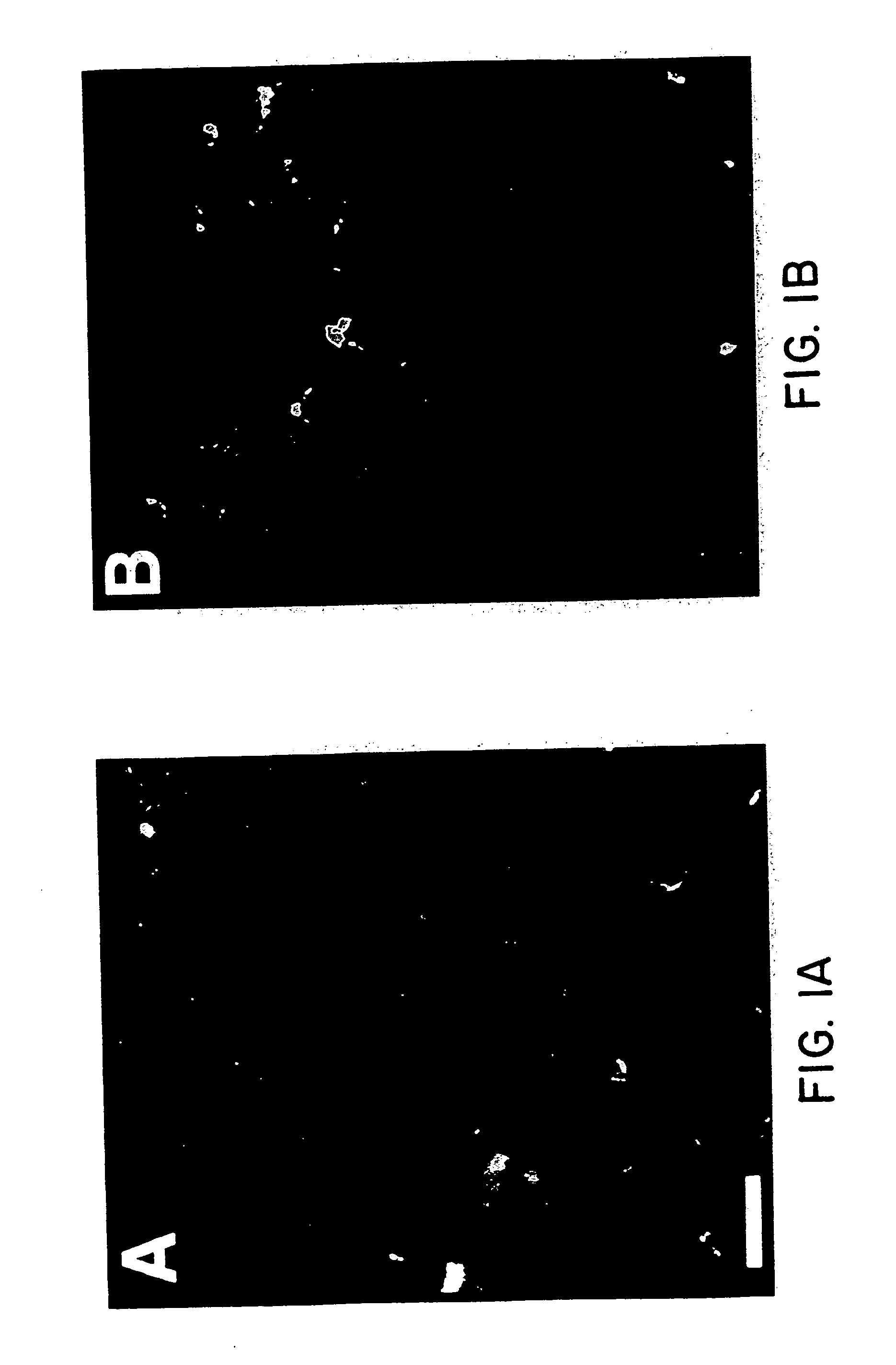
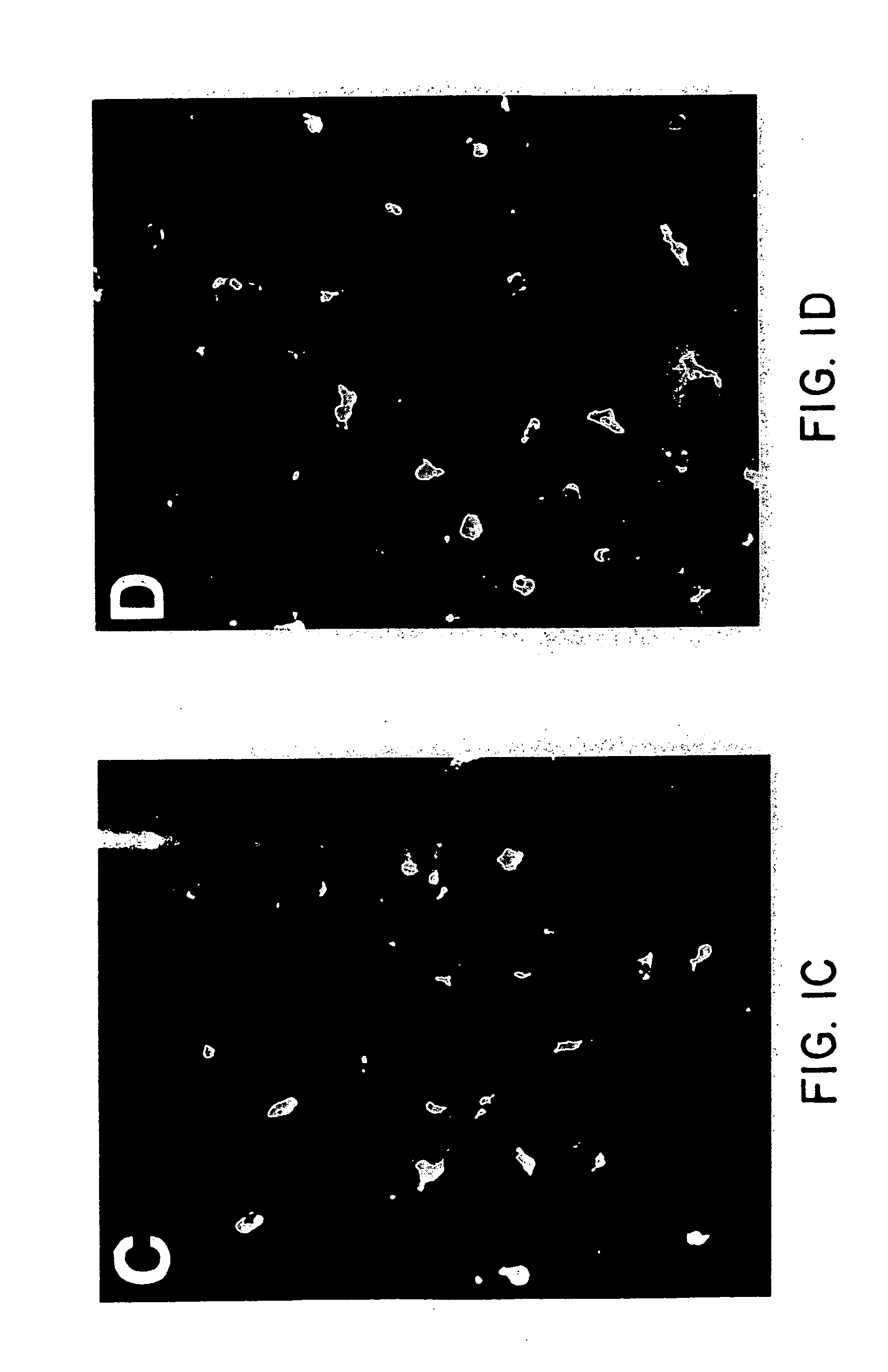
Adjuvant for transcutaneous immunization
a technology of adjuvant and immunization, applied in the field of adjuvant, can solve the problem of not being expected to achieve immunization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0115] BALB / c mice at 6 to 8 weeks of age were immunized transcutaneously as described above for “Immunization Procedure”, in groups of five mice. The mice were immunized using 100 μl of immunization solution which was prepared as follows: liposomes prepared as described above for “Liposome Preparation” were mixed with saline to form the liposomes. The pre-formed liposomes were then diluted in either saline (liposome alone group) or with CT in saline to yield an immunizing solution containing liposomes at 10-150 mM phospholipid with 100 μg of CT per 100 μl of immunizing solution. CT was mixed in saline to make an immunizing solution containing 100 μg of CT per 100 μg of solution for the group receiving CT alone. Solutions were vortexed for 10 seconds prior to immunization.
[0116] The mice were immunized transcutaneously at 0 and 3 weeks. Antibody levels were determined using ELISA as described above for “ELISA IgG(H+L)” 3 weeks after the boosting immunization, and compared against p...
example 2
[0117] BALB / c mice at 6 to 8 weeks of age were immunized transcutaneously as described above for “Immunization Procedure”, in groups of five mice. The mice were immunized at 0 and 3 weeks using 100 μl of immunization solution prepared as follows: BSA was mixed in saline to make an immunizing solution containing 200 μg of BSA per 100 μl of saline for the group receiving