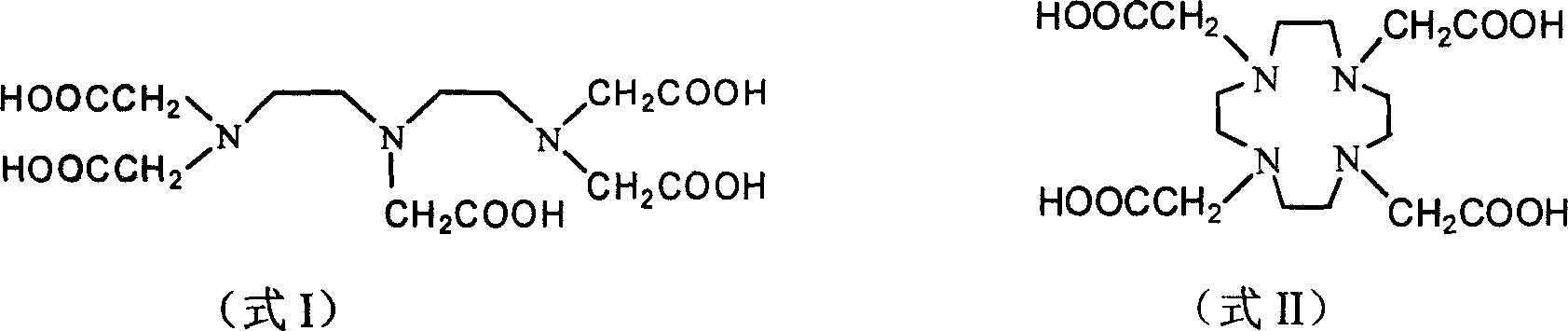Patents
Literature
46 results about "Tricine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
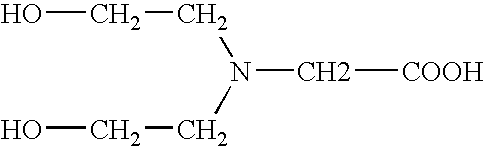
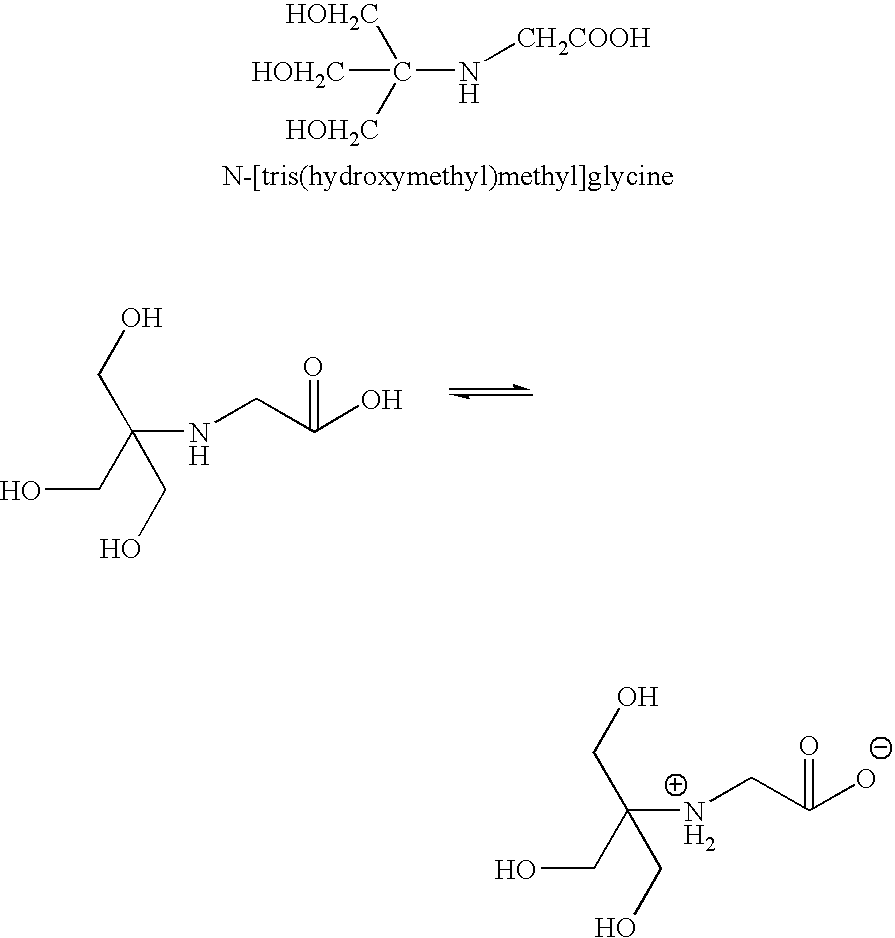
Tricine is an organic compound that is used in buffer solutions. The name tricine comes from tris and glycine, from which it was derived. It is a white crystalline powder that is moderately soluble in water. It is a zwitterionic amino acid that has a pKa1 value of 2.3 at 25 °C, while its pKa2 at 20 °C is 8.15. Its useful buffering range of pH is 7.4-8.8. Along with bicine, it is one of Good's buffering agents. Good first prepared tricine to buffer chloroplast reactions.
Truncated fragments of alpha-synuclein in Lewy body disease
ActiveUS20050198694A1Useful pharmacological activityBiocideNervous disorderGel electrophoresisC-terminus
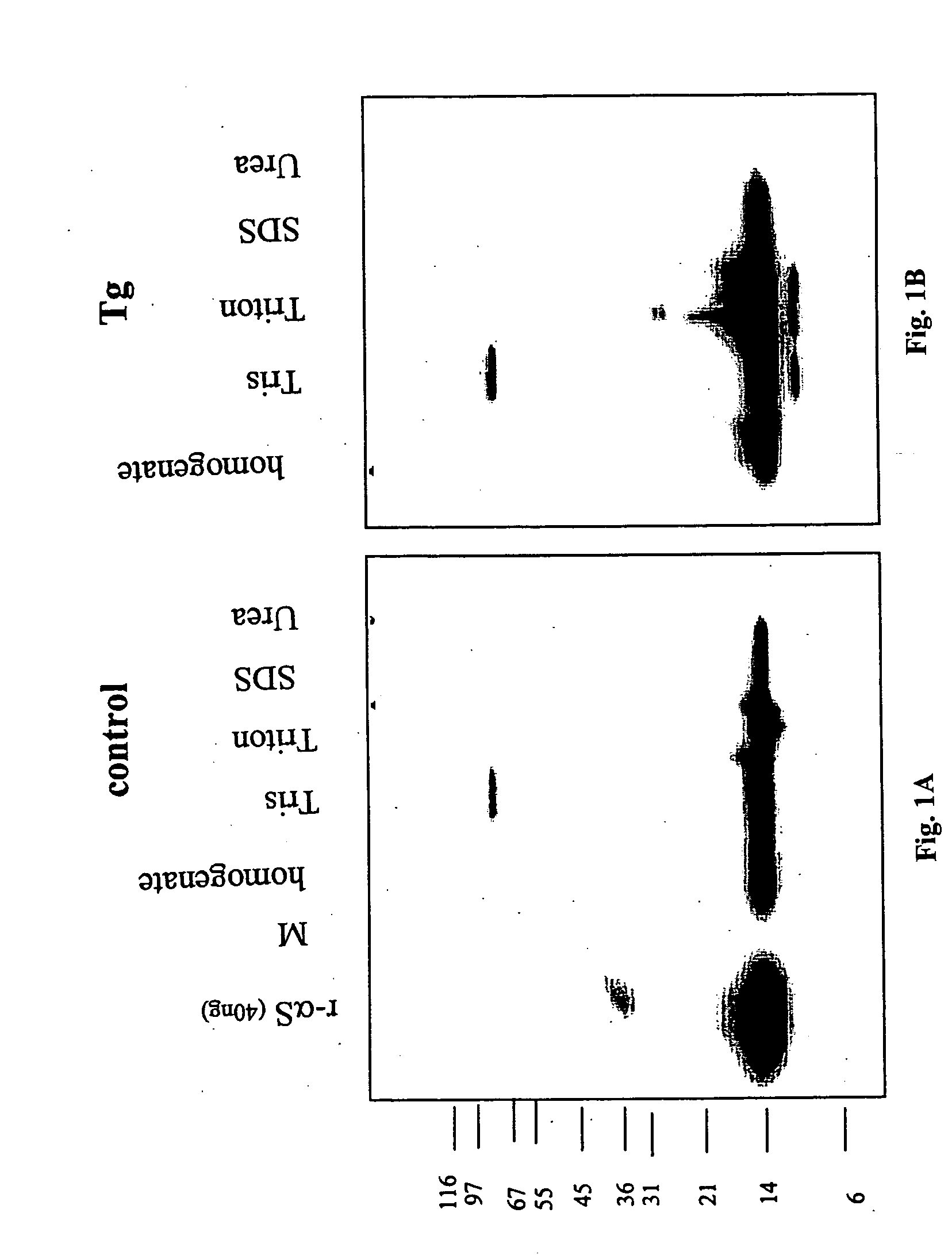
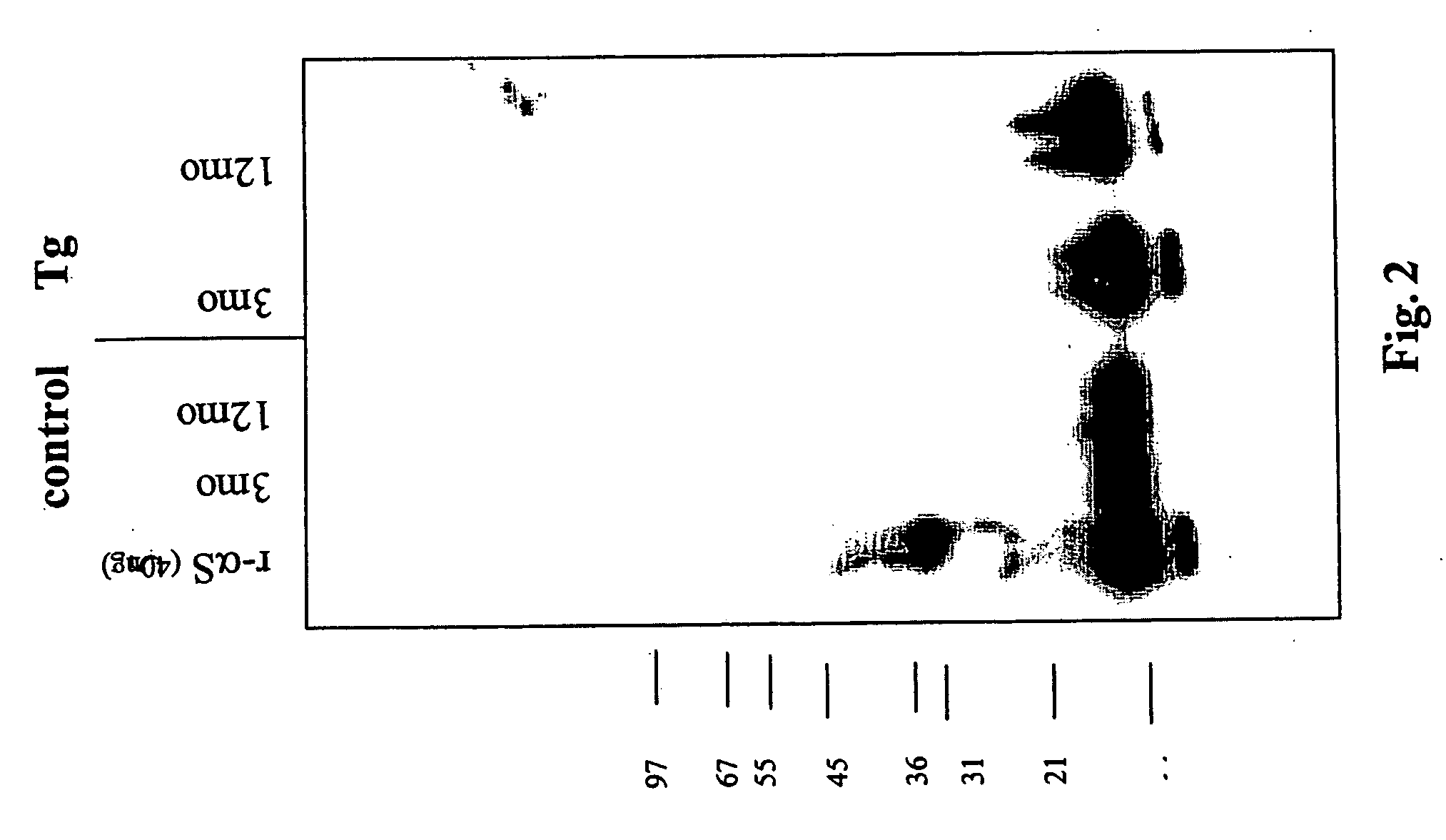
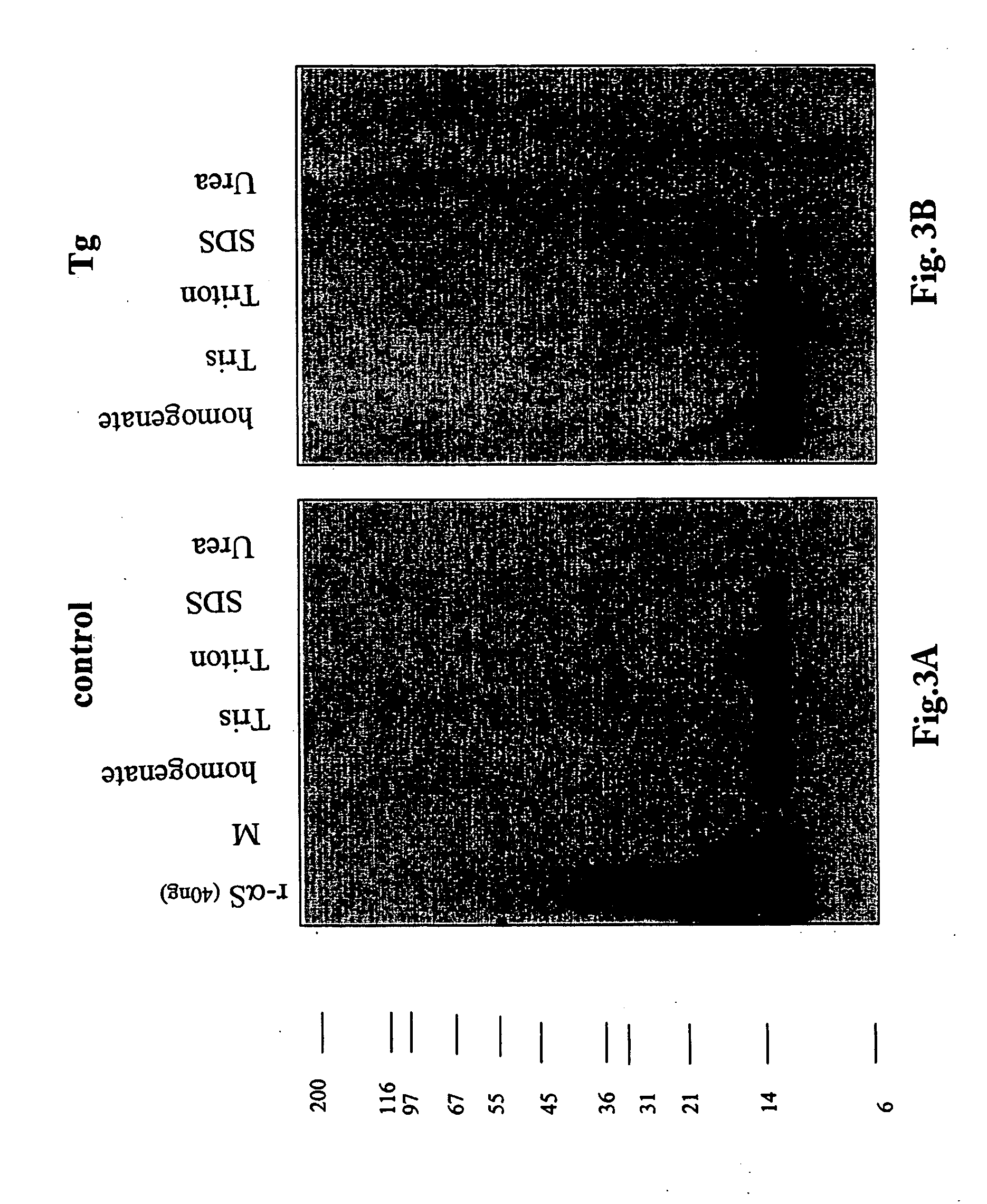
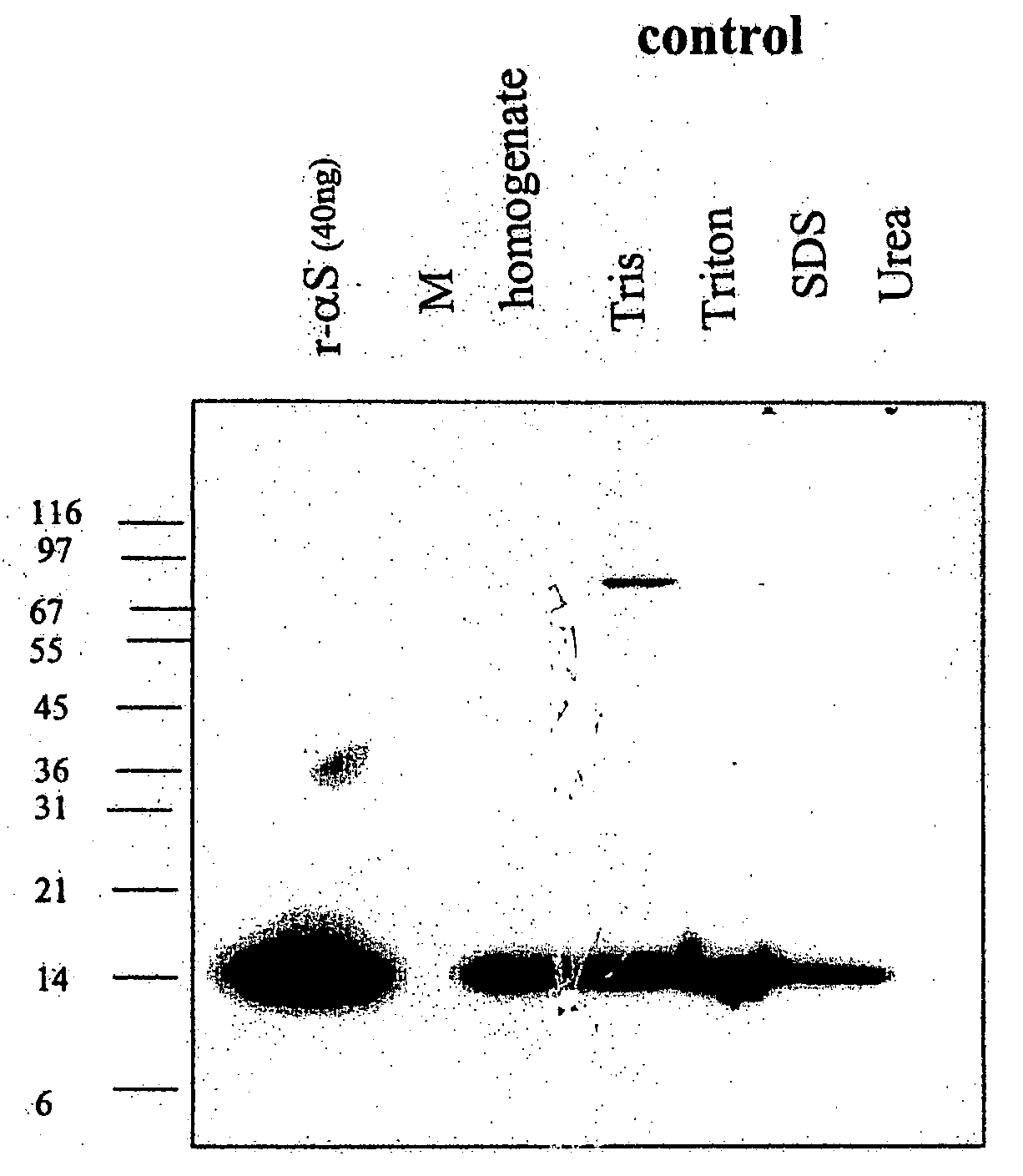
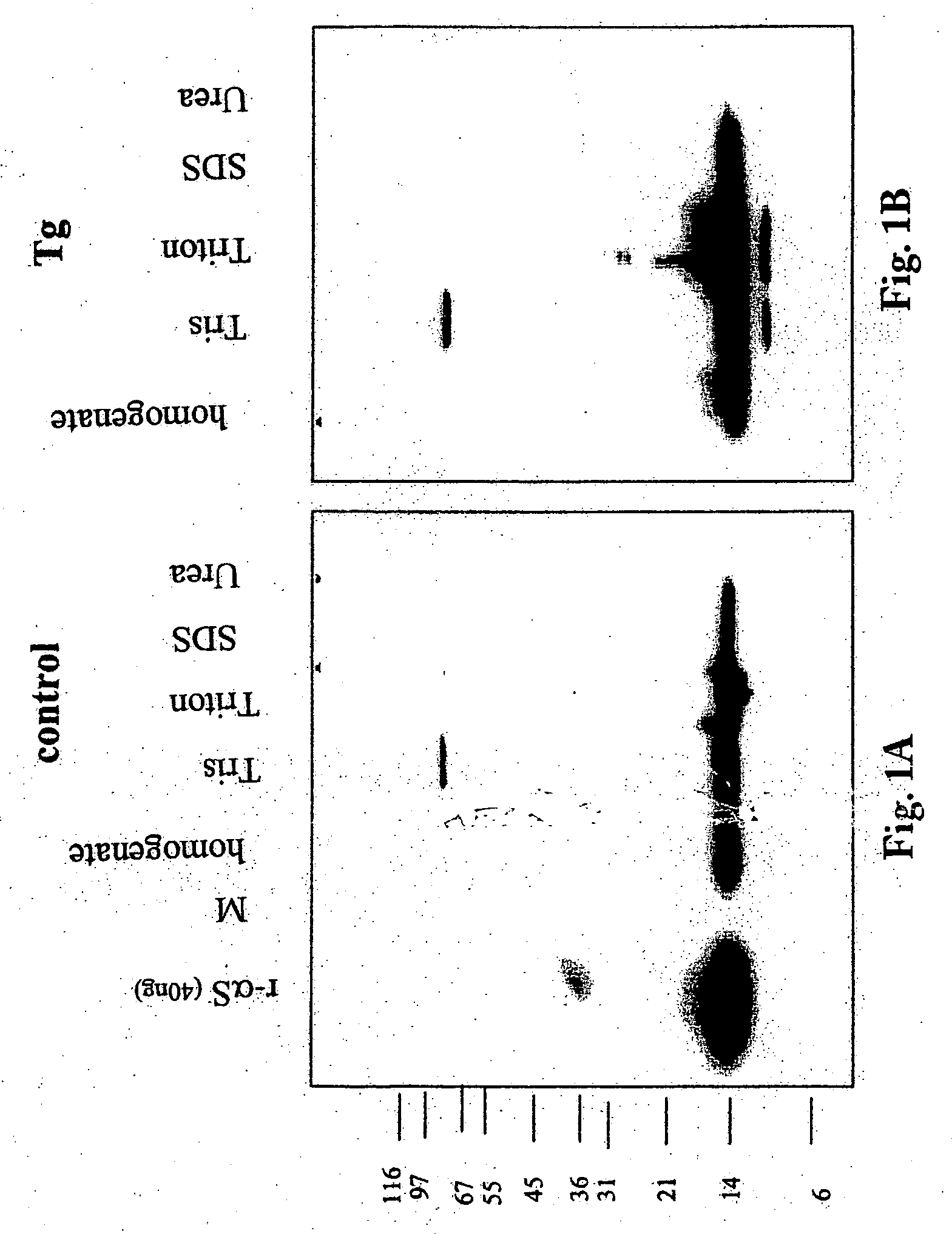
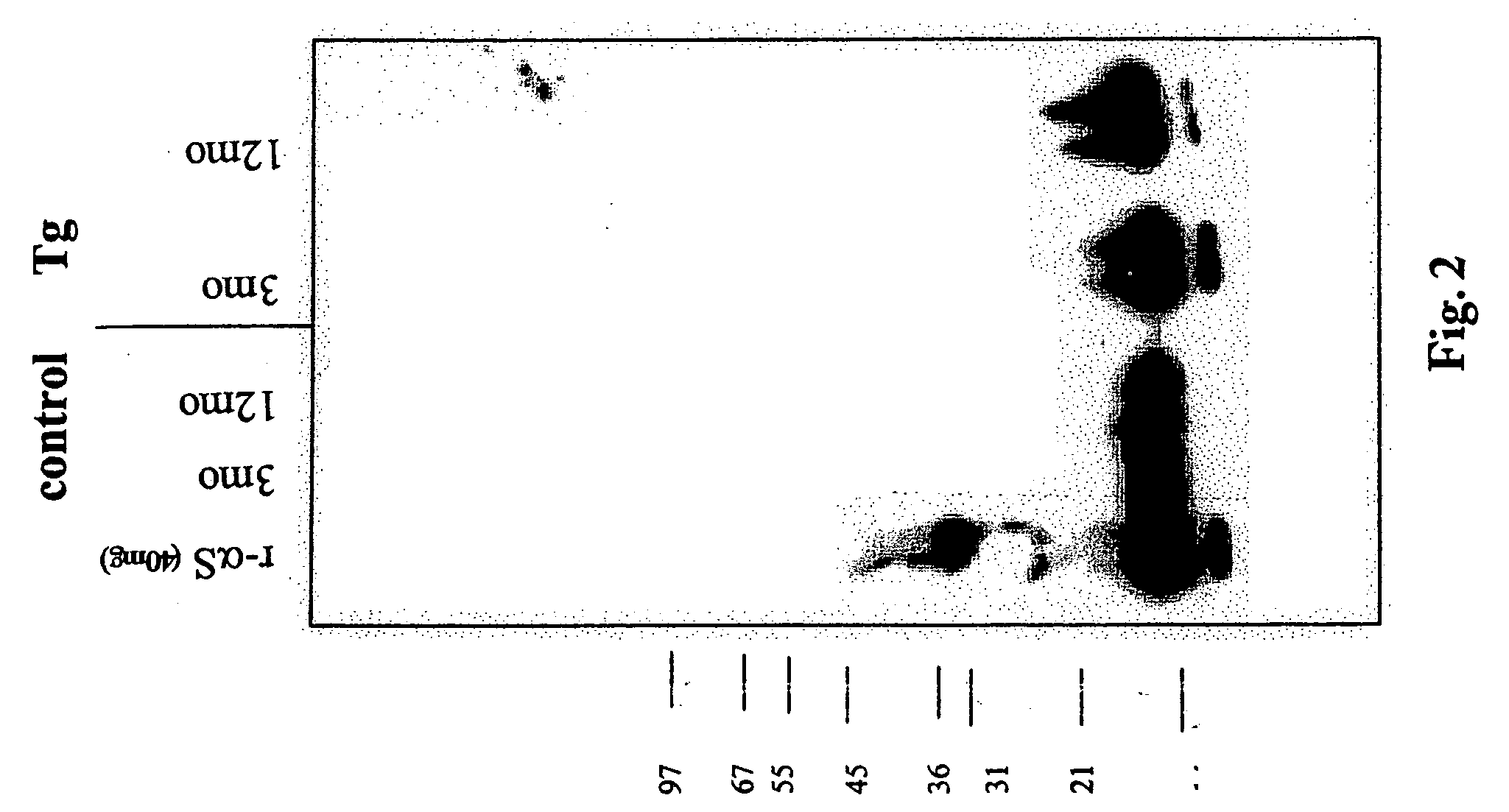
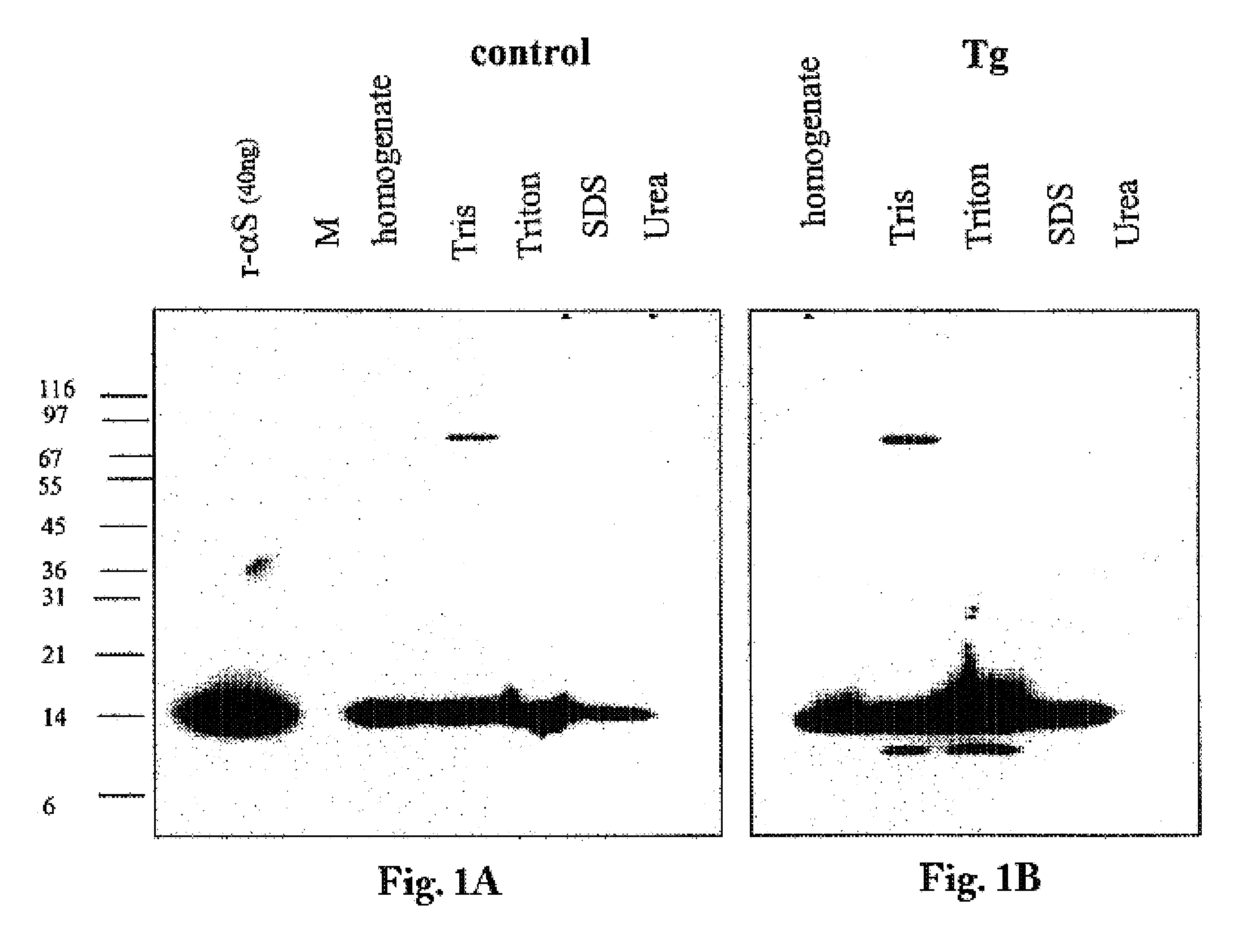
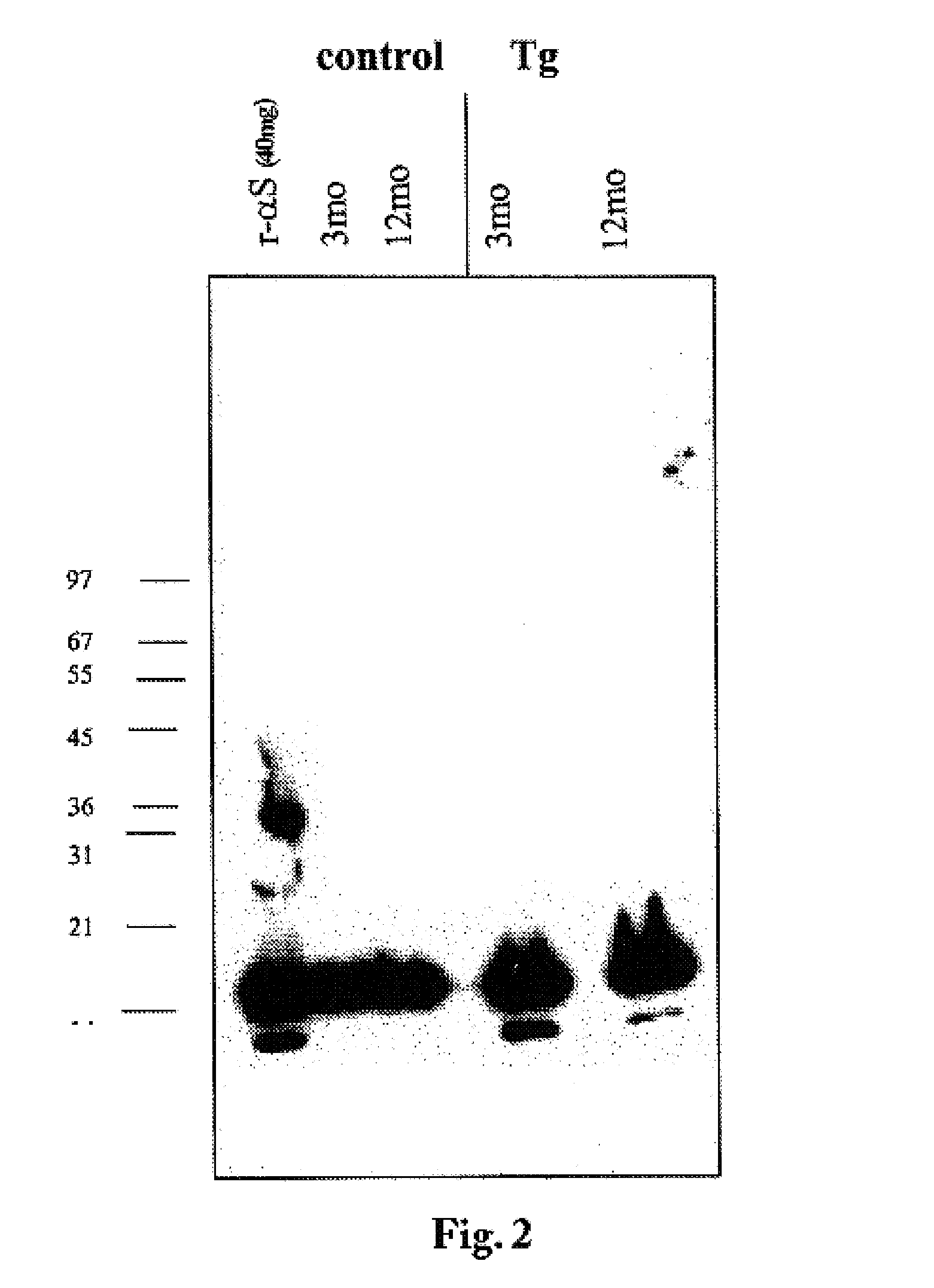
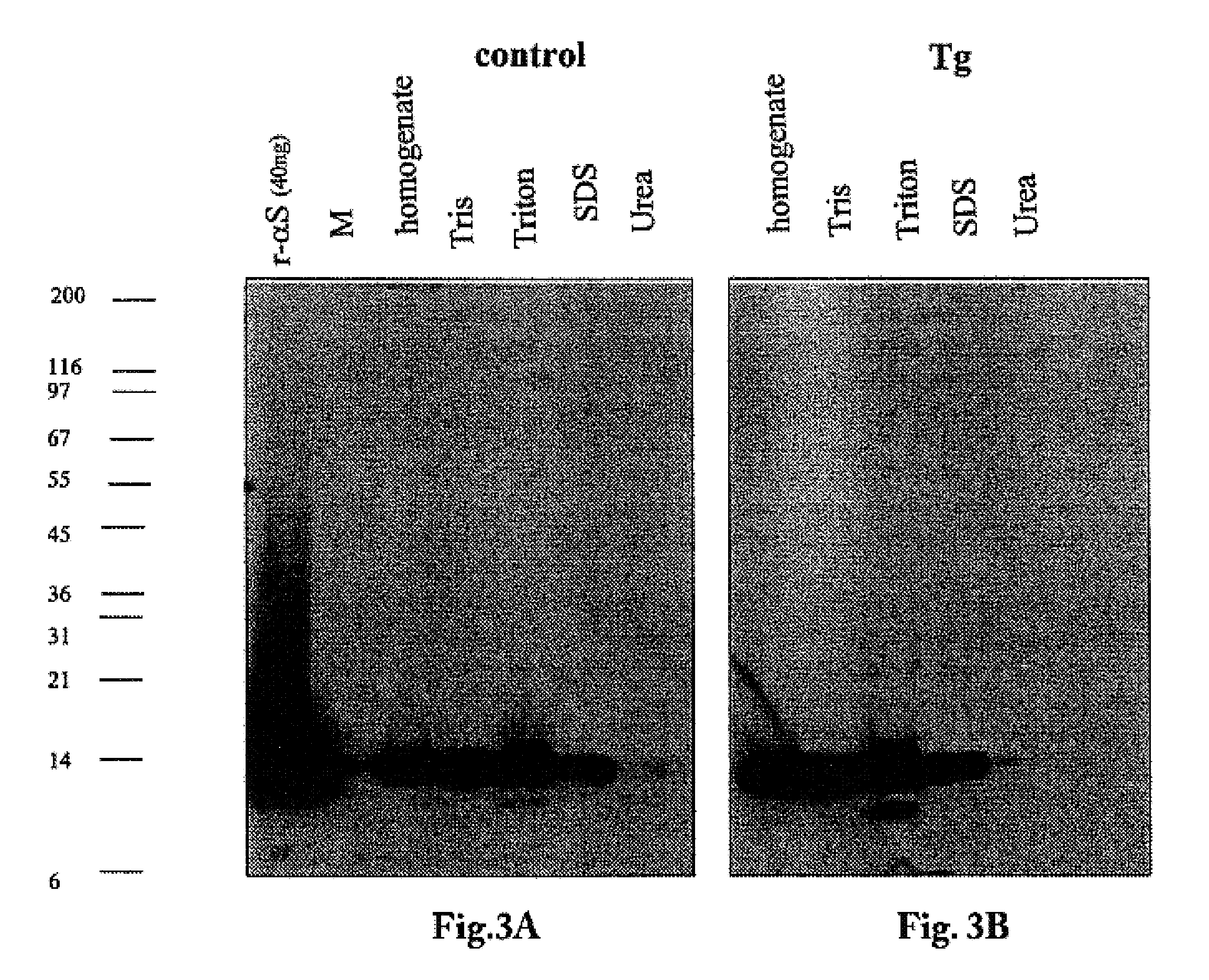
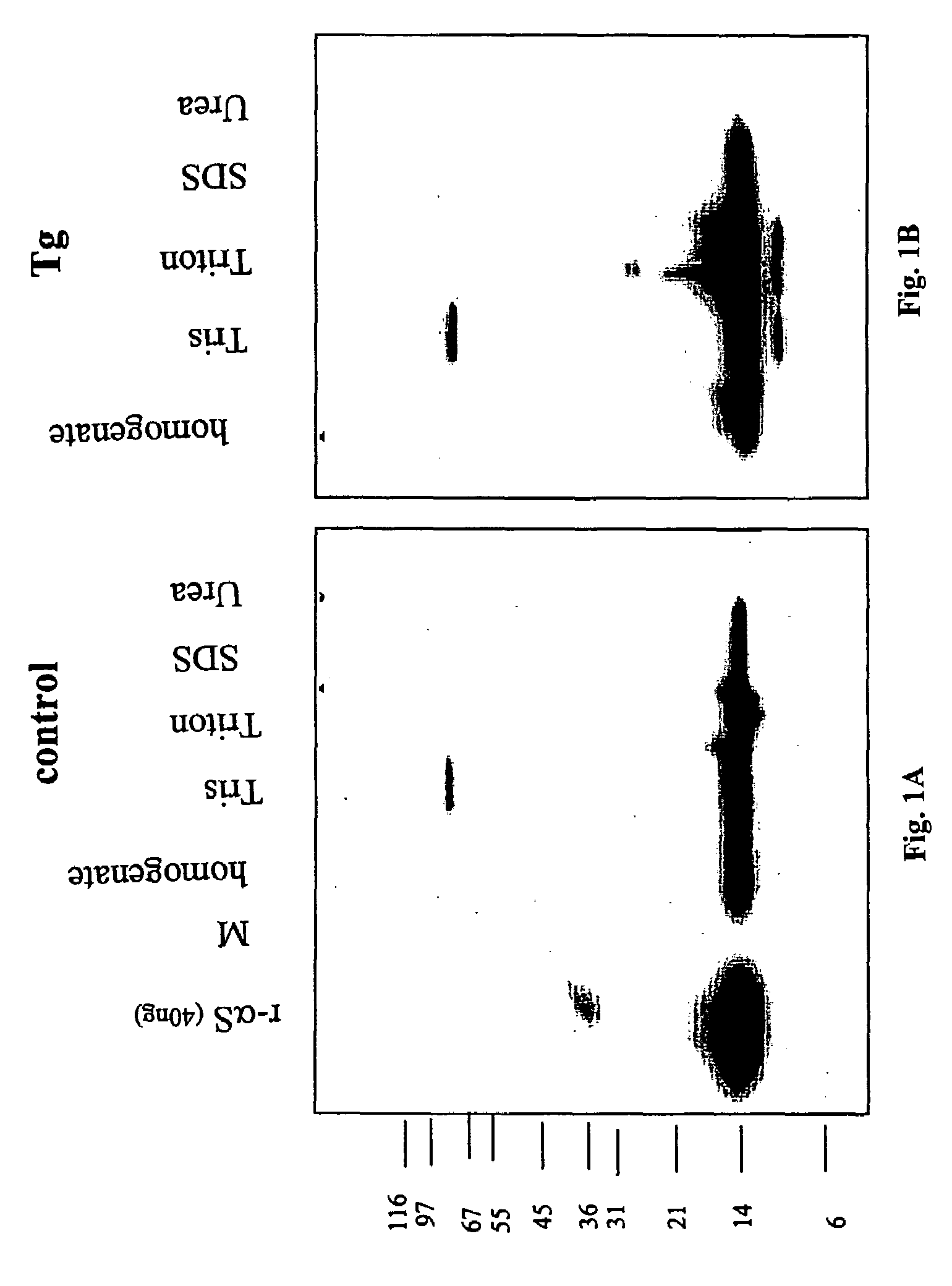
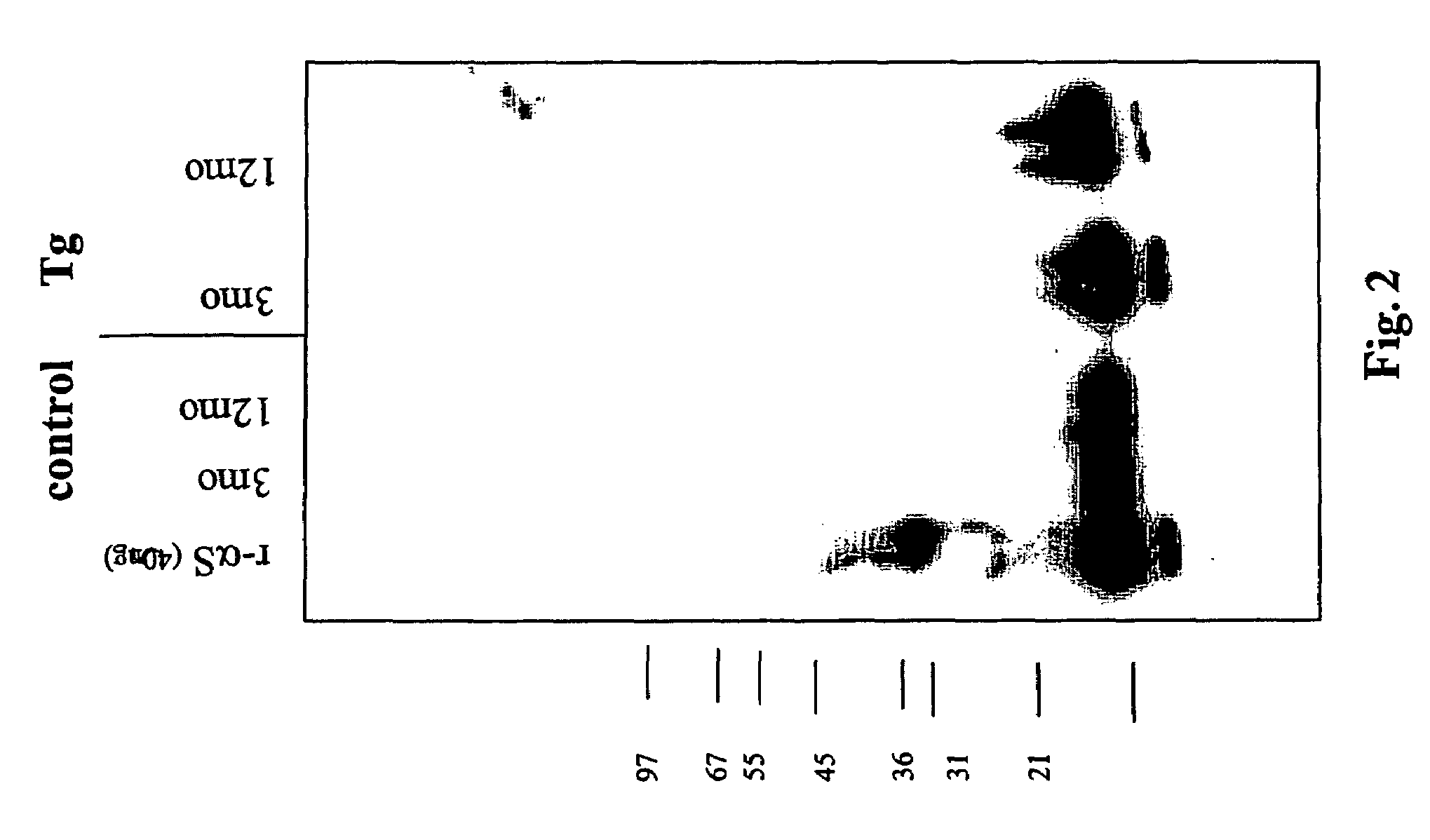
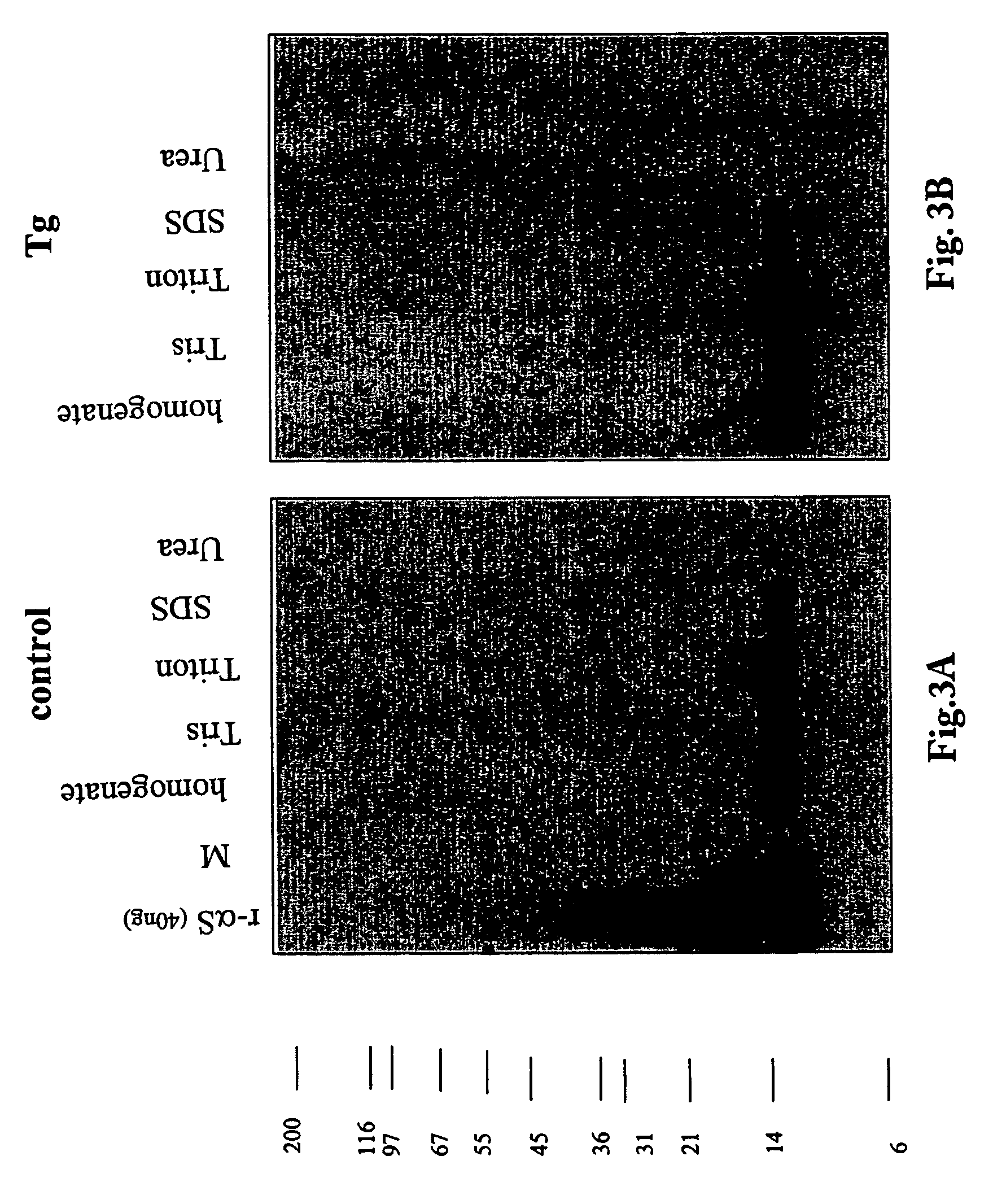
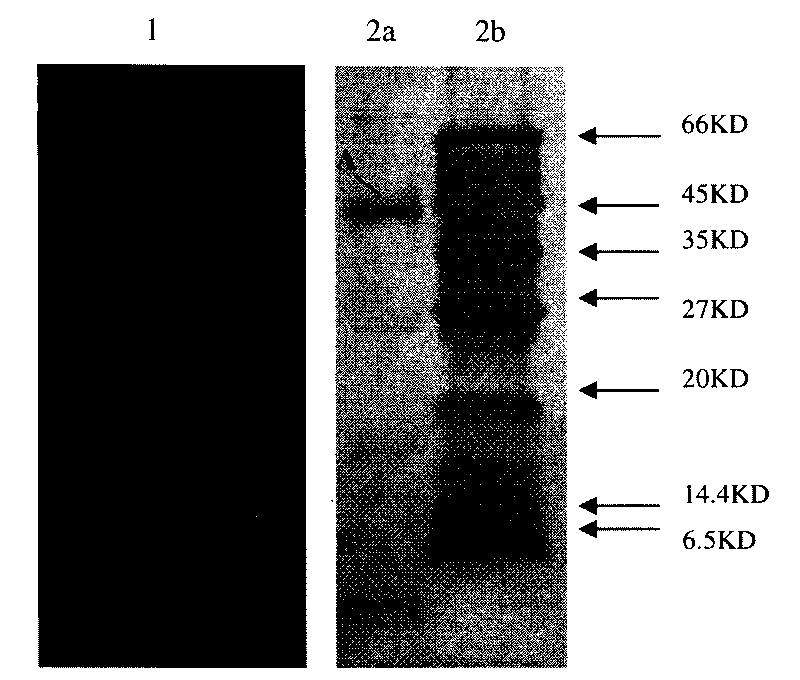
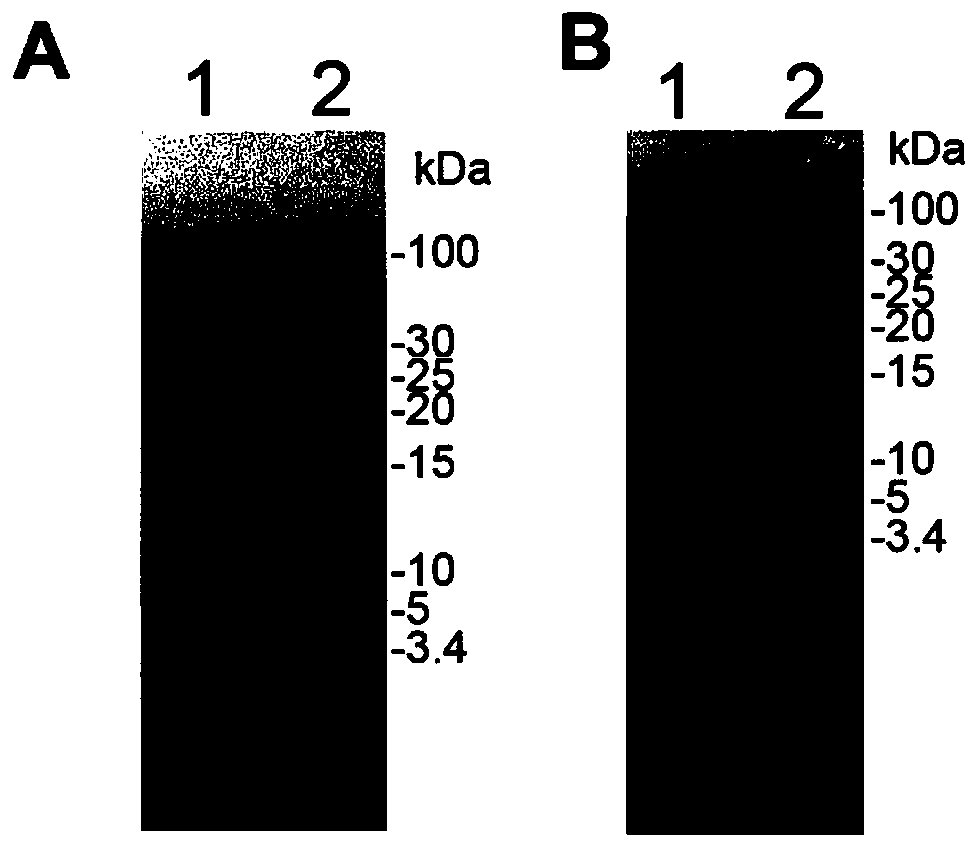
The application identifies novel fragments of alpha-synuclein in patients with Lewy Body Disease (LBD) and transgenic animal models thereof. These diseases are characterized by aggregations of alpha-synuclein. The fragments have a truncated C-terminus relative to fill-length alpha-synuclein. Some fragments are characterized by a molecular weight of about 12 kDa as determined by SDS gel electrophoresis in tricine buffer and a truncation of at least ten contiguous amino acids from the C-terminus of natural alpha-synuclein. The site of cleavage preferably occurs after residue 117 and before residue 126 of natural alpha-synuclein. The identification of these novel fragments of alpha-synuclein has a number of application in for example, drug discovery, diagnostics, therapeutics, and transgenic animals.
Owner:TRUSTEES OF FLINDERS UNIV +2
Truncated fragments of alpha-synuclein in Lewy Body Disease
The application identifies novel fragments of alpha-synuclein in patients with Lewy Body Disease (LBD) and transgenic animal models thereof. These diseases are characterized by aggregations of alpha-synuclein. The fragments have a truncated C-terminus relative to full-length alpha-synuclein. Some fragments are characterized by a molecular weight of about 12 kDa as determined by SDS gel electrophoresis in tricine buffer and a truncation of at least ten contiguous amino acids from the C-terminus of natural alpha-synuclein. The site of cleavage preferably occurs after residue 117 and before residue 126 of natural alpha-synuclein. The identification of these novel fragments of alpha-synuclein has a number of application in for example, drug discovery, diagnostics, therapeutics, and transgenic animals.
Owner:PROTHENA BIOSCI LTD
Cleaning Composition
