Application of dipeptide base peptidase IV effector for reducing blood sugar level of mammal
A dipeptidyl peptidase and mammalian technology, applied in the direction of active ingredients of heterocyclic compounds, medical preparations containing active ingredients, drug combinations, etc., can solve the problem of expensive transplantation and achieve pharmaceutically useful effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
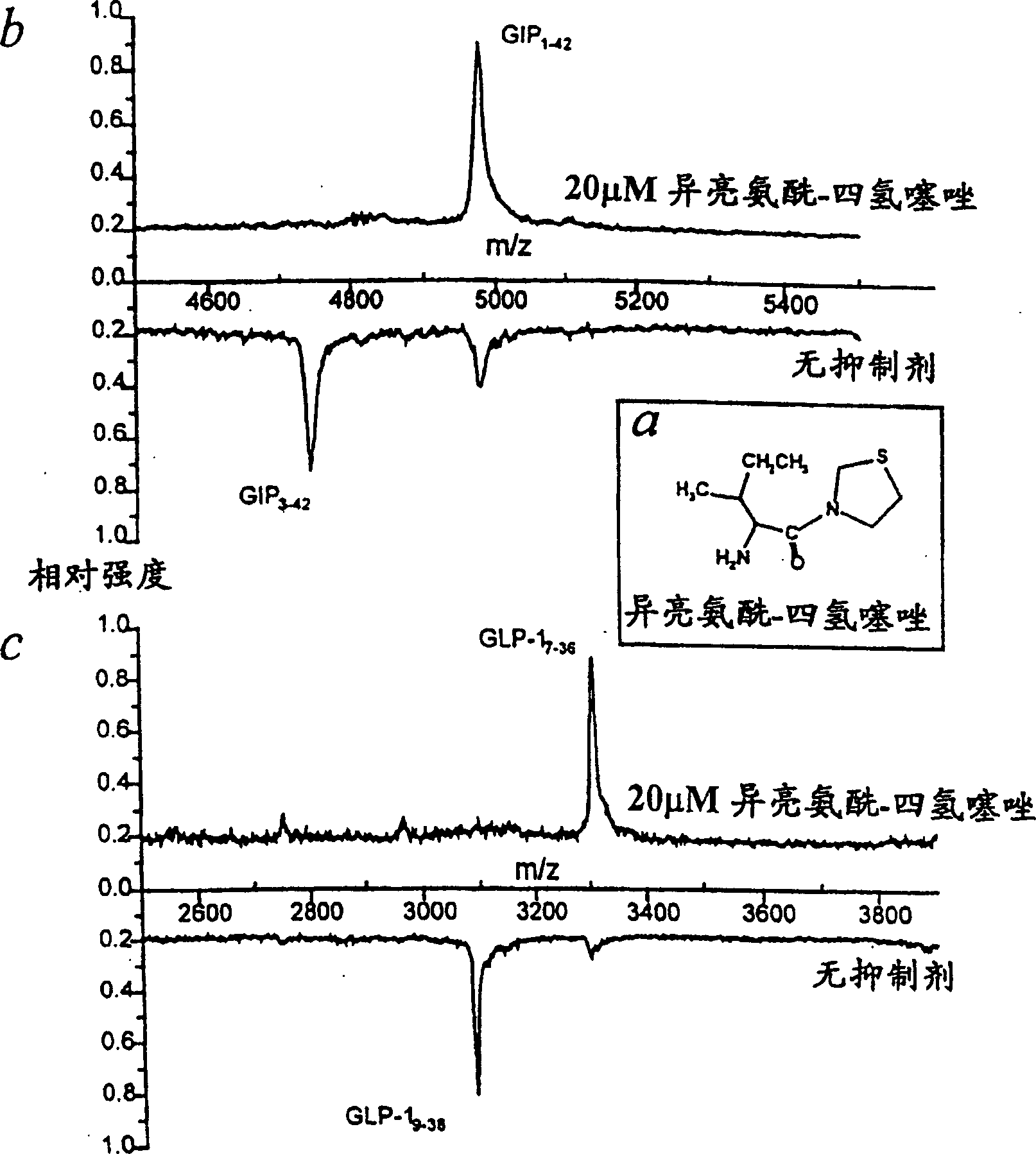
[0027] Example 1. In vivo incretin GIP- 1-42 and GLP-1 7-36 Inhibition of DP IV-catalyzed hydrolysis
[0028] Hydrolysis of incretins by DP IV- and DP IV analogue-enzymatic activity, or its inhibition by inhibitors, could be demonstrated in vivo using purified enzymes or in situ using pooled human serum ( figure 1 ).