BSA alone; BSA and CT were mixed in saline to make an immunizing solution containing 200 μg of BSA and 100 μg of CT per 100 μl of saline for the group receiving BSA and CT. Where liposomes were used, the liposomes were prepared as described above for “Liposome Preparation”, and were first mixed with saline to form the liposomes. They were then diluted in BSA or BSA and CT in saline to yield an immunizing solution containing liposomes at 50 mM phospholipid with 200 μg of BSA per 100 μl of immunizing solution, or 200 μg BSA+100 μg CT per 100 μl of immunizing solution. Solutions were vortexed for 10 seconds prior to immu...
example 3
[0119] BALB / c mice at 6 to 8 weeks of age were immunized transcutaneously as described above for “Immunization Procedure”, in groups of five mice. The mice were immunized at 0 and 3 weeks using 100 μl of immunization solution prepared as follows: LT was mixed in saline to make an immunizing solution containing 100 μg of LT per 100 μl of saline for the group receiving LT alone. Where liposomes were used the liposomes prepared as described above for “Liposome Preparation”, and were first mixed with saline to form the liposomes. The pre-formed liposomes were then diluted in LT in saline to yield an immunizing solution containing liposomes at 50 mM phospholipid with 100 μg of LT per 100 μl of immunizing solution. Solutions were vortexed for 10 seconds prior to immunization.
[0120] The anti-LT antibodies were determined using ELISA as described above for “ELISA IgG(H+L)” 3 weeks after the second immunization. The results are shown in Table 3. LT was clearly immunogenic both with and with...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com