly GLP-17-37 or analogues thereof) are decomposed in a causal sequence to a reduced extent by DP IV enzymes or those similar to DP IV. Consequently, the fall in the concentration of said peptide hormones or the analogues thereof is reduced or retarded. The increased stability, achieved by the action of DP IV effectors, of the incretine or the analogues thereof which are available endogenously or exogenously and consequently provided in increased numbers for insulinotropic stimulation of the incretine receptors of the Langerhans cells in the pancreas, changes the power of endogenous insulin thereby stimulating the metabolism of carbohydrates in the treated organism. The blood sugar level therefore drops below the glucose concentration, characteristic of hyperglycaemia, in the serum of the treated organism.
Owner:PROSIDION LIMITED
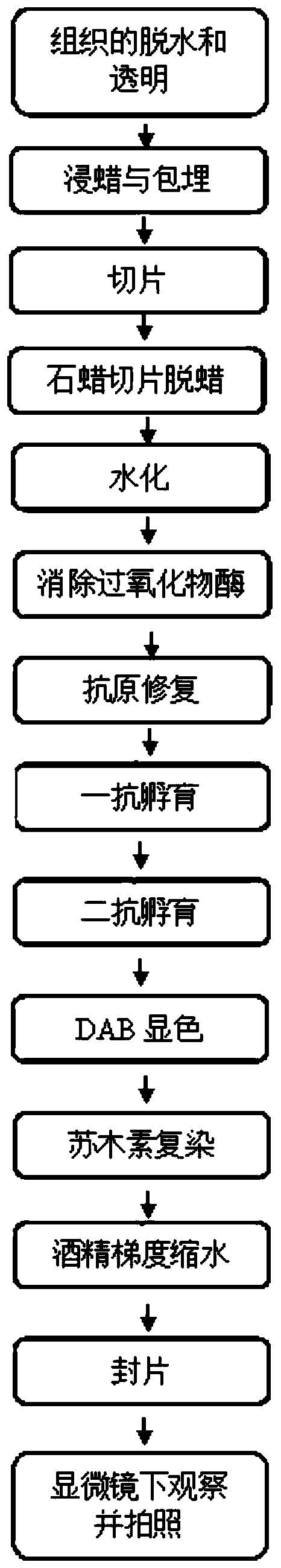
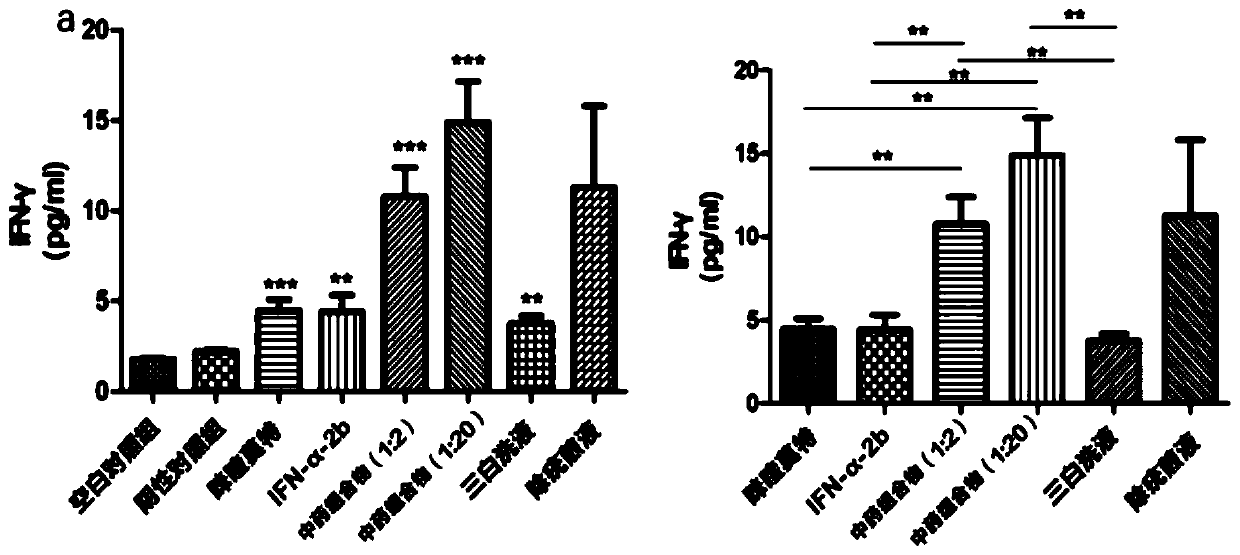
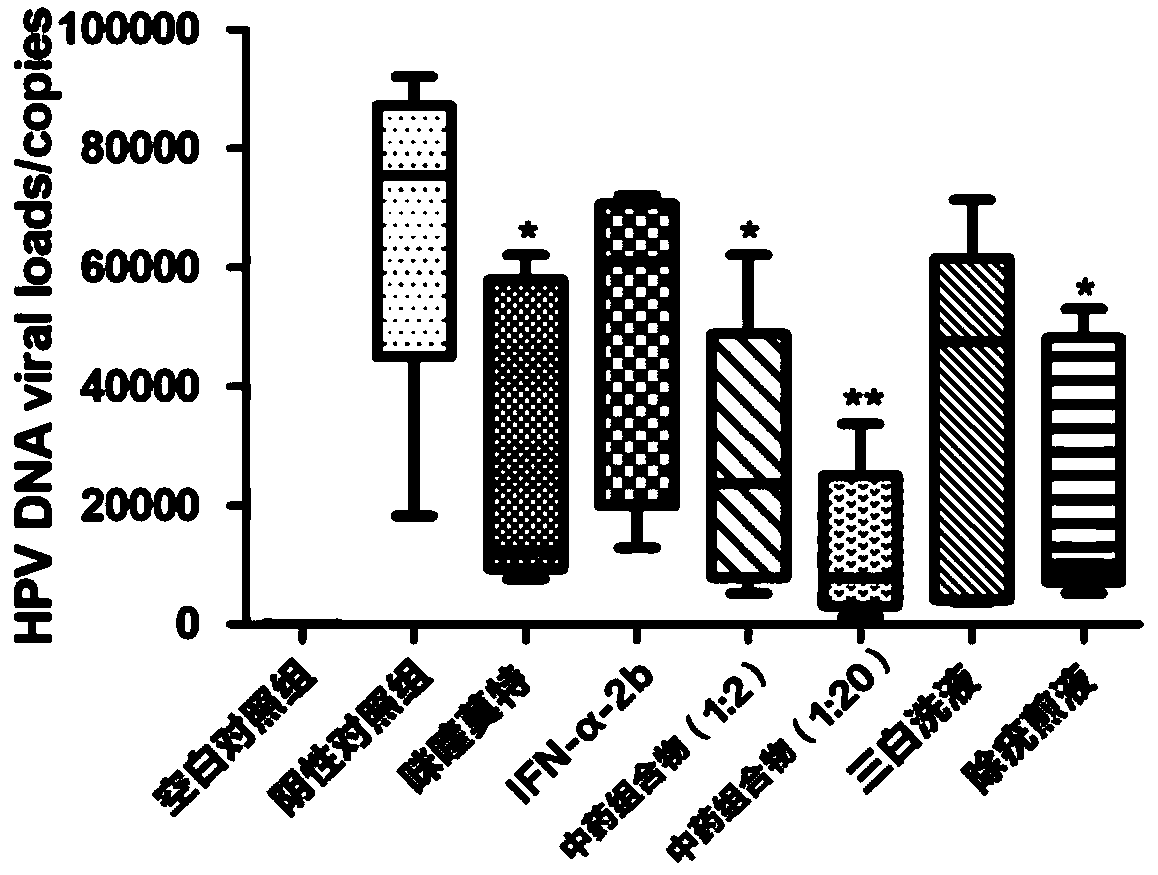
Traditional Chinese medicine composition for treating human papilloma virus (HPV) infection and preparing method and application thereof
InactiveCN110368463AEfficient removalIncrease the number ofAnthropod material medical ingredientsAntiviralsLangerhan cellTreatment effect
The invention relates to traditional Chinese medicine compositions, and particularly discloses a traditional Chinese medicine composition for treating human papilloma virus (HPV) infection and a preparing method and application thereof. Effective components of the traditional Chinese medicine composition are derived from sinopodophyllum hexandrum, cortex magnoliae officinalis, rhizoma polygoni cuspidati, air-plant herb, liquorice root, fructus kochiae, fructus bruceae, rhizoma smilacis glabrae and herba centellae. The traditional Chinese medicine composition is combined with the clinical treatment effect and condyloma acuminate animal model tests to constantly adjust the use quantities of all active pharmaceutical ingredients in the formula, and full play is given to the compatibility andsynthesis effects of all the active pharmaceutical ingredients in the traditional Chinese medicine prescription. The traditional Chinese medicine composition can effectively remove the human papillomavirus, has a significant treatment effect on HPV low-risk infection and HR-HPV positive cervical intraepithelial neoplasia low-grade lesions, and can increase the number of langerhans cells, and thusthe immunity of the body is enhanced.
Owner:BEIJING PATBORN BIOTECH DEV CO LTD +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com