ActiveUS20090281017A1Improve abilitiesGood removal effectOrganic detergent compounding agentsNon-surface-active detergent compositionsIminodiacetic acidGlycine
The invention relates to compositions and methods for cleaning integrated circuit substrates. The compositions are in the form of an aqueous solution and include a quaternary ammonium hydroxide compound and a chelating compound. The chelating compound includes either boric acid or at least one N-substituted aminocarboxylate selected from the group consisting of N-bis(2-hydroxyethyl)glycine(bicine), N-tris(hydroxymethyl)methyl glycine (tricine) and mixtures thereof, and can optionally include glycine, Iminodiacetic acid (IDA), Nitrilo trizacetic acid (NTA), Ethylenediammine Tetraacetic acid (EDTA), or mixtures thereof.
Owner:EKC TECH
Truncated fragments of alpha-synuclein in Lewy body disease
The application identifies novel fragments of alpha-synuclein in patients with Lewy Body Disease (LBD) and transgenic animal models thereof. These diseases are characterized by aggregations of alpha-synuclein. The fragments have a truncated C-terminus relative to full-length alpha-synuclein. Some fragments are characterized by a molecular weight of about 12 kDa as determined by SDS gel electrophoresis in tricine buffer and a truncation of at least ten contiguous amino acids from the C-terminus of natural alpha-synuclein. The site of cleavage preferably occurs after residue 117 and before residue 126 of natural alpha-synuclein. The identification of these novel fragments of alpha-synuclein has a number of application in for example, drug discovery, diagnostics, therapeutics, and transgenic animals.
Owner:PROTHENA BIOSCI LTD
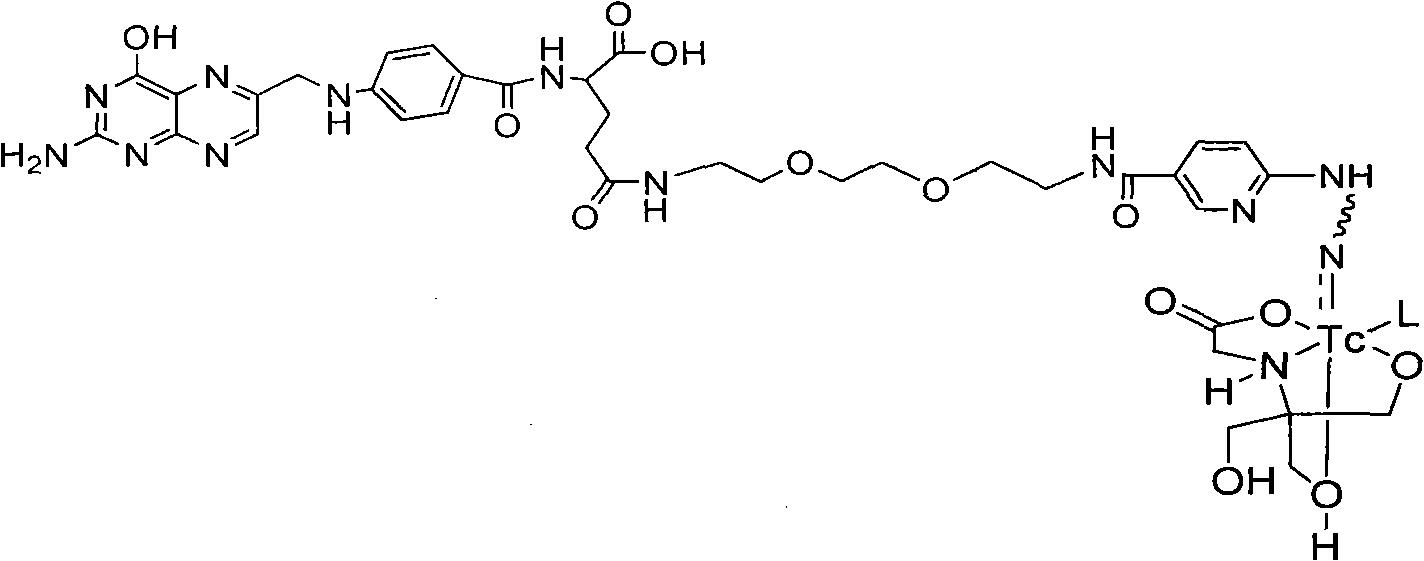
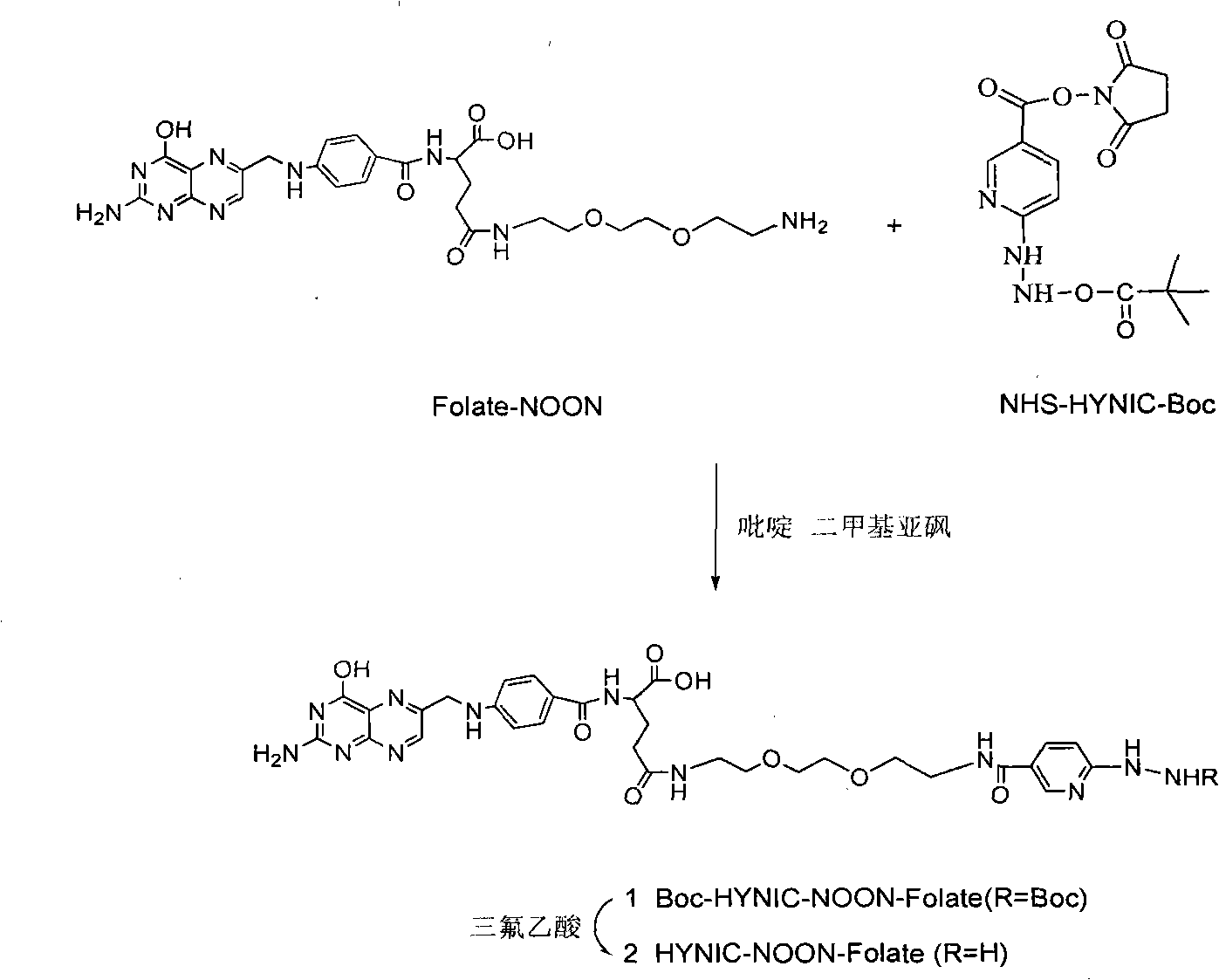
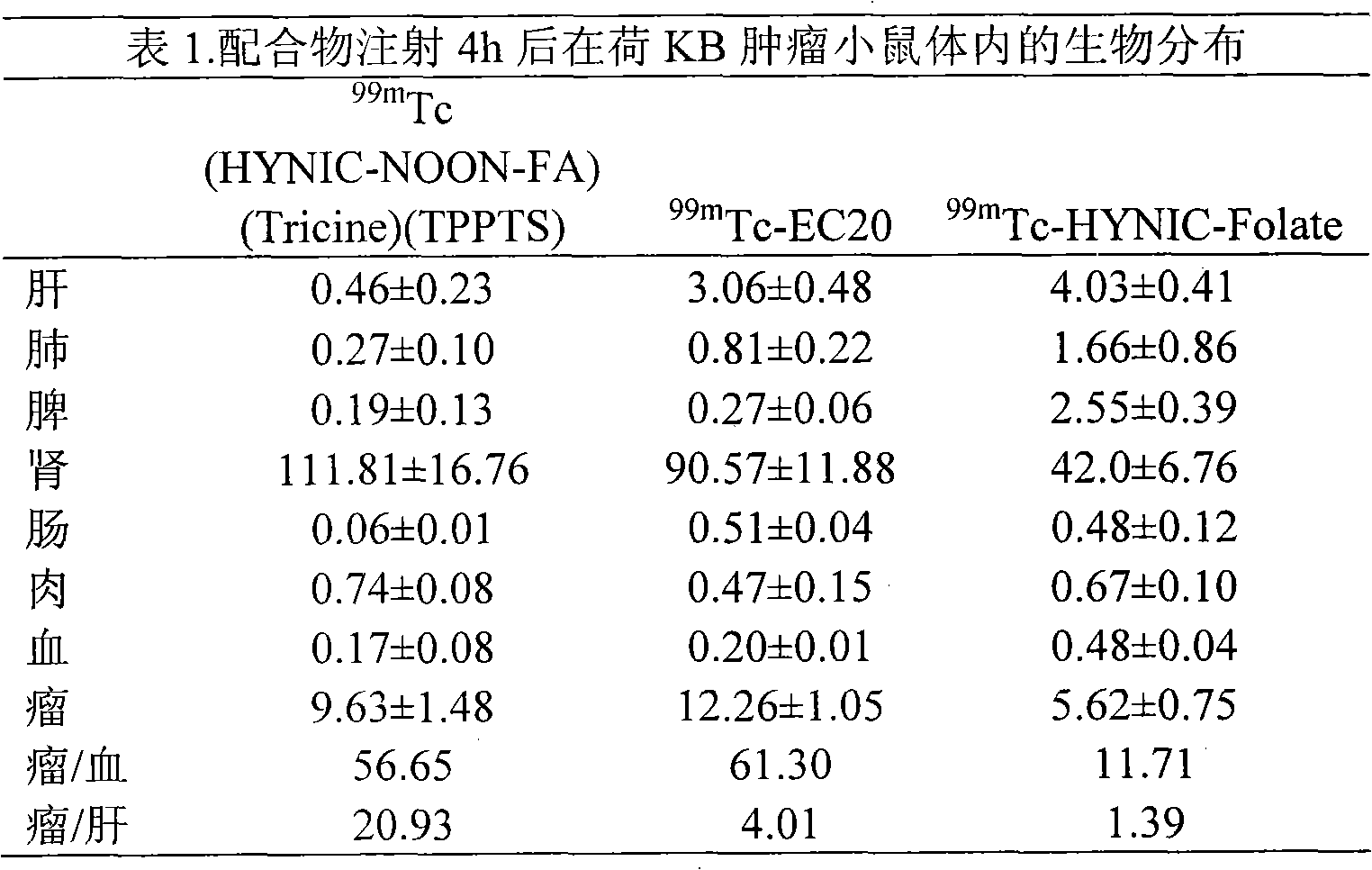
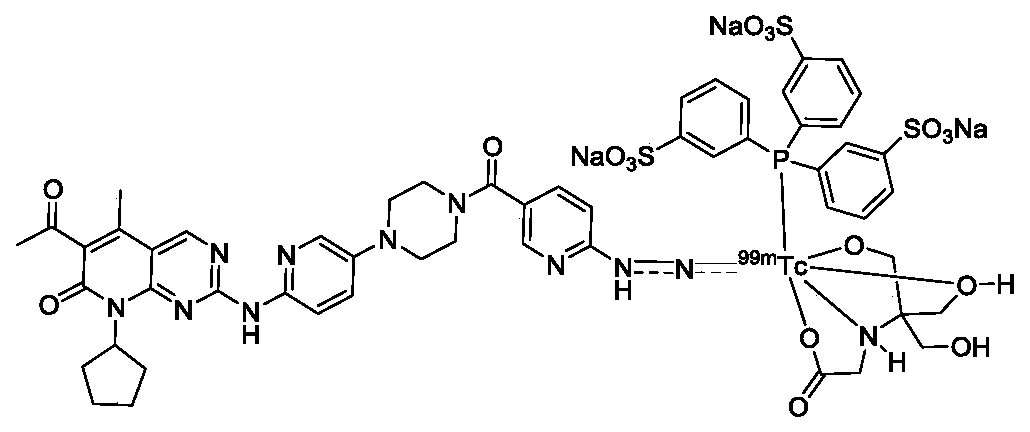
Labeled 99mTc hydrazino-nicotinamide-dioxodecoyl-folic acid coordination compound and preparation method
InactiveCN101863924AImprove performanceRadioactive preparation carriersGroup 7/17 element organic compoundsSodium phosphatesNiacin
The invention discloses a labeled 99mTc hydrazino-nicotinamide-dioxodecoyl-folic acid coordination compound with a general formula of 99mTc(HYNIC-NOON-FA)(Tricine)(L). In the structural formula, L is triphenyl sodium phosphate or triphenyl sodium photrisulfonic acid, wherein 1,8-diamido-3,6-octane dioxide is used as a connecting chain for generating a hydrazino-nicotinamide-3,6-dioxodecoyl-folic acid coupler respectively with folic acid and hydrazino-niacin through amido bonds and coordinating with oxygen atoms and phosphorus atoms in a co-ligand Tricine and an L molecule and 99mTc, and the 99mTc(HYNIC-NOON-FA)(Tricine)(L) coordination compound is obtained through two steps of: (a) synthesizing the hydrazino-nicotinamide-3,6-dioxodecoyl-folic acid coupler used as a ligand; and (b) labeling the 99mTc-hydrazino-nicotinamide-dioxodecoyl-folic acid coordination compound. The coordination compound has the advantages of high radiochemical purity, good stability, high tumor intake, good retention, low non-target organ background and clear tumor SPECT (Single Photon Emission Computed Tomography) development and can be prepared into a novel 99mTc labeled folic acid receptor tumor developer widely applied to the technical field of radioactive pharmaceutical chemistry and nuclear medicine.