[0029] According to the present invention, in vivo by incubating 30 μM GIP 1-42 or 30 μM GLP-1 7-36 and 20 μM isoleucyl-tetrahydrothiazole (1a), within 24 hours to achieve complete inhibition of the enzymatic hydrolysis of the two peptide hormones (1b and 1c, each see the above spectrum), the incubation at pH7. Reversible DP IV-inhibitor in 20% pooled serum at 6 and 30°C. At pH 7.6 and 30°C, the synthetic gastric inhibitory polypeptide GIP 1-42 (5 μM) and synthetic GLP-1 7-36 (15 μM) were incubated with human serum (20%) in 0.1 mM TRICINE buffer for 24 hours. After various time intervals, samples for incubation analysis (for GIP 1-42 2.5pmol for GLP-1 ...
Embodiment 2
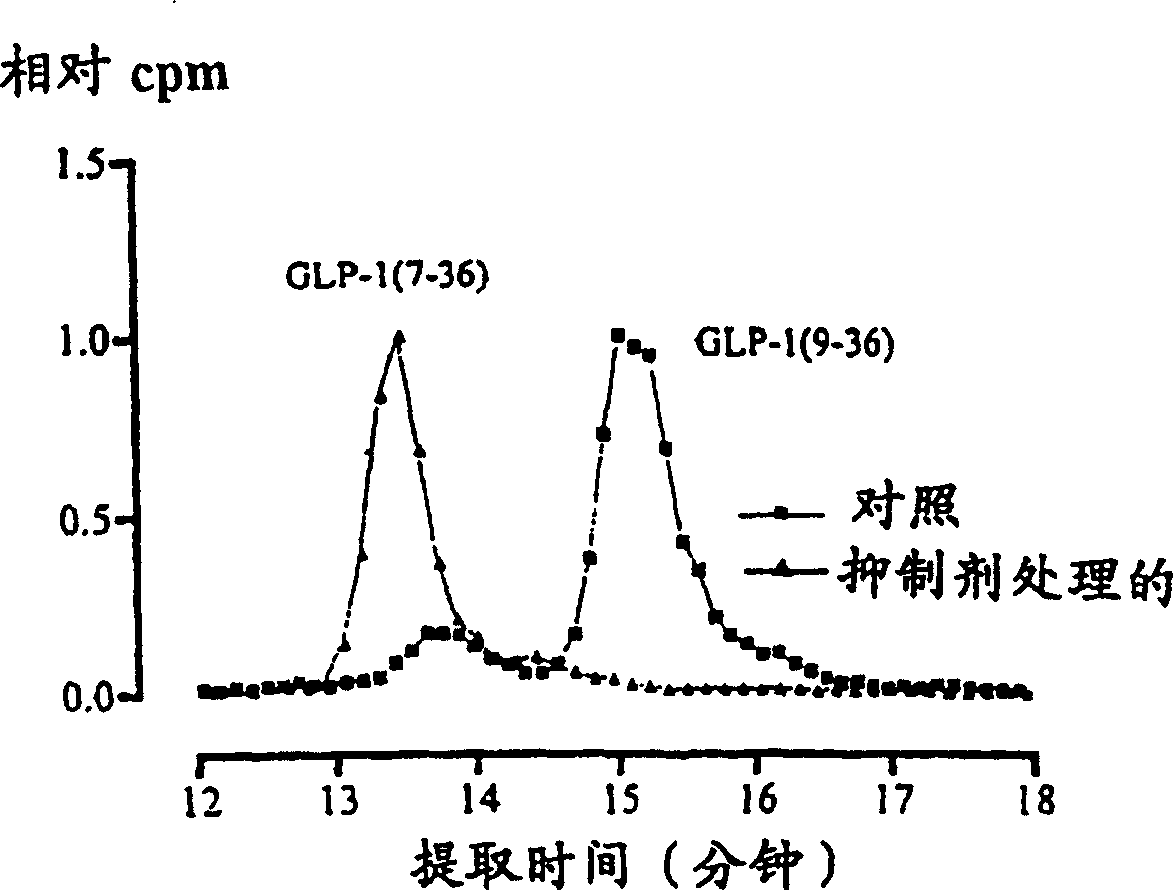
[0033] Example 2. Inhibition of GLP-1 by the DP IV-inhibitor isoleucyl-tetrahydrothiazole in vivo 7-36 Degradation
[0034] Compared with a control sample, the natural incretin (here GLP-1 7-36 ) dependence of the metabolism of the DP IV-inhibitor isoleucyl-tetrahydrothiazole (intravenous injection of 0.9% saline solution containing 1.5 μM inhibitor). In test animals (N=5) treated with inhibitors, the insulinotropic peptide hormone GLP-1 7-36 No degradation was observed during the experiment ( figure 2 ).
