Pichia pastoris gene engineering bacteria for recombinant expression of human glutamic acid decarboxylase
A technology of glutamic acid decarboxylase and genetically engineered bacteria, applied in the field of genetic engineering, can solve the problems of formation of inclusion bodies, unstable expression of plasmids, and low enzyme activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
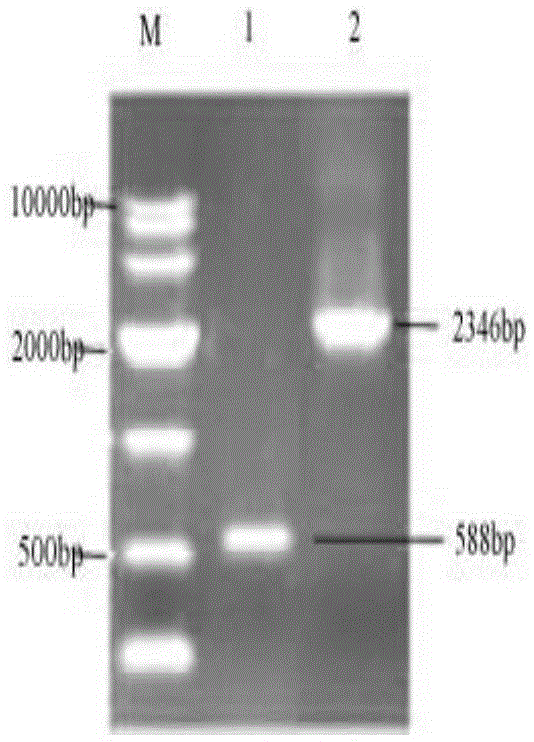
[0026] Embodiment 1: Construction of recombinant bacteria
[0027] (1) Recombinant plasmid pMD19-T-GAD 65 build
[0028] The GAD enzyme gene with nucleotides such as SEQIDNO:1 is connected to the pMD19-T plasmid, and transformed into Escherichia coli, and the correct transformant is verified to obtain the recombinant plasmid pMD19-T-GAD 65 . Extract pMD19-T-GAD 65 and pPICZα plasmids, and then double-enzyme pMD19-T-GAD with XhoI and NotI respectively 65 (Synthetic GAD 65 The gene was cloned into pMD19-T vector) and pPICZα plasmid for 4h, and analyzed by 0.7% agarose gel electrophoresis. Use the rubber tapping recovery kit to recover the target fragment, connect at 16°C for 4 hours, use the chemical transformation method to transform Escherichia coli DH5α, spread the transformed bacterial solution on a low-salt LB plate with a final concentration of 25 μg / mL zeocin, and store at 37°C After culturing for 24 hours, select the colonies grown on several plates, inoculate them...
Embodiment 2
[0045] Embodiment 2: Recombinant bacterial fermentation produces glutamic acid decarboxylase GAD 65
[0046] fusion protein hGAD 65 Induced expression and product identification
[0047] (1) Induced expression
[0048] Extraction and sequencing of the correct recombinant plasmid pPICZα-hGAD 65 Transform into Pichia pastoris X-33 to obtain gene engineering expression bacteria.
[0049] (a) Inoculate a single colony of the selected recombinant Pichia pastoris in a 50mL growth medium BMGY / 500mL Erlenmeyer flask, and culture at 30°C and 200r / min for 20h;
[0050] (b) Leave the bacterial solution in the above-mentioned BMGY growth medium at room temperature for 1 h, discard the supernatant, resuspend the bacterial cells with 30 mL of BMMY medium, add 100% methanol to a final concentration of 1% (v / v), Cultivate at 30°C, 200r / min, add 100% methanol every 24h to a final concentration of 1% (v / v) for induction;
[0051] (c) After methanol induction for 48h, centrifuge at 12000r / ...
Embodiment 3
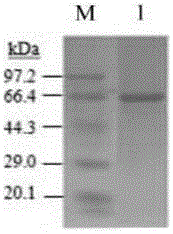
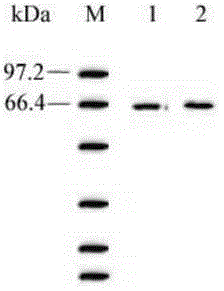
[0087] Embodiment 3: the glutamic acid decarboxylase GAD of recombinant bacterial fermentation 65 activity detection
[0088] Identification of fusion protein hGAD by WesternBlot 65 .
[0089] (1) Use the serum of diabetic patients positive for GAD antibody as the primary antibody:
[0090]①Tricine-SDS-PAGE: Take 200 μl sink sample of shake flask horizontal protein sample for Tricine-SDS-PAGE;
[0091] ②Transfer: Take a nitrocellulose filter membrane (NC) with the same size as the gel and soak six pieces of 3mm filter paper in the electrotransfer buffer for 10-15min. After electrophoresis, remove the gel, cut off the stacking gel for marking, rinse with electrotransfer buffer and equilibrate for 20 minutes. Install in the following order: anode - sponge - 3 pieces of filter paper - NC membrane - gel - 3 pieces of filter paper - sponge - cathode. Every time a layer is placed, the air bubbles between the layers should be removed, so as not to affect the transfer effect. Cl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com