Owner:BEIJING NORMAL UNIVERSITY +1
Truncated fragments of alpha-synuclein in Lewy body disease
The application identifies fragments of alpha-synuclein in patients with Lewy Body Disease (LBD) and transgenic animal models thereof. These diseases are characterized by aggregations of alpha-synuclein. The fragments have a truncated C-terminus relative to full-length alpha-synuclein. Some fragments are characterized by a molecular weight of about 12 kDa as determined by SDS gel electrophoresis in tricine buffer and a truncation of at least ten contiguous amino acids from the C-terminus of natural alpha-synuclein. The site of cleavage preferably occurs after residue 117 and before residue 126 of natural alpha-synuclein. The identification of these novel fragments of alpha-synuclein has a number of application in for example, drug discovery, diagnostics, therapeutics, and transgenic animals.
Owner:TRUSTEES OF FLINDERS UNIV +2
Antibacterial lipopeptide of endophytic Bacillus subtilis and separation and purification method
InactiveCN101724014ABroad antibacterial spectrumImprove thermal stabilityMicroorganism based processesFungicidesPesticide residueAntifungal drug
The invention relates to an extracellular antibacterial lipopeptide of plant endophytic Bacillus subtilis Jaasedl and a separation and purification method. The distribution range of the molecular weight of the extracellular antibacterial lipopeptide is mainly between 1,000Da and 2,200Da. The extracellular antibacterial lipopeptide contains an Iturin homologue, a Fengycin homologue and a Surfactin-like Compound homologue. The extracellular antibacterial lipopeptide is a group of uncommon antibacterial lipopeptide mixture produced by a single bacterial strain. The separation and purification method of the extracellular antibacterial lipopeptide comprises the following steps of: after salting out to obtain the crude extract of the antibacterial lipopeptide by using ammonium sulfate from the fermentation liquor of the endophytic Bacillus subtilis Jaasedl, performing Sephedex G-25 molecular sieve chromatography, Cellulose DEAE-52 anion exchange chromatography and FPLC300SB-C18 column chromatography successively, wherein 34 to 37 min of collecting peak has bacteriostatic activity; detecting by Tricine-SDS-PAGE after concentrating; and achieving electrophoretically pure at only one strip to obtain pure extracellular antibacterial lipopeptide. The antibacterial lipopeptide has extremely high research and application value for the development of broad-spectrum antifungal medicaments, and has wide application prospect for natural quality protection of crops and pesticide residue reduction.
Owner:JIANGSU ACAD OF AGRI SCI
Cleaning composition comprising a chelant and quaternary ammonium hydroxide mixture
ActiveUS7825079B2Improve abilitiesGood removal effectOrganic detergent compounding agentsSemiconductor/solid-state device manufacturingGlycineIminodiacetic acid