[0035] To detect metabolites of incretins in the presence and absence of DP IV-inhibitors, test and reference animals were given a further iv injection of 50-100 pM 20 min after initiation of iv administration of the inhibitor or saline 125 I-GLP-1 7-36 (approximately 1 μCi / pM specific activity). Blood samples were collected after 2-5 minutes of incubation, and protoplasts were extracted using 20% acetonitrile. Subsequently, the peptide extracts were separated on ...
Embodiment 3
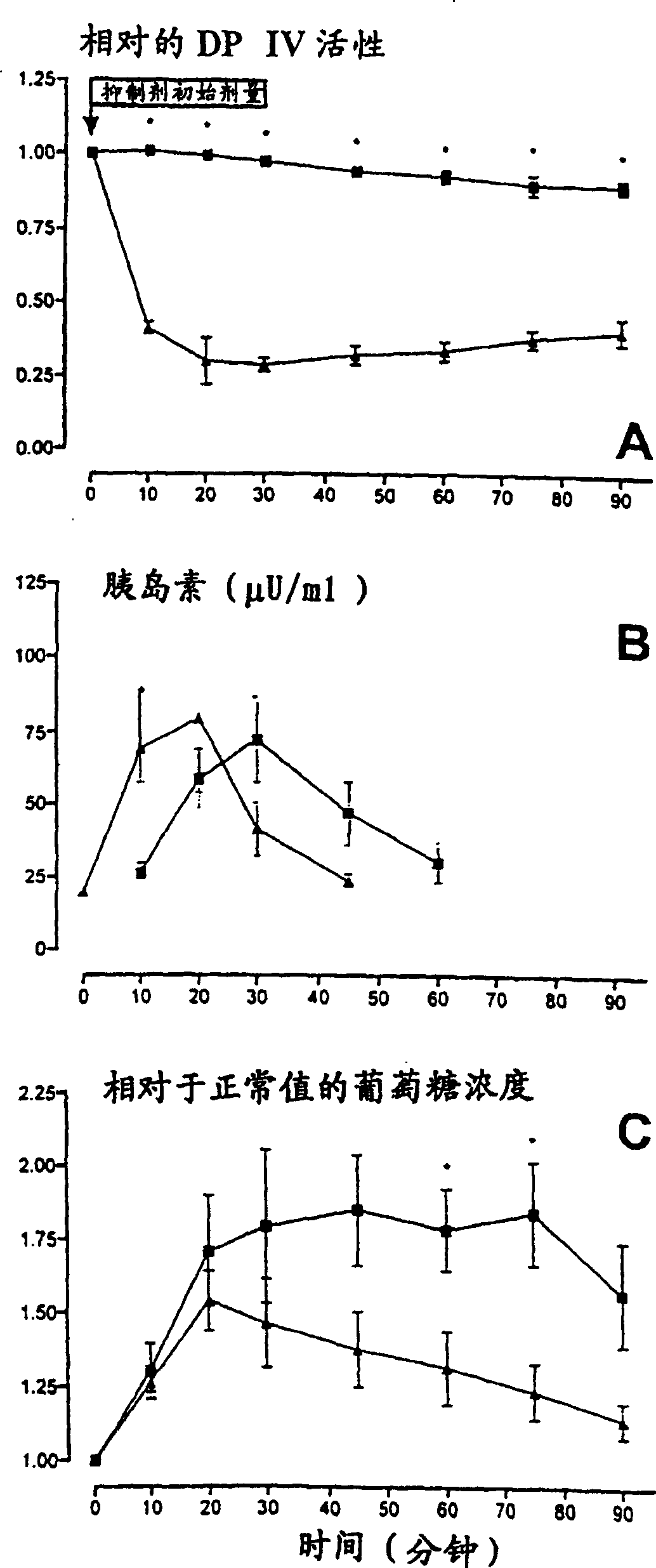
[0036] Example 3. Modulation of insulin action and reduction of blood glucose levels following intravenous administration of the DP IV-inhibitor isoleucyl-tetrahydrothiazole in vivo.
[0037] In mice stimulated by intraduodenal (i.d.) injection of glucose, by in vivo administration of different DP IV-effectors such as 0.1 mg of isoleucyl-tetrahydrothiazole per kg of mouse weight, observed attributable The temporally delayed onset of action of the inhibitor effect is a decrease in glucose levels. This effect was dose dependent and was reversible after cessation of injection of the DP IV-inhibitor isoleucyl-tetrahydrothiazole at 0.05 mg / min / kg rat weight. In contrast to intraduodenal glucose-stimulated test animals, no comparable effect was observed after intravenous administration of the same amount of glucose to inhibitor-treated and control animals. image 3 These inhibitor-dependent changes in protoplasm parameters are shown: A-DP IV-activity, B-protoplasm-insulin level, C-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com