Owner:EKC TECH
Bicine/tricine containing composition and method for chemical-mechanical planarization
InactiveUS20050194563A1High selectivityOther chemical processesDecorative surface effectsTricineChemical compound
A composition and associated method for chemical mechanical planarization (or other polishing) are described. The composition comprises an abrasive and a tricine-type or bicine-type compound. The composition possesses high selectivities for removal of copper in relation to tantalum and dielectric materials whilst minimizing local dishing and erosion effects in CMP. The composition may further comprise an oxidizing agent in which case the composition is particularly useful in conjunction with the associated method for metal CMP applications (e.g., copper CMP).
Owner:DUPONT AIR PRODS NANOMATERIALS +1
Nucleic acid preserving fluid
InactiveCN111334503AAvoid degradationLong storage timeDNA preparationPathogenic microorganismTricine
The invention provides nucleic acid preserving fluid. The nucleic acid preserving fluid consists of a nuclease inhibitor, a protein denaturant, a stabilizer, a bacteriostatic agent and a Tricine buffer solution, wherein the concentration of the nuclease inhibitor is 5mg / ml-20mg / ml, the concentration of the protein denaturant is 20mg / ml-80mg / ml, the concentration of the stabilizer is 30mg / ml-90mg / ml, the concentration of the bacteriostatic agent is 100mg / ml-500mg / ml, and the concentration of the Tricine buffer solution is 20nM-120nM. The nucleic acid preserving fluid has the beneficial effectsthat the nucleic acid preserving fluid is capable of effectively preventing degradation of nucleic acid at a normal temperature and providing a stable environment for nucleic acid, so that the preservation time of the nucleic acid is prolonged, and the safety during collection, transportation and detection can be guaranteed; and meanwhile, pathogenic microorganisms can be inactivated, and the nucleic acid preserving fluid has the advantages of low preparation cost, stable performance, easiness in preservation and the like.
Owner:JIANGSU COWIN BIOTECH CO LTD
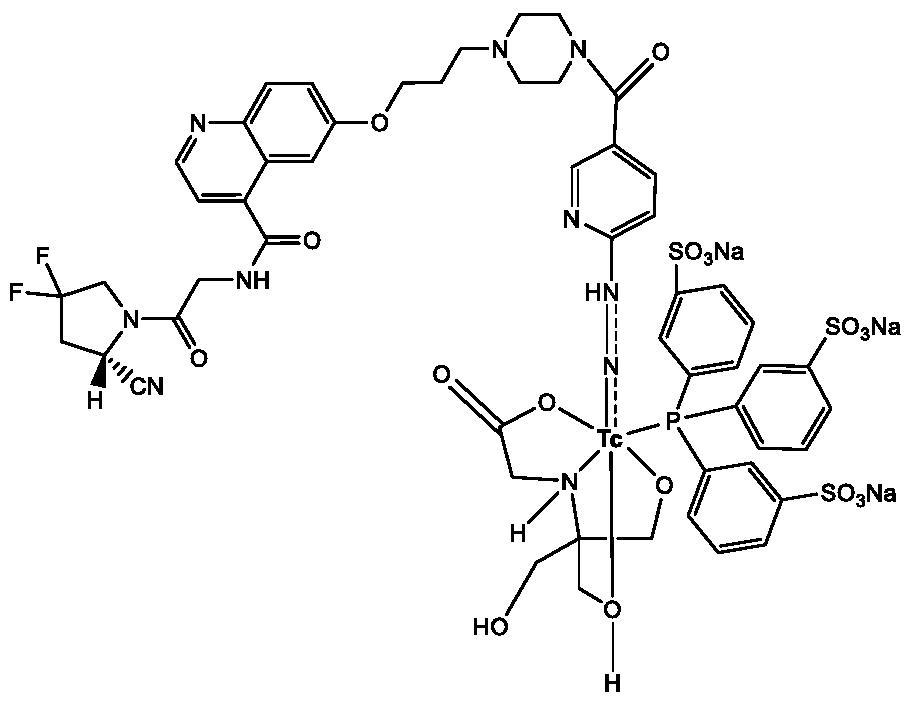
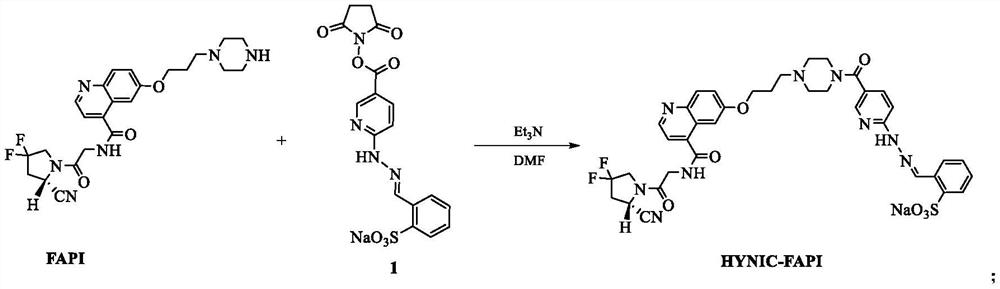
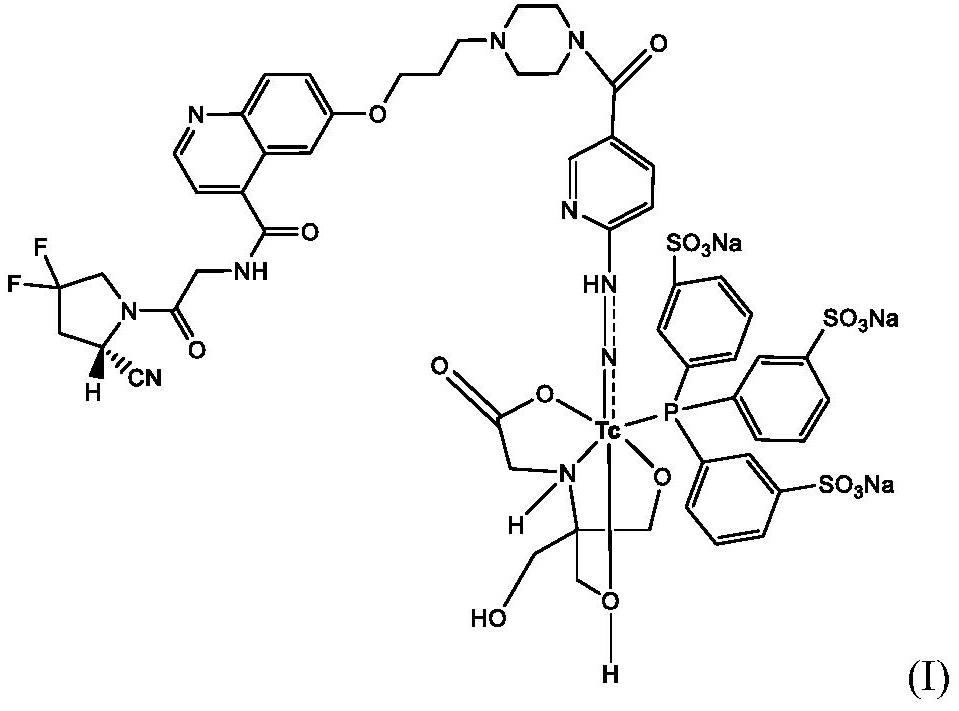
Technetium-99m labeled hydrazine-based nicouramido-containing FAPI derivative as well as preparation method and application thereof
PendingCN112625065AReduce intakeConcentrated obviouslyRadioactive preparation carriersIsotope introduction to organic compoundsTPPTSTricine
The invention discloses a compound with a general formula of 99mTc (HYNIC-FAPI) (tricine / TPPTS) technetium-99 m labeled FAPI derivative containing hydrazino-nicotinamide, and a preparation method and application thereof. By two steps of synthesis of ligand HYNIC-FAPI and 99mtc (HYNIC-FAPI) (tricine / TPPTS) preparation, the 99mTc (HYNIC-FAPI) (tricine / TPPTS) complex is obtained. The complex has the advantages of simple preparation, high radiochemical purity and good stability, has high uptake and good detention at tumor parts of tumor-bearing mice, has specific uptake in tumors, and is a novel tumor radiopharmaceutical with clinical application value.
Owner:BEIJING NORMAL UNIVERSITY
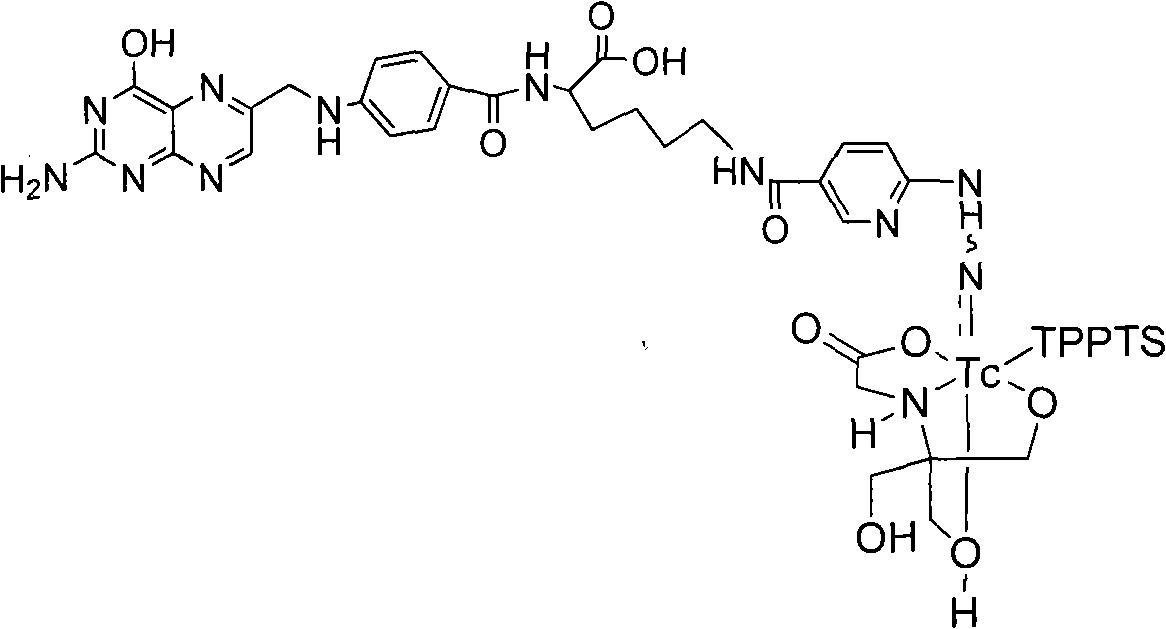
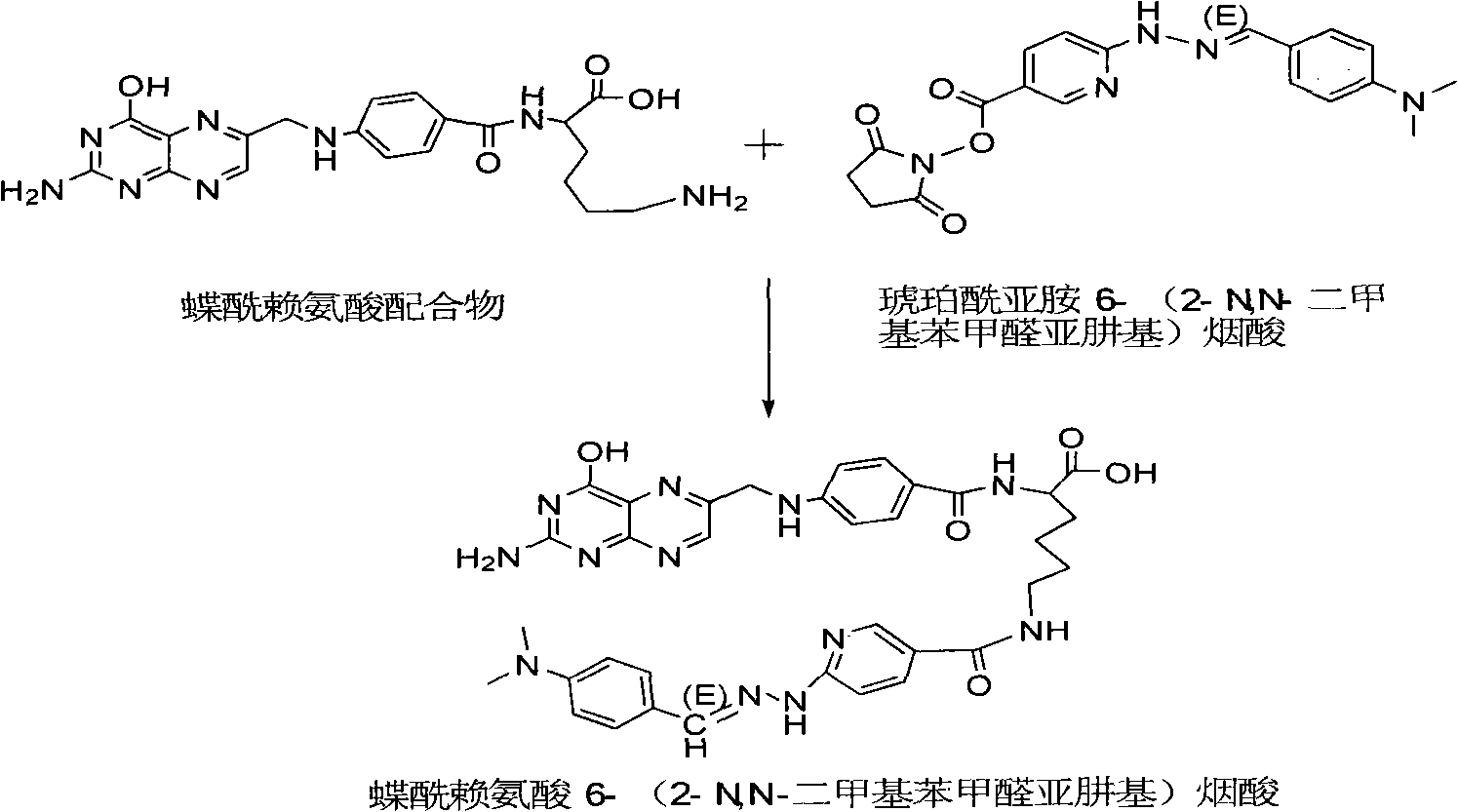
Preparation method of 99mTc labeled hydrazinonicotinamide group-pteroyllysine coordination compound
InactiveCN101885744AHigh target to non-target ratioIn-vivo radioactive preparationsGroup 7/17 element organic compoundsTPPTSHydrazino nicotinamide
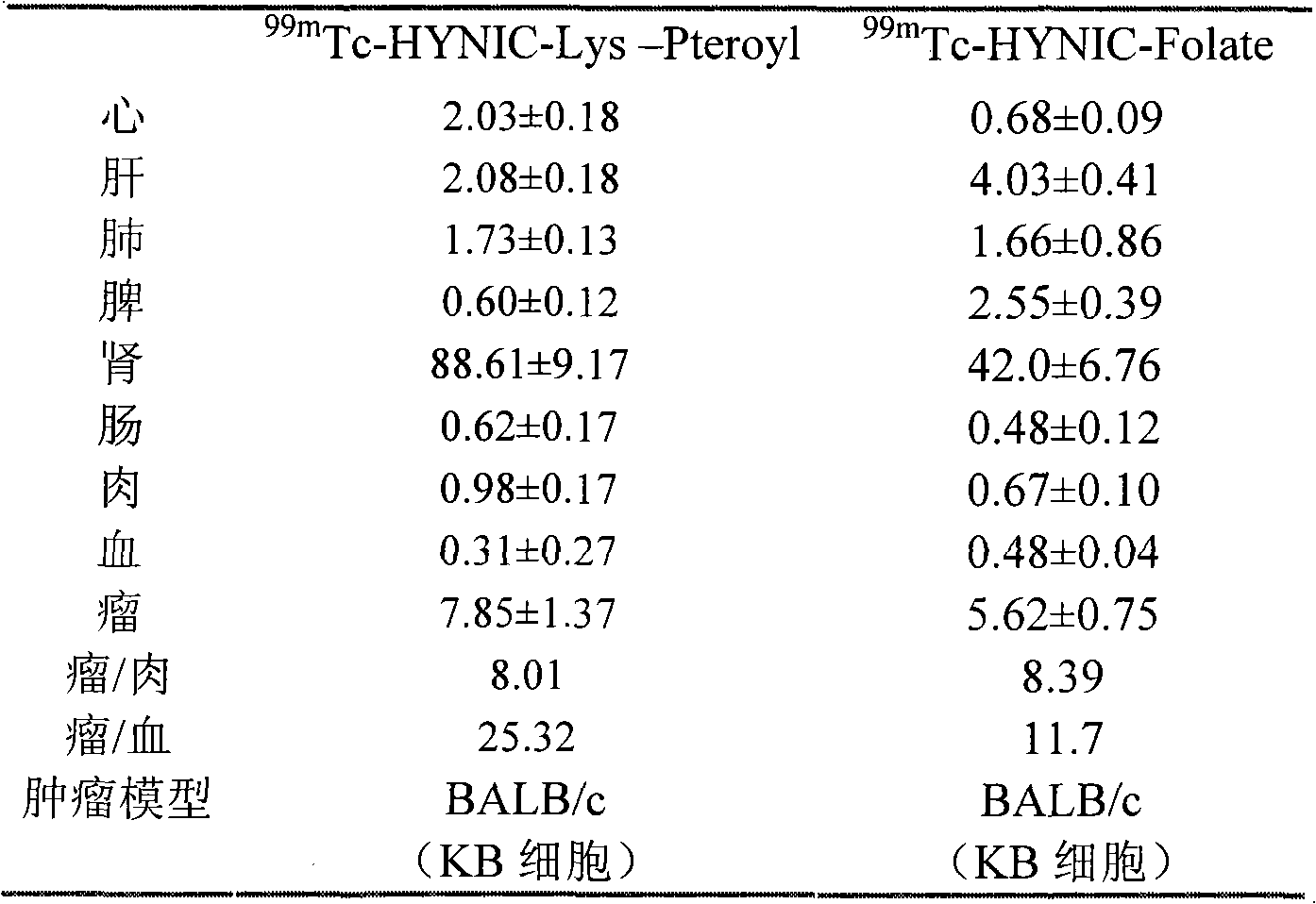
The invention discloses a 99mTc labeled hydrazinonicotinamide group-pteroyllysine coordination compound with an expression of 99mTc (HYNIC-Lys-Pteroyl)(Tricine)(TPPTS), a preparation method and application. The target coordination compound is obtained through the following two steps of: (a) synthesizing pteroyllysine 6-(2-N,N-dimethylbenzaldehydehydrazono) niacin as a ligand; and (b) obtaining the 99mTc labeled hydrazinonicotinamide group-pteroyllysine coordination compound. The coordination compound has the advantages of high radiochemical purity, good stability, low price, high tumor uptakerate, good retention and good target / non-target specific values of tumor / blood, tumor / muscle, and the like and can be popularized and applied by being used as a novel 99mTc labeled folate receptor tumor image developer.
Owner:BEIJING NORMAL UNIVERSITY +1
Imroved alkanolamines for co2 removal from gas streams
InactiveUS20110116997A1Large CO absorption capacityPoor rateGas treatmentHydrogen sulfidesCo2 removalTricine
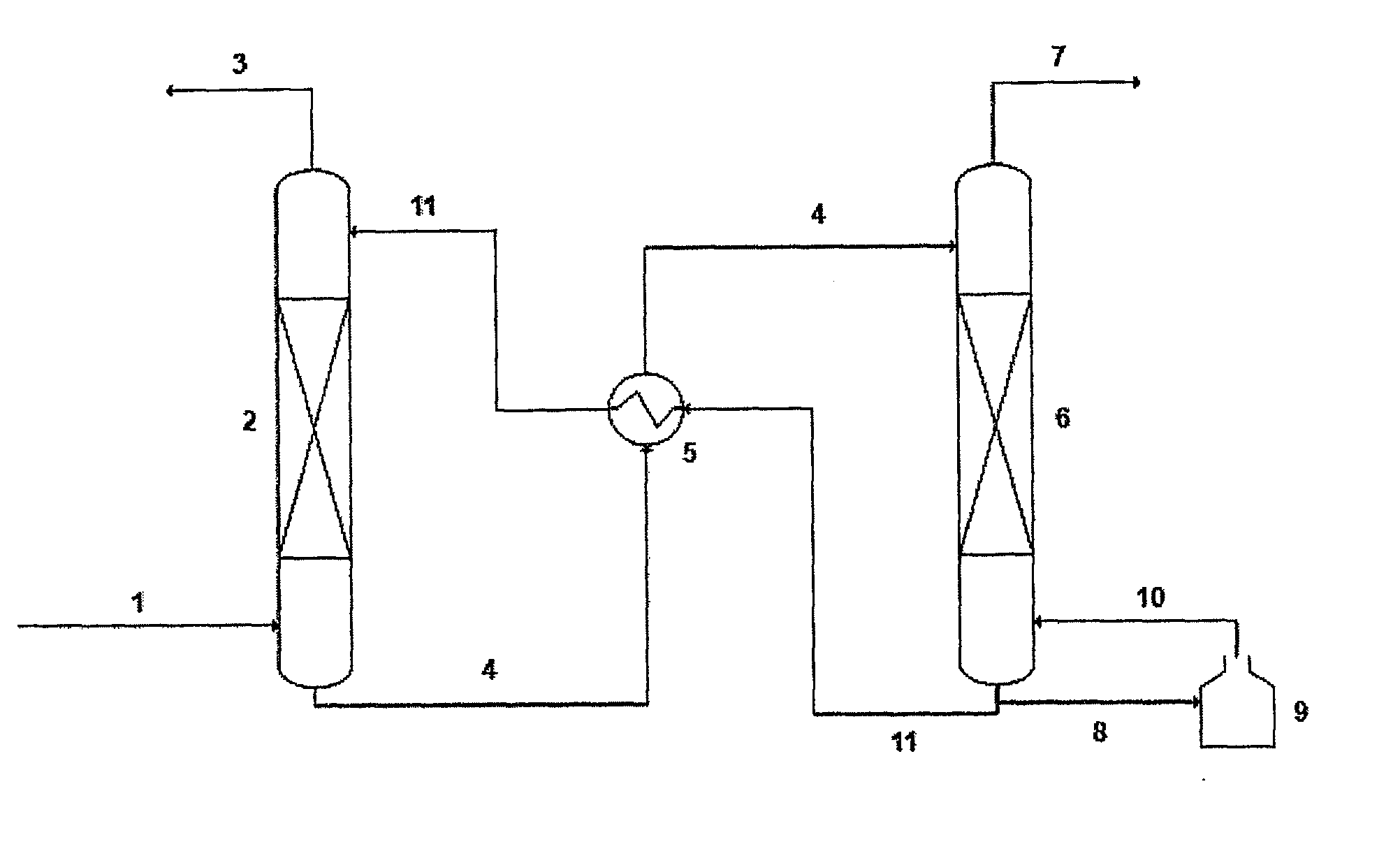
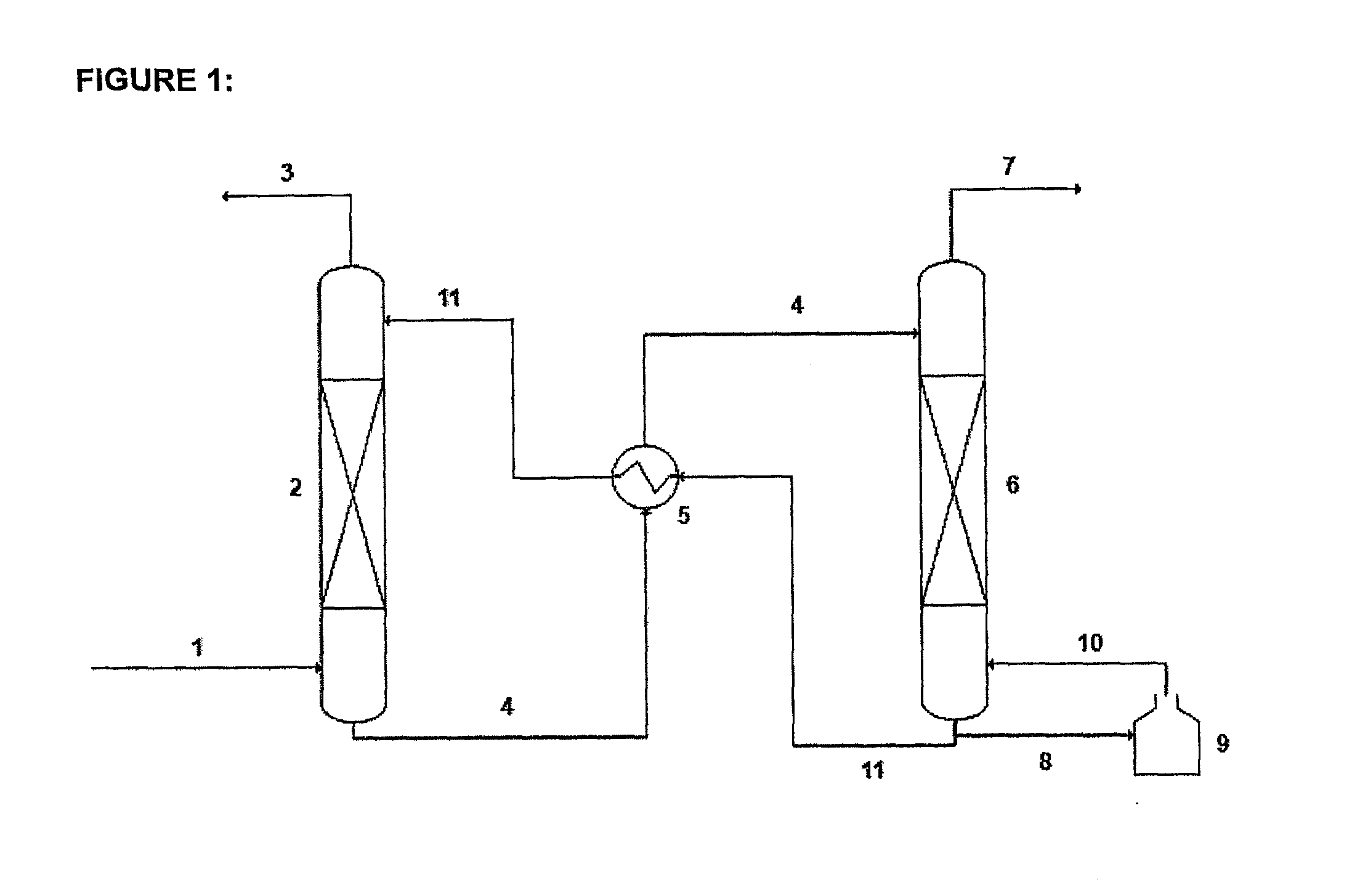
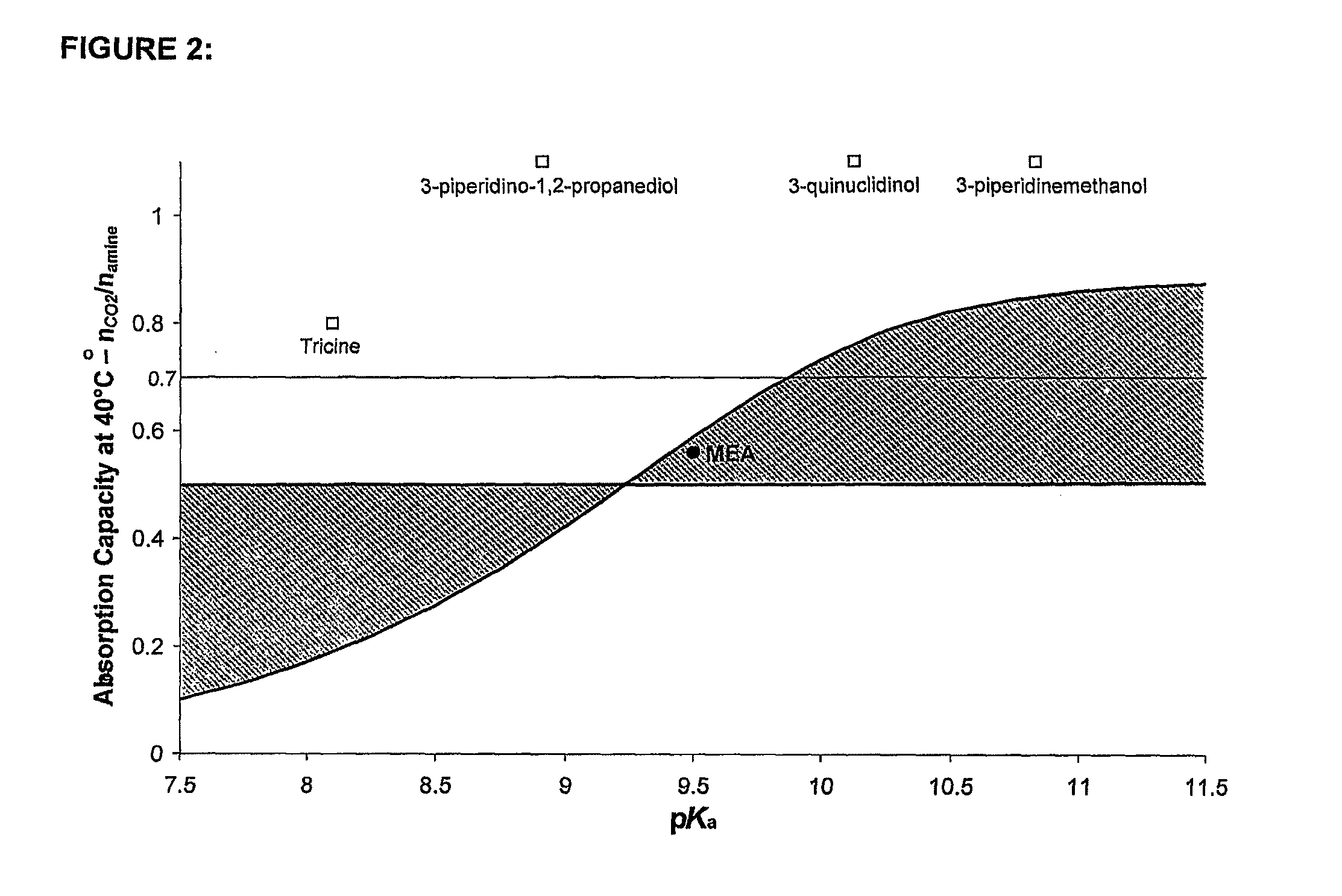
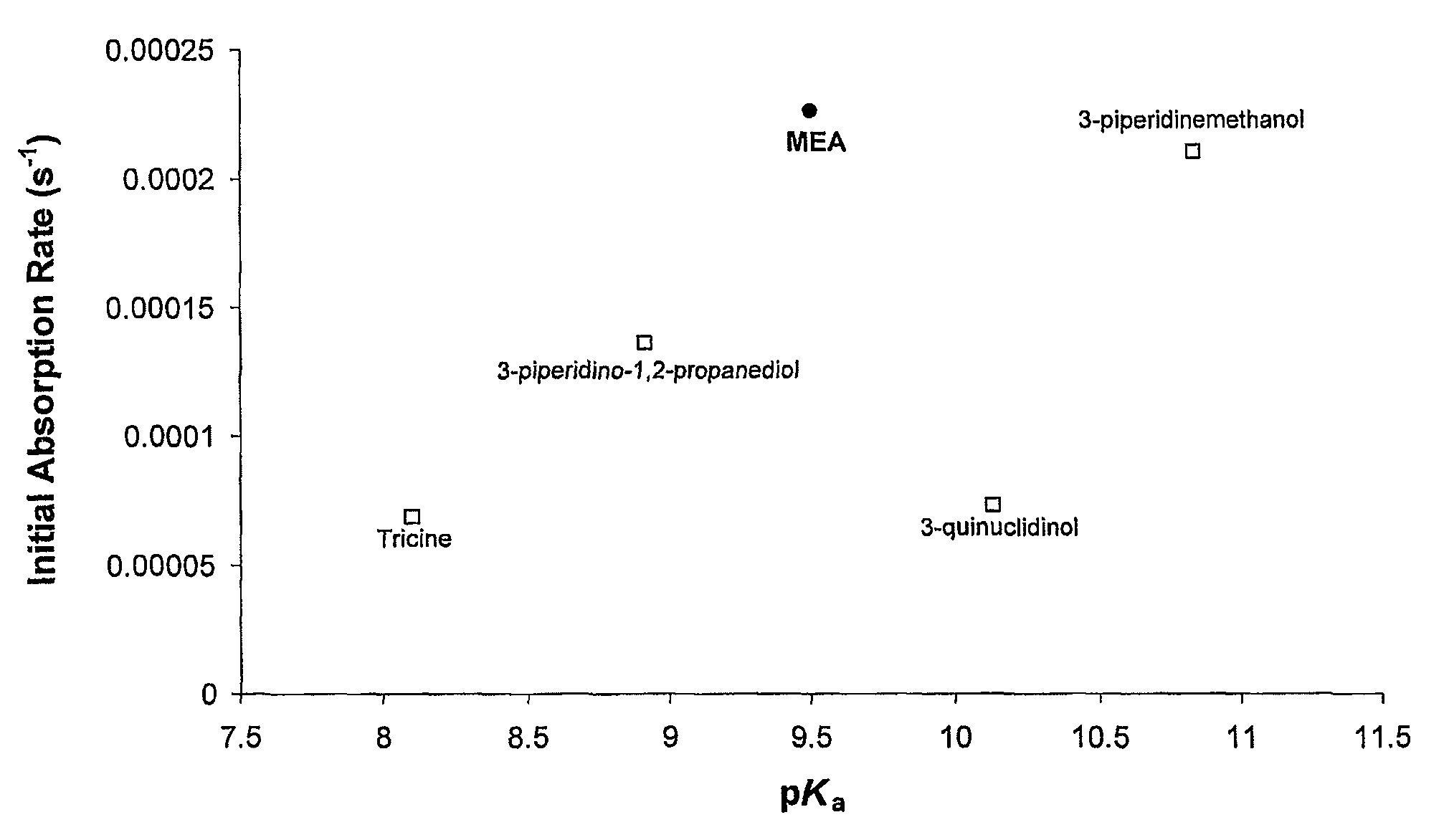
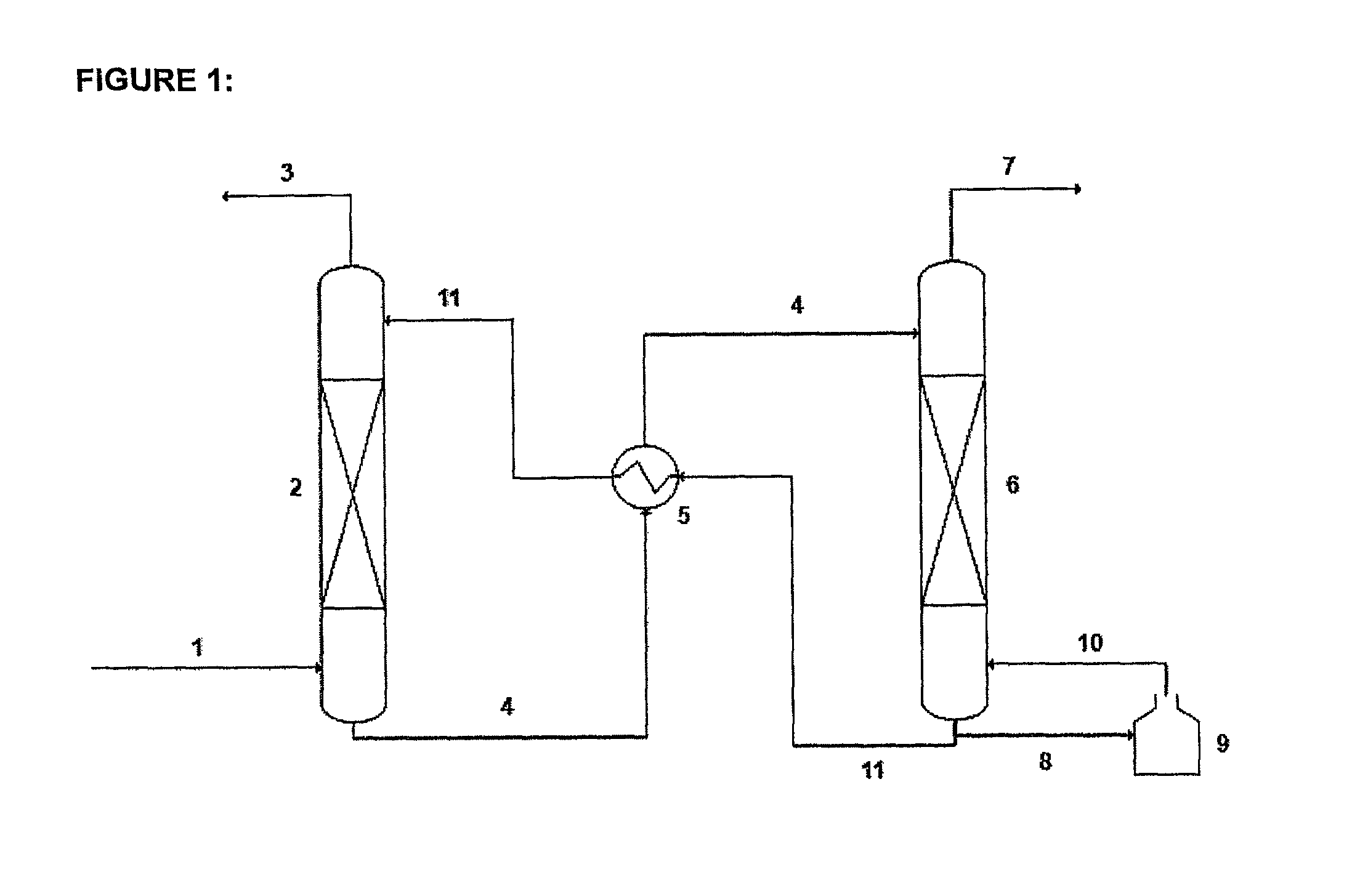
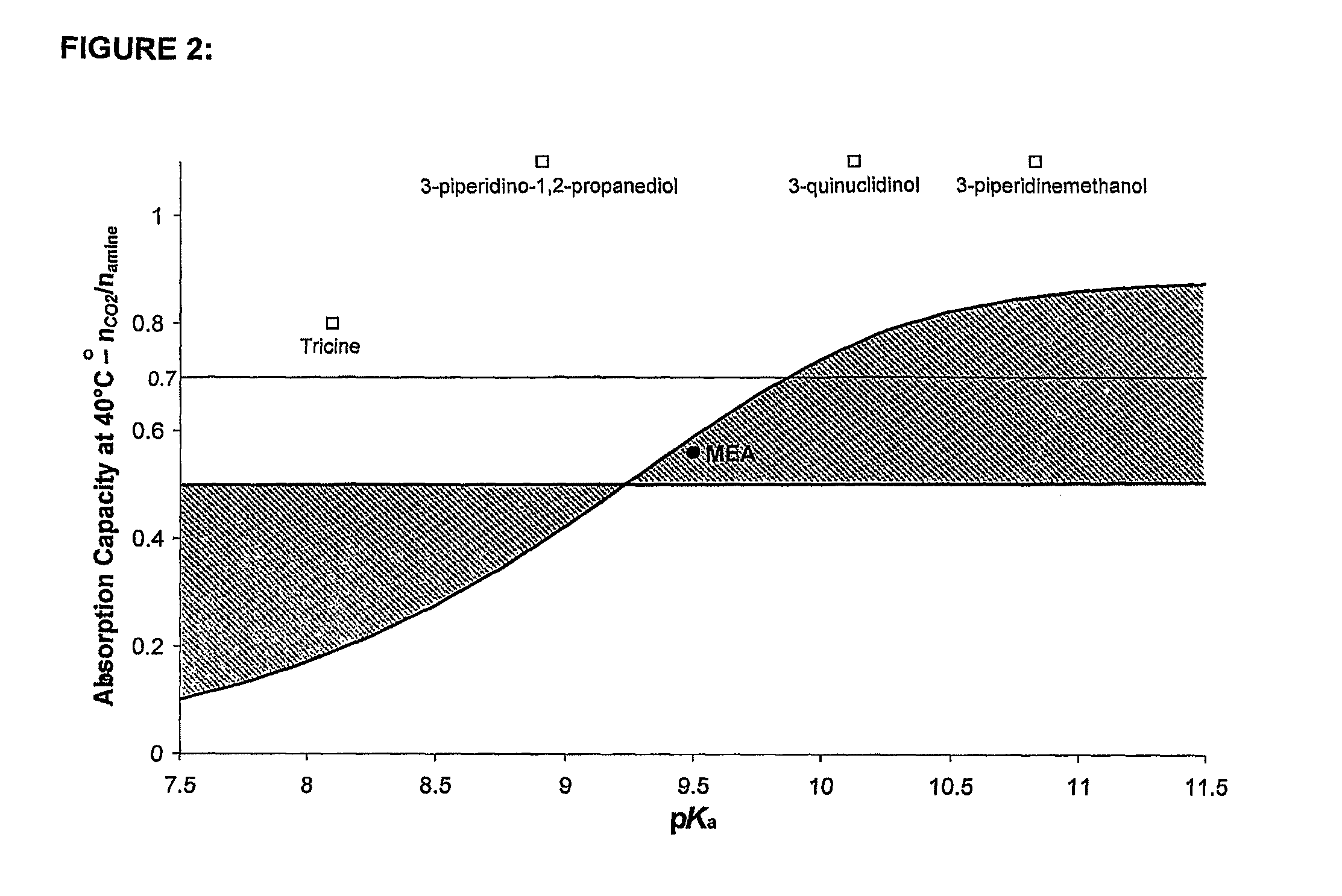
A process for the capture of CO2 from gas streams comprising contacting a CO2 containing gas stream with an aqueous alkanolamine solution, wherein the alkanolamine is selected from the group consisting of: 3-piperidinemethanol, Tricine, 3-quinuclidinol, 3-piperidino-1,2-propanediol and their salts.
Owner:COMMONWEALTH SCI & IND RES ORG
MRI molecular image probe and its preparing method
InactiveCN100998506ASmall molecular weightImprove biological performanceMagnetic property measurementsDiagnostic recording/measuringSuberedamine BBiological body
Owner:PEOPLES HOSPITAL PEKING UNIV
Insoluble carrier particle nephelometric immunoassay reagent
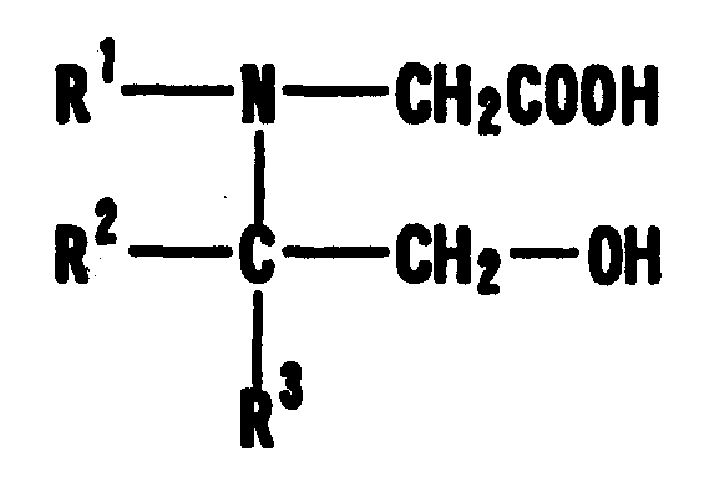
Turbidimetric immunoassay of insoluble carrier particles that can inhibit the agglutination reaction of insoluble carrier particles such as latex by inhibiting the effect of plasma components that affect the measured value, stabilize the agglutination reaction, stabilize the absorbance of the reaction solution, and obtain accurate measurement results A reagent for measurement, a kit for insoluble carrier particle turbidimetric immunoassay, and a method for insoluble carrier particle turbidimetric immunoassay using the reagent or kit. In the presence or absence of a buffer containing a compound containing the following groups in the molecule, such as broad bean pyrimidine glucoside, tricoflavone, etc., the insoluble carrier particles are loaded with antibodies or antigens, and then, in the presence of the above buffer , the insoluble carrier particle suspension sensitive to the antibody or antigen is in contact with the test body, and an immunoagglutination reaction occurs, and the concentration produced is measured through the agglutination reaction of the insoluble carrier particles, and the antigen or antibody in the test body is quantified. (In the formula, R1, R2, and R3 can be the same or different, and represent a hydrogen atom, a hydroxyalkyl group, etc.)
Owner:KYOWA MEDEX CO LTD +1
Pichia pastoris gene engineering bacteria for recombinant expression of human glutamic acid decarboxylase
InactiveCN105400813AAchieve inducible expressionFungiPeptide/protein ingredientsPichia pastorisGlutamate decarboxylase
The invention discloses pichia pastoris gene engineering bacteria for recombinant expression of human glutamic acid decarboxylase and belongs to the technical field of gene engineering. A human glutamic acid decarboxylase gene GAD65 and a vector pPICZalpha are connected to establish an expression vector pPICZalpha-hGAD65 which is placed in pichia pastoris X-33 in an electricity facing mode, and a recombinant strain for excessively expressing human glutamic acid decarboxylase (GAD65) is obtained. Through shake flask fermentation, Tricine-SDS-PAGE and Western Blot identification on the recombinant strain, it is proved that human glutamic acid decarboxylase (GAD65) achieves inducible expression, fusion protein is obtained after ferementaiton supernate is separated and purified, and the concentration of protein hGAD65 reaches 2.36 g / L through measurement on the protein concentration.
Owner:JIANGNAN UNIV
Amino acid sequence and polynucleotide sequence for chicken intestinal canal beta alexin and extraction method thereof
InactiveCN101210243ANo pollution in the processNo side effectsPeptide preparation methodsDepsipeptidesSodium acetateAntibacterial activity
A chicken intestinal tract Beta-defensin cDNA is characterized in that the cDNA has the following sequence: tcagacagcc agctgtgcag gaacaaccat ggccactgcc ggaggctctg cttccacatg gagagctggg ctgggagctg catgaacggc cgcctgcgct gctgcaggtt ctccaccaag cagccctttt ccaaccctaa acattcagtg ctgcacacag cagagcagga cccttcccca agccttggag ggacgtga. The amino acid sequence of the Beta-defensin is Ser-Asp-Ser-Gln-Leu Cys-Arg-Asn-Asn-His Gly-His-Cys-Arg- Arg Leu-Cys-Phe-His-Met Glu- Ser-Trp-Ala-Gly Ser-Cys-Met-Asn-Gly Arg-Leu-Arg-Cys-Cys Arg-Phe-Ser-Thr-Lys-Gln Pro-Phe-Ser-Asn-Pro Lys-His-Ser-Val-Leu His-Thr-Ala-Glu-Gln Asp-Pro-Ser-Pro-Ser Leu-Gly-Gly-Thr. The extraction method comprises the following steps of: (1) collecting broken mucosa cells of chicken intestinal tract; (2) breaking vesicles; (3) leaching with 5% acetic acid under stirring, centrifuging, collecting supernatant, removing sediment, subpackaging the supernatant and freeze-storing to obtain crude chicken intestinal tract Beta-defensin; (4) separating the supernatant with Sephadex G-100 gel column at low temperature, eluting with 0.2mol / L sodium acetate (constant flow pump speed 3*1), detecting with nucleic acid-protein detector, collecting the eluate with an automatic collector (1.5mL each tube), and recording with a recorder (speed 6cm / h, and range 20mV); (5) detecting the antibacterial activity of the liquid in each tube to Pasteurella with agarose diffusion method, collecting the eluate with bacteriostatic activity, and storing under vacuum freeze drying; (6) purifying the the eluate with bacteriostatic activity with Tricine-PAGE, PVDF membrane blotting the protein bands, and performing amino acid sequence analysis with Sanger partial hydrolysis method; and (7) deriving chicken intestinal tract Beta-defensin cDNA with BLAST software.
Owner:HENAN AGRICULTURAL UNIVERSITY
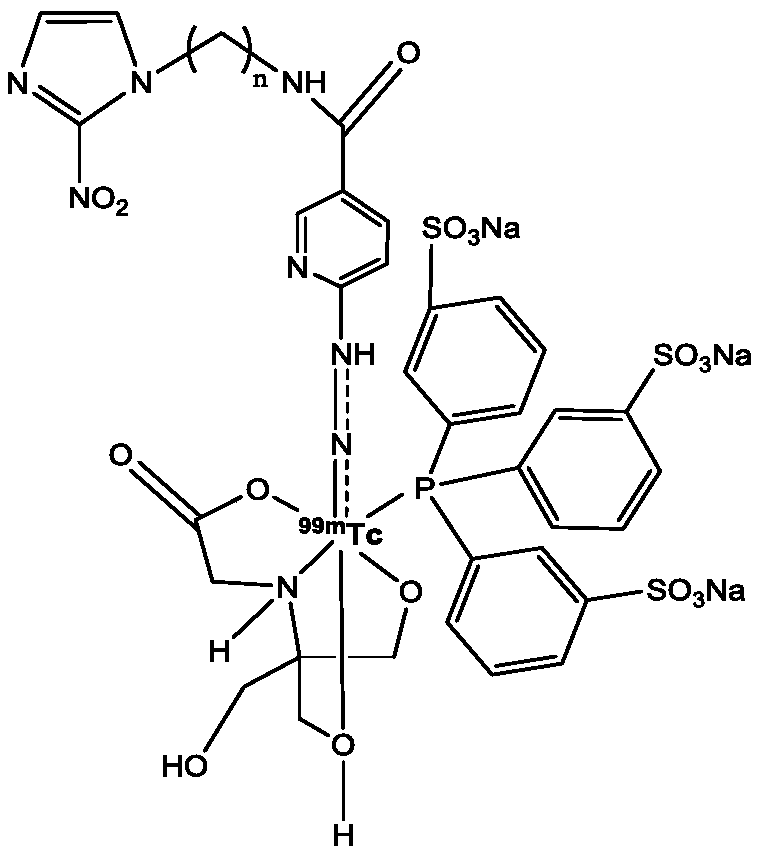
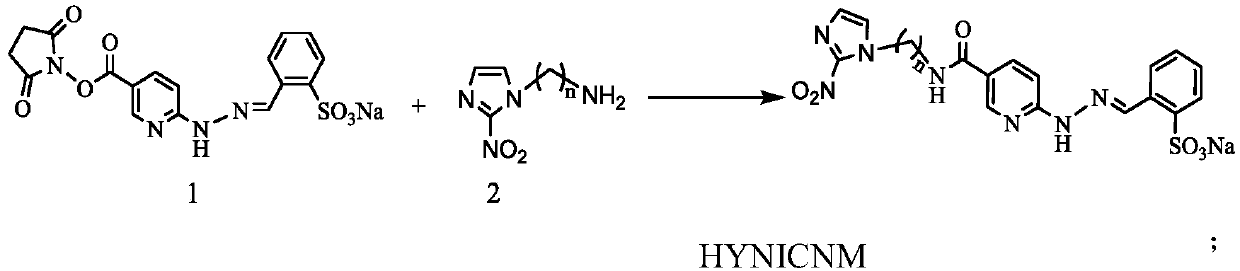
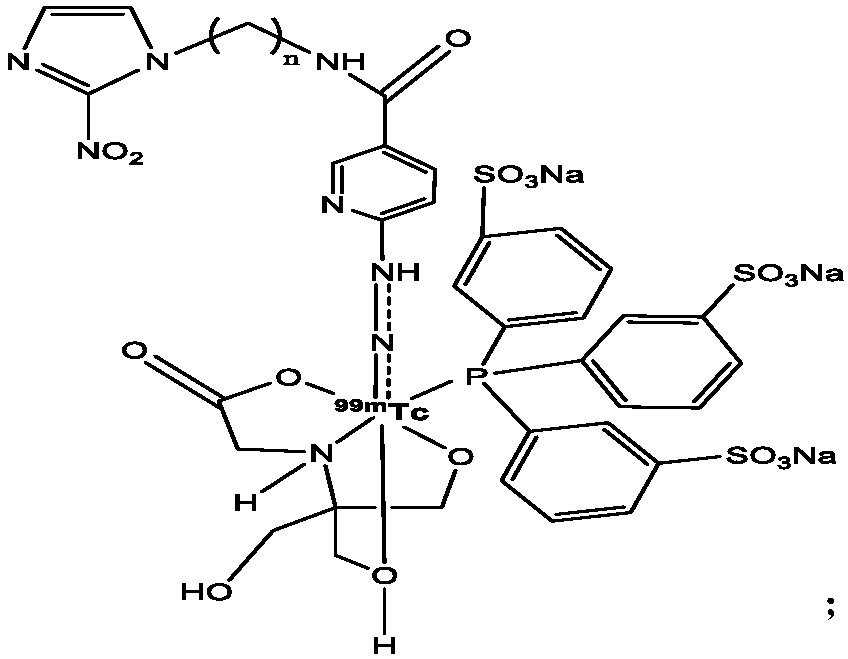
Technetium-99m labeled 2-nitroimidazole complex containing hydrazinyl nicotinamide group and preparation method and application of technetium-99m labeled 2-nitroimidazole complex
ActiveCN110078767AGood stability in vitroImprove performanceRadioactive preparation carriersIsotope introduction to organic compoundsTPPTSNitroimidazole
The invention discloses a 99mTc (HYNICNM) (tricine / TPPTS) complex and a preparation method and application thereof. The 99mTc (HYNICNM) (tricine / TPPTS) complex is obtained through two steps of synthesis of a ligand HYNICNM and preparation of a 99mTc (HYNICNM) (tricine / TPPTS) complex. The complex is simple and convenient to prepare and has oxygen deficiency selectivity. Tumor-bearing mice have highuptake and good retention in tumor parts, the tumor / non-target ratio is high, the uptake of non-target organs such as the liver and intestines is obviously reduced, and the 99mTc (HYNICNM) (tricine / TPPTS) complex is a novel tumor hypoxia imaging agent with excellent performance.
Owner:BEIJING NORMAL UNIVERSITY +1
Polyacrylamide gel for separating small-molecular-weight proteins and preparation method of polyacrylamide gel
ActiveCN110527017AEasy to separateReduce manufacturing costPeptide preparation methodsPolyacrylamideAmmonium sulfate
The invention belongs to the technical field of protein polyacrylamide gel electrophoresis, and especially relates to a polyacrylamide gel for small molecular weight protein separation. The polyacrylamide gel comprises acrylamide, N, N-methylene bisacrylamide, ultrapure water, a gel buffer solution, glycerin, an ammonium persulfate solution and tetramethylethylenediamine; the gel buffer solution comprises boric acid and a Tris-HCl solution, and the pH value of the gel buffer solution is 7.0. The polyacrylamide gel is prepared by taking Tris-boric acid as a buffer system; small molecular proteins can be well separated; protein strips are clear and sharp; the separation effect is equivalent to that of currently common small molecular protein separation gel Tris-Tricine polyacrylamide gel; the separation effect is even better than that of Tris-Tricine; the use cost of reagents is greatly reduced; and a better separation effect can be obtained.
Owner:武汉赛维尔生物科技有限公司
Use of organic buffering agents to enhance the antimicrobial activity of pharmaceutical compositions
InactiveUS20050124702A1Enhanced anti-microbial activityReduce the amount requiredAntibacterial agentsBiocideTricineBuffering agent
The use of organic buffers to enhance the antimicrobial activity of aqueous pharmaceutical compositions is described. The buffers have tri-hydroxy functional groups and terminal acid groups, and are zwitterionic at physiological pH conditions. The most preferred buffer is tricine. The invention is particularly directed to the use of tricine to enhance the antimicrobial activity of ophthalmic compositions, such as solutions for disinfecting contact lenses and artificial tear compositions.
Owner:ALCON INC
Preparation method of anti-aging whey protein peptide
InactiveCN107574214ABest enzymatic hydrolysis processPeptide preparation methodsFermentationDPPHDesorption
The invention discloses a preparation method of anti-aging whey protein peptide, belonging to the technical field of bioactive peptide. The preparation method comprises the following steps: hydrolyzing whey protein concentrate (WPC 80) by adopting alkaline protease, by adopting the degree of hydrolysis and the DPPH free radical scavenging rate as the indexes of evaluation, the hydrolysis and the DPPH free radical scavenging rate as the indexes, adjusting the influences of the following conditions on the hydrolysis effect: the enzyme dosage: 400-10000 U / g, the pH: 8.0, 8.5 and 9.0, the temperatures: 55 DEG C, 60 DEG C and 65 DEG C, the concentration of the substrate: 3-7 %, and the hydrolysis time: 1-6 h, thus the optimal hydrolysis process is obtained, and then enzymolysis is carried out according to the optimal process; hydrolyzing the whey protein concentrate (WPC 80) under the optimal process conditions, desalting the whey protein zymolyte by adopting DA201-C macroporous resin, carrying out dynamic desalting experiment under the following conditions: deionized water and 40-70 % alcohol are adopted as desorption reagents, the sample loading flow rate is 0.5-2.5 mL / min, and the sample loading concentration is 10-50 mg / mL, collecting peptide liquid obtained after desalting, carrying out ultra-filtration separation, and identifying the molecular weight of whey protein peptide byadopting Tricine-SDS-PAGE electrophoresis.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Method for improving content of peanut secretory peptide of peanut hairy roots
PendingCN110408651AEasy to operateImprove conversion efficiencyVector-based foreign material introductionAngiosperms/flowering plantsRhizobium rhizogenesGenetic engineering
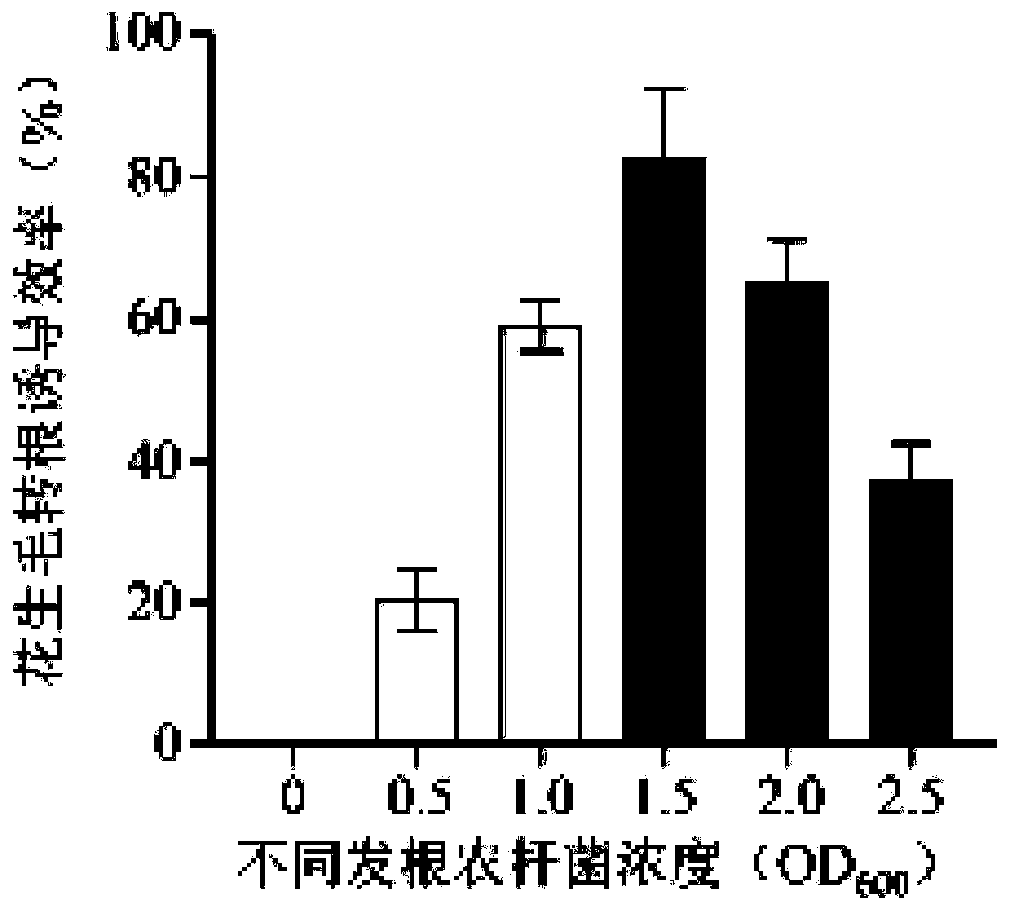
The invention provides a method for improving the content of peanut secretory peptide of peanut hairy roots, and belongs to the field of genetic engineering. A secretory peptide gene is cloned from peanut, a plant gene expression vector is constructed, the peanut hairy roots containing the peanut secretory peptide gene are obtained by an agrobacterium rhizogenes mediated method, the expression ofthe peanut secretory peptide gene in the transgenic peanut hairy roots is identified, the biomass of the peanut hairy roots is determined, the expression level of the peanut secretory peptide is determined by a Tricine SDS-PAGE method, and the content of the peanut secretory peptide is determined by a BCA protein quantitative detection method. The method adopts the peanut hairy root system to produce the peanut secretory peptide, has the advantages of simple operation, short cycle, high output, low cost, biological activity and the like, and is a relatively economic and efficient preparation method of the peanut secretory peptide.
Owner:SHANDONG PEANUT RES INST
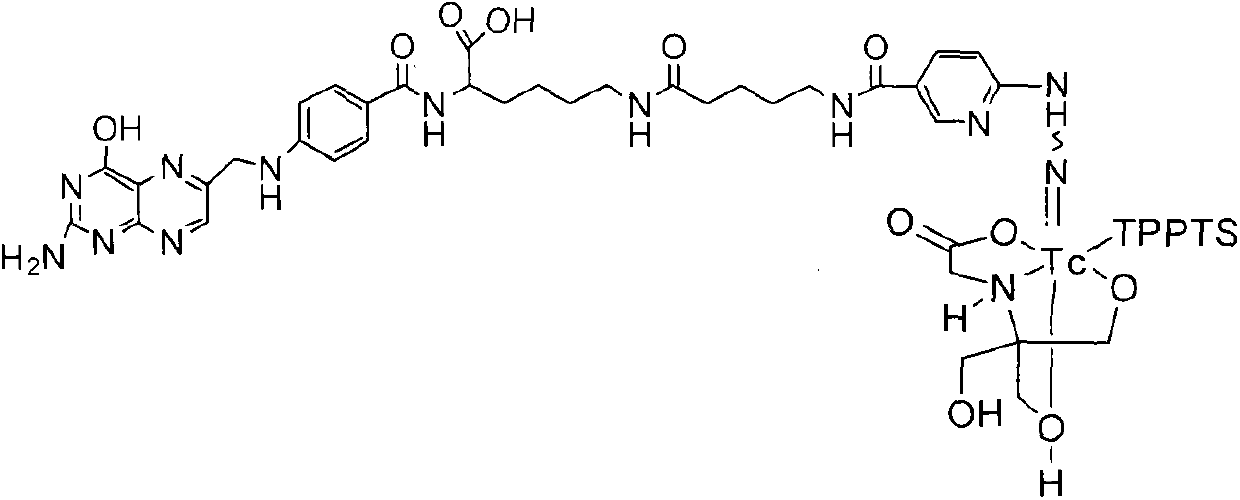
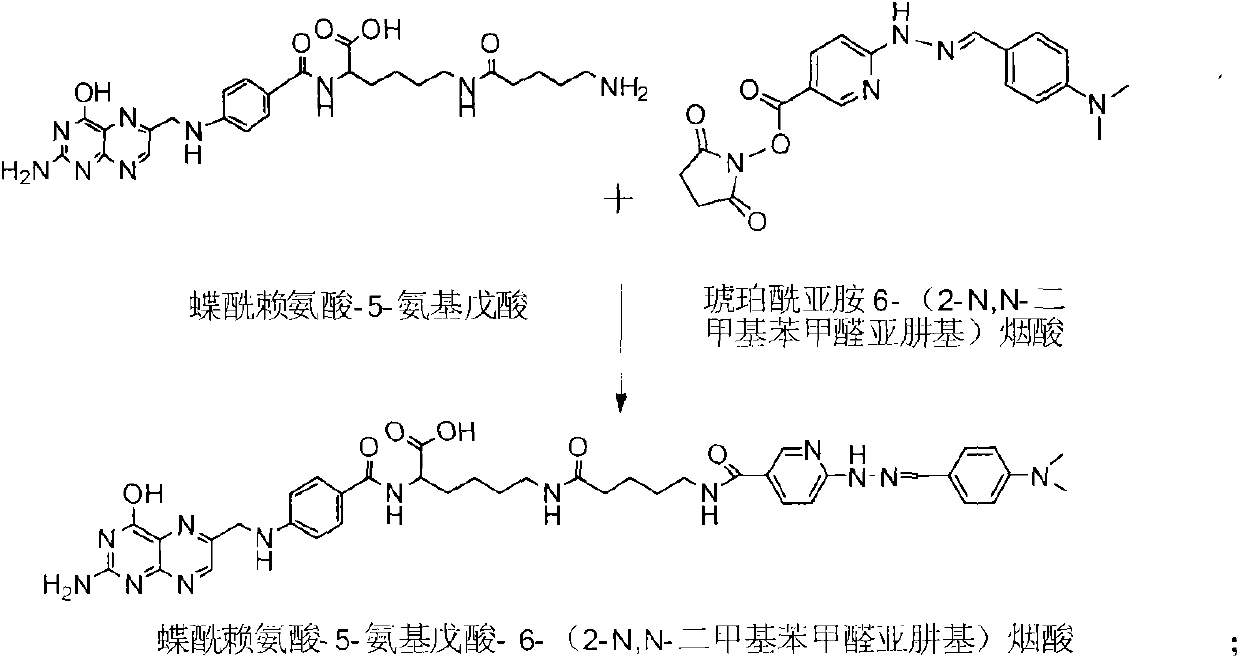
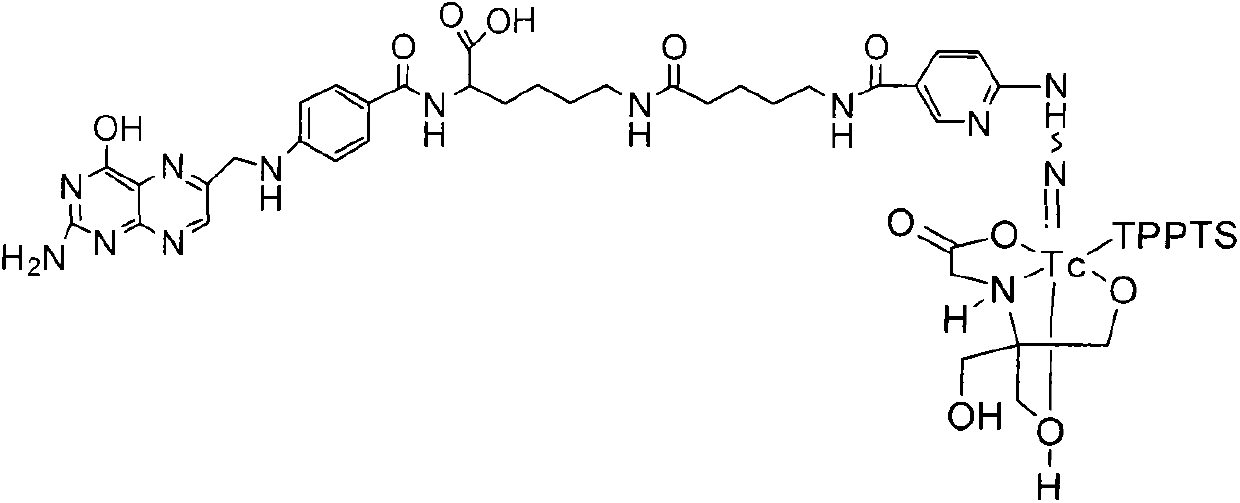
Preparation method and application for 99mTc-labelled hydrazino-nicotinamide-5-aminopentanoic acid-pteroyl lysine complex
ActiveCN103342724AIncrease in absolute uptakeIncreased uptakeOrganic chemistryIn-vivo radioactive preparationsTPPTSHydrazino nicotinamide
The invention discloses a 99mTc-labelled hydrazino-nicotinamide-5-aminopentanoic acid-pteroyl lysine complex having an expression of 99mTc(HYNIC-penta-lys-Pteroyl) (Tricine / TPPTS), as well as a preparation method and an application, wherein the target complex is obtained by preparation comprising the following two steps: step a, synthesising a ligand, namely, pteroyl lysine-5-aminopentanoic acid-6-(2-N,N-dimethyl benzaldehyde hydrazono) nicotinic acid; and step b, preparing the 99mTc-labelled hydrazino-nicotinamide-5-aminopentanoic acid-pteroyl lysine complex. The complex has the advantages of being high in radiochemical purity, good in stability, low in price, high in tumour uptake, good in retention, and high in target / non-target ratios such as tumour / blood ratio and tumour / liver ratio, and can be popularized and applied as a novel 99mTc-labelled folate receptor tumour developer.
Owner:BEIJING NORMAL UNIVERSITY +1
Alkanolamines for CO2 removal from gas streams
InactiveUS8486356B2Desirable performanceIncrease in sizeGas treatmentHydrogen sulfidesCo2 removalTricine
A process for the capture of CO2 from gas streams comprising contacting a CO2 containing gas stream with an aqueous alkanolamine solution, wherein the alkanolamine is selected from the group consisting of: 3-piperidinemethanol, Tricine, 3-quinuclidinol, 3-piperidino-1,2-propanediol and their salts.
Owner:COMMONWEALTH SCI & IND RES ORG
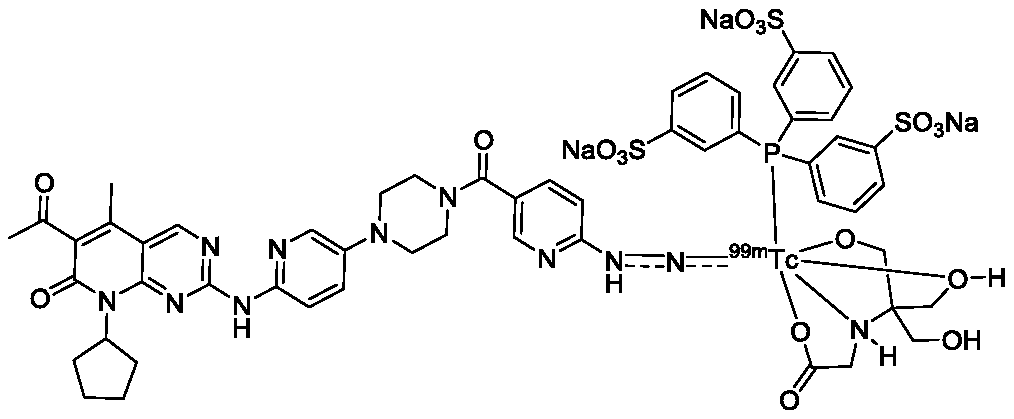
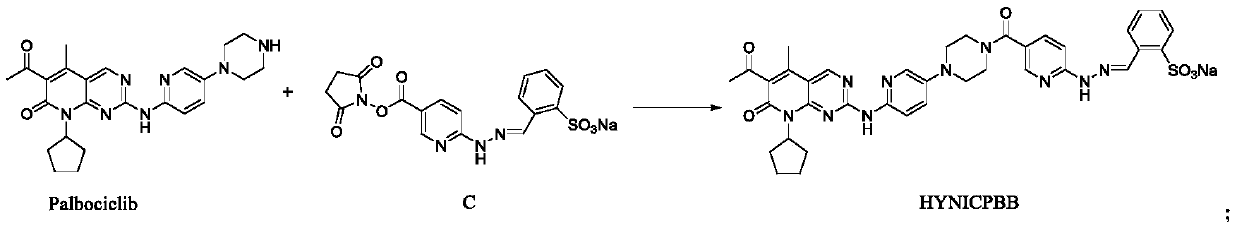
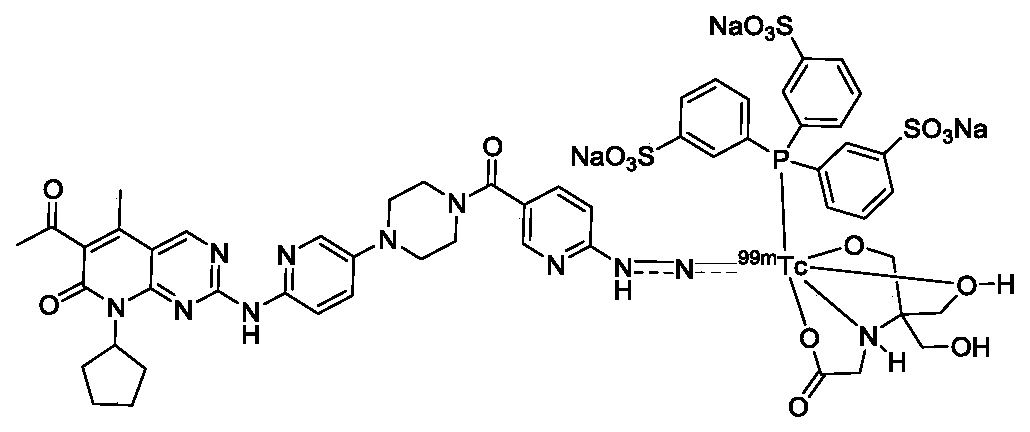
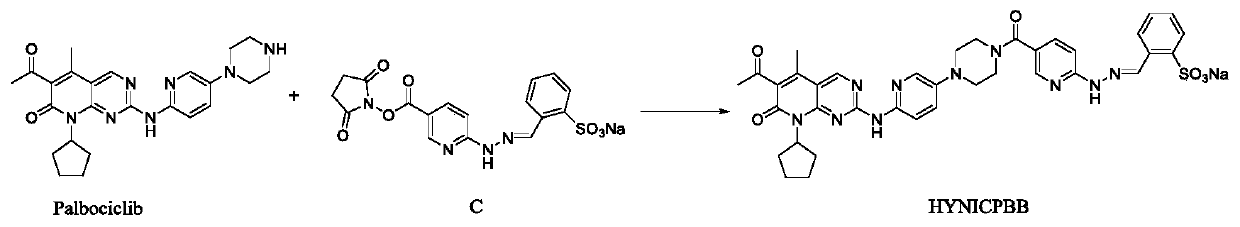
Technetium-99m labeled palbociclib derivative containing HYNIC as well as preparation method and application
The invention discloses a <99m>Tc(HYNICPBB)(tricine / TPPTS) complex as well as a preparation method and an application. The <99m>Tc(HYNICPBB)(tricine / TPPTS) complex is obtained through two steps including synthesis of a ligand HYNICPBB and preparation of <99m>Tc(HYNICPBB)(tricine / TPPTS). The complex is simple to prepare, high in radiochemical purity and good in stability, has better water solubility and has certain uptake in MCF-7 cells, the uptake can be significantly inhibited by palbociclib, which indicates that the complex has a CDK4 / 6 specificity. The complex has higher uptake and retention and better tumor / muscle ratios at tumor parts of female Balb / c nude mice carrying MCF-7 tumor, has obviously reduced uptake of non-target organs such as liver and can be applied to preparation of anovel CDK4 / 6 targeting tumor developer.
Owner:BEIJING NORMAL UNIVERSITY
Evaluation method of specificity removal effect of haze sensitive protein in yellow wine
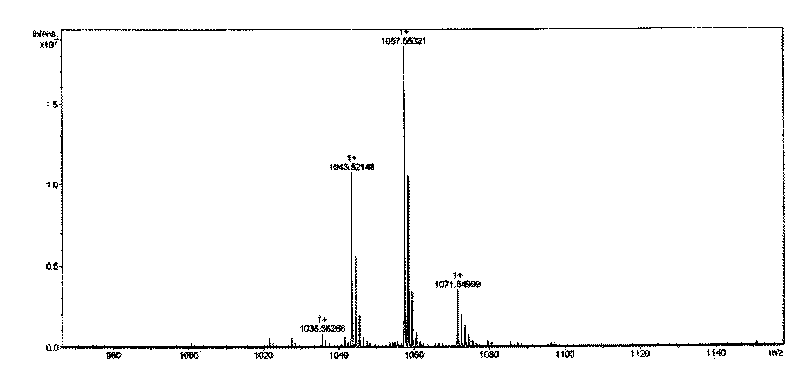
InactiveCN104483451AAccurately evaluate the removal effectMaterial analysisDesorptionDecreased protein S
The invention discloses an evaluation method of specificity removal effect of haze sensitive protein in yellow wine, belonging to the field of yellow wine production process. The method comprises the steps of adding a clarifying agent into the yellow wine, collecting protein adsorbed by the clarifying agent, comparing the distribution of molecular weights of haze sensitive protein and protein adsorbed by the clarifying agent by use of the Tricine-SDS-PAGE (Tricine-sodium dodecyl sulfate-polyacrylamide gelelectrophoresis) technology, identifying the types of the haze sensitive protein and the protein adsorbed by the clarifying agent by the MALDI-TOF / TOF MS (Matrix-Assisted Laser Desorption / Ionization Time of Flight Mass) technology, measuring the total nitrogen content of the yellow wine body before and after the treatment of the clarifying agent by use of a kjeldah method finally, and evaluating that whether the haze sensitive protein is removed and evaluating the removal effect strength according to the identification result that whether the protein adsorbed by the clarifying agent is consistent to the haze sensitive protein by the MALDI-TOF / TOF MS technology and the reduction amount of the protein in the wine body by the kjeldah method. The method can directly and accurately evaluate the removal effect of the clarifying agent on the haze sensitive protein.
Owner:JIANGNAN UNIV
Technetium-99m-labeled hynic-containing palbociclib derivatives, preparation method and application
Owner:BEIJING NORMAL UNIVERSITY
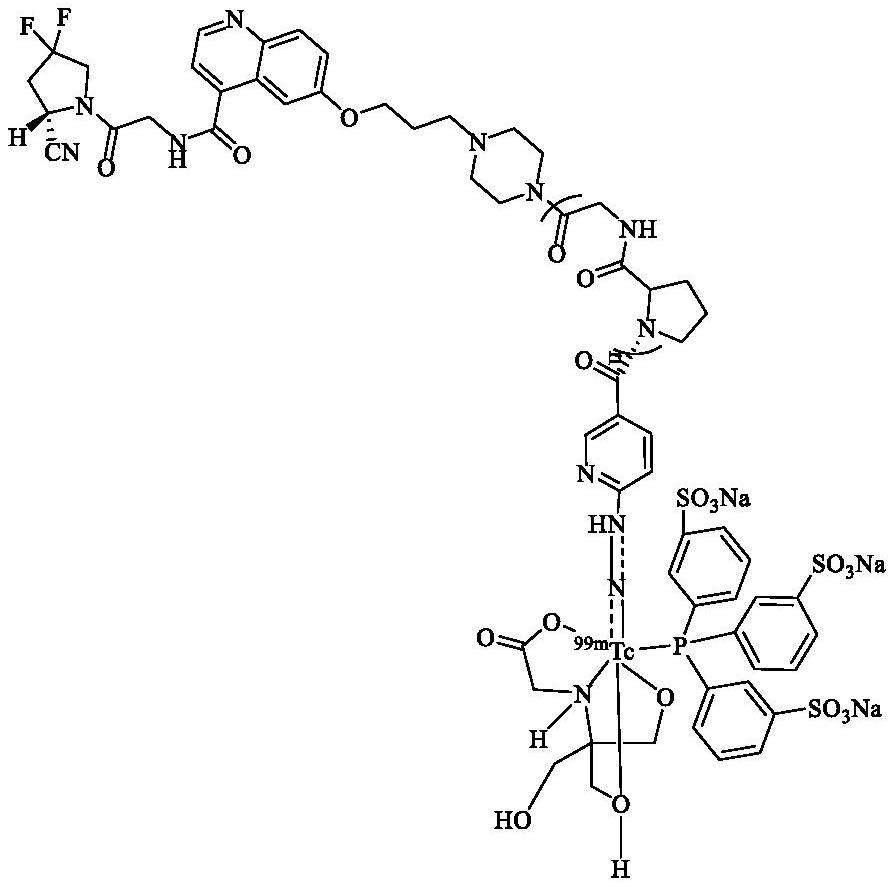
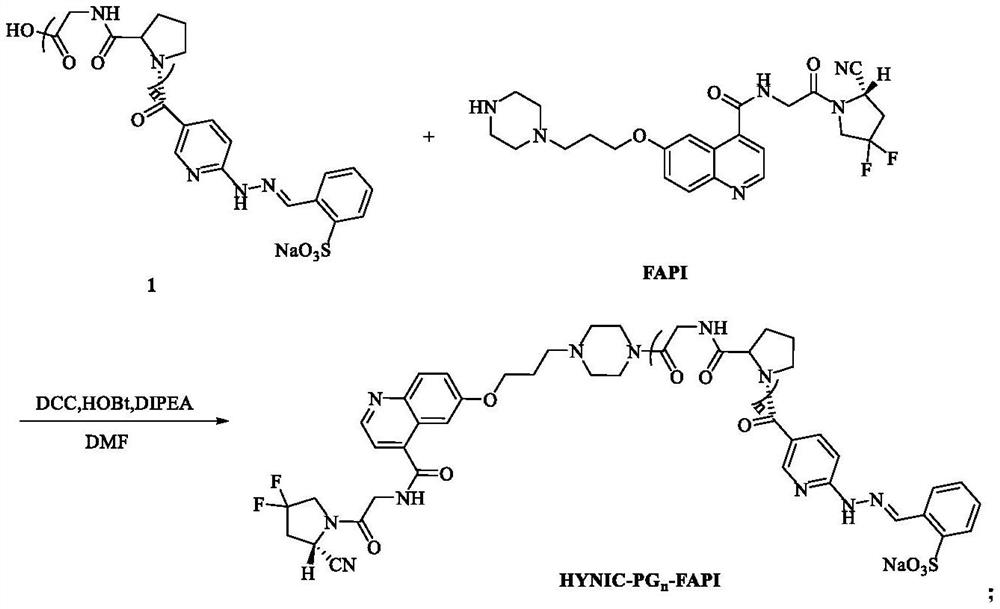
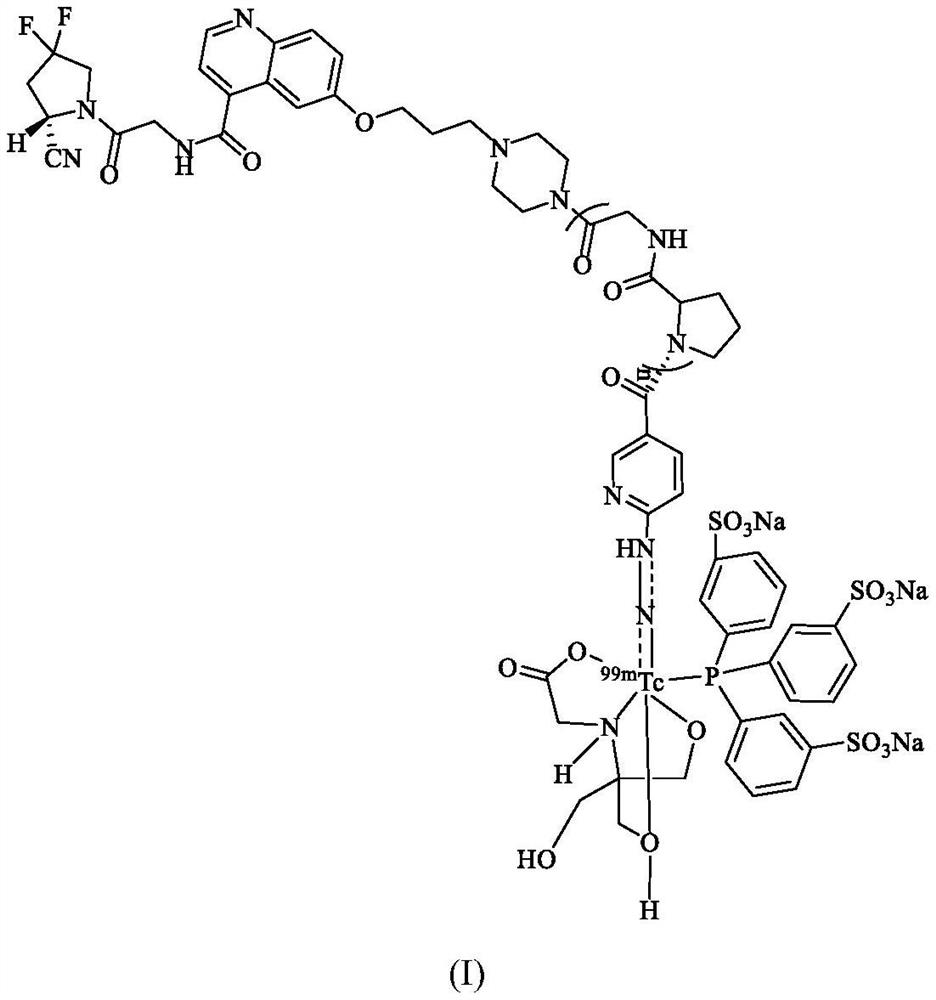
Technetium-99m labeled FAPI derivative modified by D-proline glycine containing polypeptide as well as preparation method and application of technetium-99m labeled FAPI derivative modified by D-proline glycine containing polypeptide
ActiveCN114456227AIncrease intakeReduce the significanceRadioactive preparation carriersPeptide preparation methodsGlycineU87
The invention discloses a technetium-99m labeled FAPI derivative containing D-proline glycine polypeptide modification and a preparation method and application of the technetium-99m labeled FAPI derivative containing D-proline glycine polypeptide modification, wherein the structural general formula of the technetium-99m labeled FAPI derivative is 99mTc (HYNIC-PGn-FAPI) (Tricine / TPPTS). The < 99m > Tc (HYNIC-PGn-FAP) (trickine / TPPTS) complex is prepared by the following two steps: synthesis of a ligand HYNIC-PGn-FAPI and preparation of the < 99m > Tc (HYNIC-PGn-FAP) (trickine / TPPTS), and the < 99m > Tc (HYNIC-PGn-FAP) (trickine / TPPTS) complex is obtained. The complex is simple and convenient to prepare, high in radiochemical purity and good in stability, has very high uptake and target-to-non-target ratio at the tumor site of a U87MG tumor-bearing mouse, has specific binding with FAP in tumors, and is a novel tumor radiopharmaceutical with clinical application value.
Owner:BEIJING NORMAL UNIVERSITY
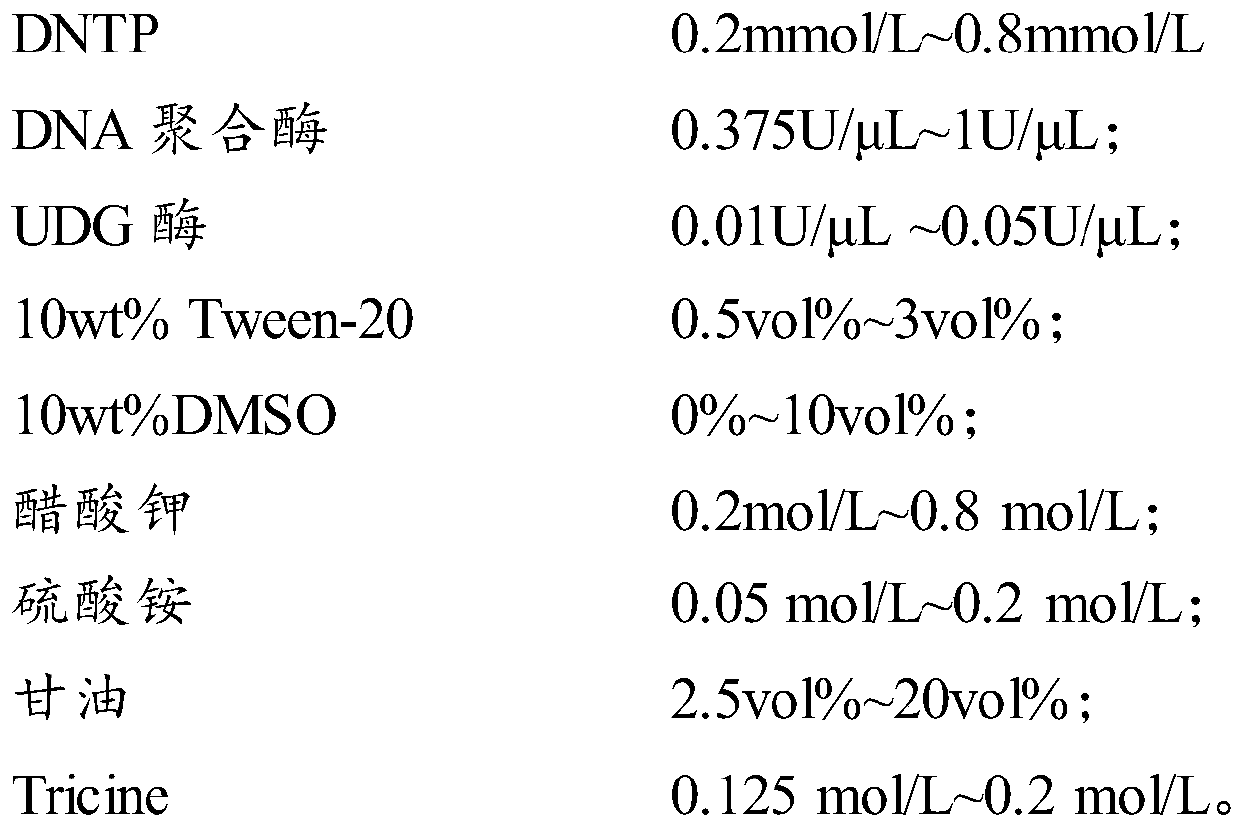
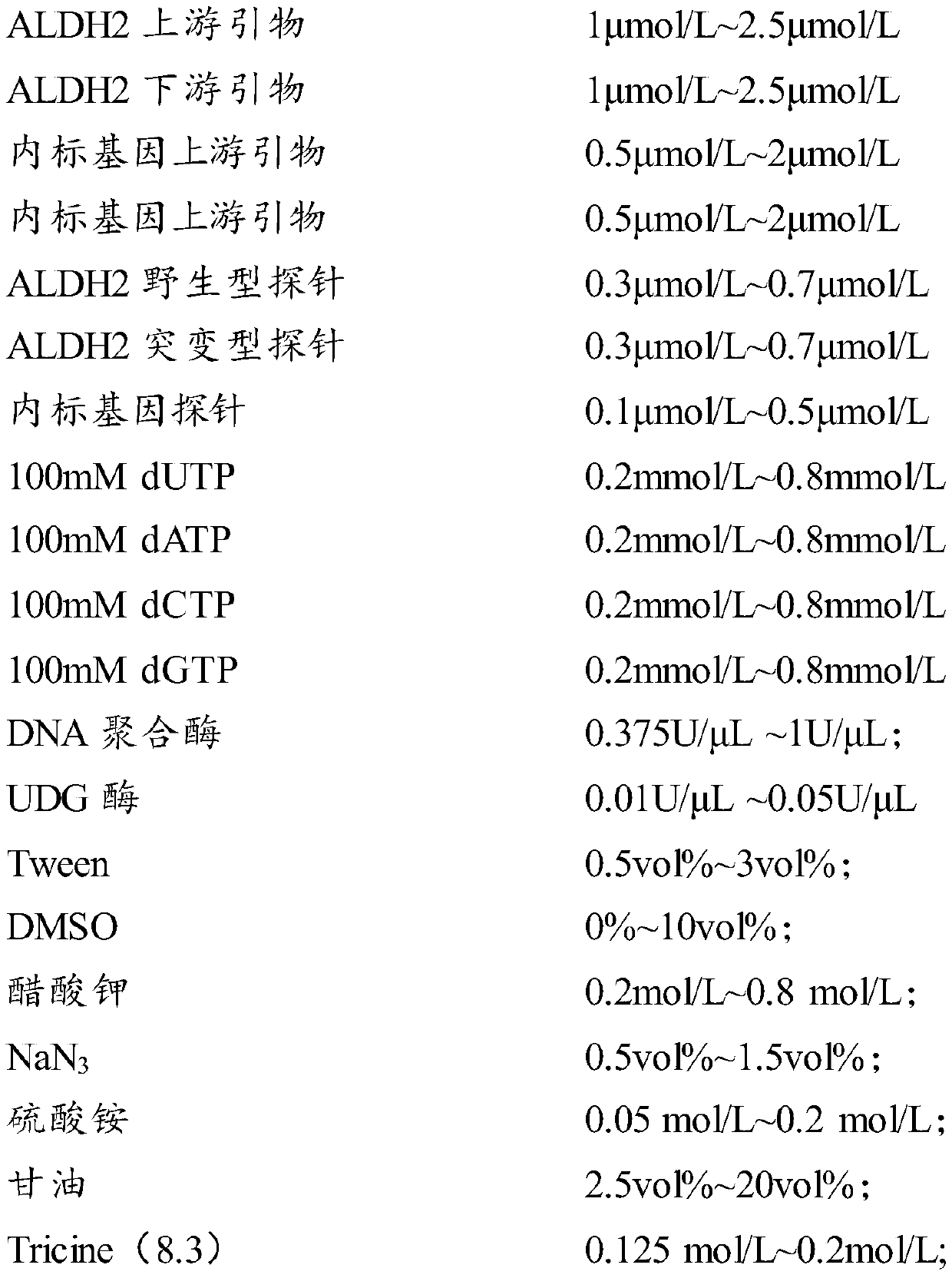
Gene polymorphism detection primer, probe and kit
PendingCN110982885AStable storageGuaranteed preservationMicrobiological testing/measurementDNA/RNA fragmentationMANGANESE ACETATEGlycerol
The invention relates to the technical field of in-vitro diagnosis, in particular to a gene polymorphism detection primer, probe and kit. A gene polymorphism detection reagent provided by the invention comprises a reaction solution 1 and a reaction solution 2. The PCR reaction solution 1 comprises water, DNTP, DNA polymerase, UDG enzyme, Tween, DMSO, potassium acetate, NaN3, ammonium sulfate, glycerinum and Tricine; and the PCR reaction solution 2 comprises water, manganese acetate and NaN3. The reaction solution 1 can further comprise the primer and the probe. The components are reasonable incomposition, so that the reaction solutions provided by the invention can be stably stored at 2-8 DEG C, a sample can still be well detected after the reaction solutions are placed for 6 months, andunfreezing is not needed before reaction.
Owner:AUTOBIO DIAGNOSTICS CO LTD
Western blot kit for detection of vaccinated poultry
InactiveUS20130017536A1Microbiological testing/measurementChemiluminescene/bioluminescenceSmall peptideTricine
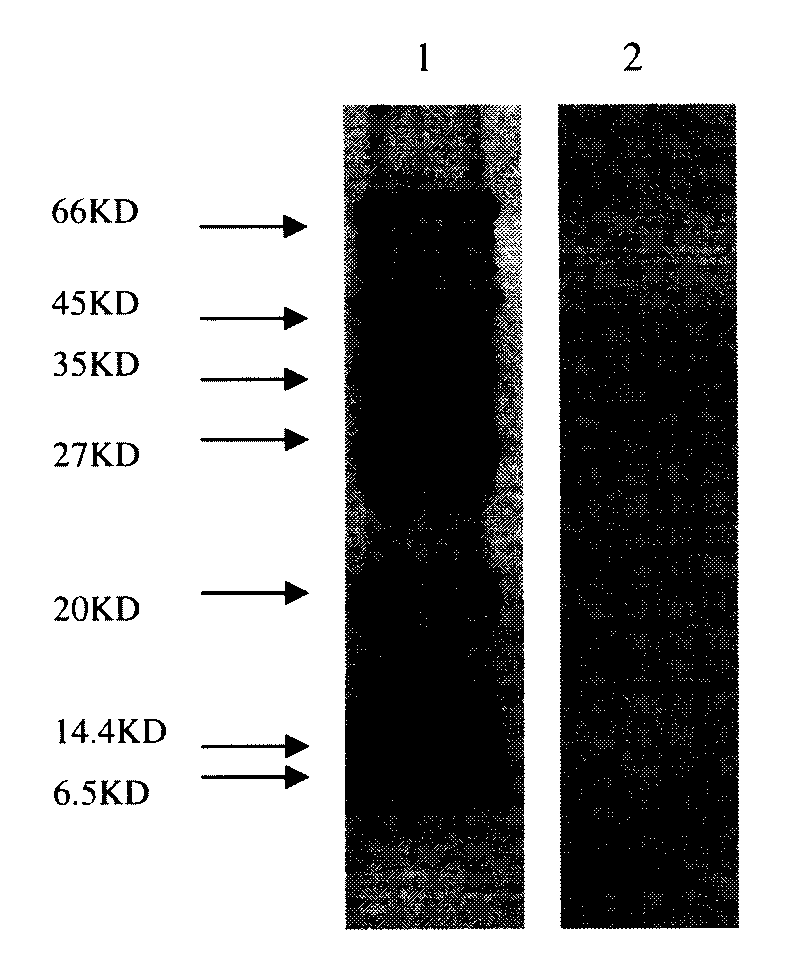
Modified western blot membranes with silver nanoparticle allow the small peptides of the NS1 protein of the poultry influenza virus to be kept in the membrane and not to diffuse during transferring from the Tricine SDS PAGE. These peptides may differentiate infected from vaccinated poultry.
Owner:NANO JAV DARU
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com