In vitro culture method for inducing human umbilical cord blood cells CD34+ to differentiate into Langerhans cell
A technology of cell differentiation and in vitro culture, applied in artificial cell constructs, blood/immune system cells, animal cells, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1 Isolation of CD34+ cells in umbilical cord blood
[0023] 1. Isolation of human cord blood mononuclear cells:
[0024] ①In the dark, add 10mL of human lymphocyte separation medium (density 1.077) into a 15mL centrifuge tube, carefully add 5mL of cord blood to the lymphocyte separation medium, and form a clear interface.
[0025] ②At room temperature, centrifuge horizontally at 400g (2500rmp, r=14.5 cm) for 25min.
[0026] ③ At this time, five layers can be seen in the centrifuge tube: the uppermost layer is plasma, the white misty layer between the plasma layer and the lymphocyte separation liquid is the mononuclear cell layer, and the white film between the lymphocyte separation liquid layer and the lowermost red blood cells The layer is the granulocyte layer.
[0027] ④ Suck off the uppermost layer of plasma, collect the mononuclear cells at the interface between the plasma layer and the lymphocyte separation medium, and try to suck out all the mononuclea...
Embodiment 2
[0042] Example 2 Induction and production of Langerhans cells in vitro
[0043] steps:
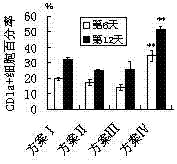
[0044] 1. Count the CD34+ cells obtained by magnetic bead sorting, and count them according to 1×10 4 cells / cm 2 Inoculated in 24-well plates, cultured with RPMI1640 complete medium plus the cytokines of the following four schemes, see Table 1. The concentration of each factor in the table is the final concentration of each factor in each mL of RPMI1640 medium.
[0045] Table 1 The scheme of inducing Langerhans cells in vitro with cytokine combination
[0046]
[0047] 2. On the first day after the separation of CD34+ cells, add RPMI-1640 culture medium containing 10% fetal bovine serum containing the cytokines in the table according to the scheme in Table 1, at 37°C, 5% CO 2 Culture; change the culture medium every 3 days. Two wells of 24-well plates were cultured in each protocol.
[0048] 3. The phenotype of Langerhans cells (CD1a) was detected by flow cytometry on the 6th day...
Embodiment 3
[0052] Example 3 Detecting the phenotypes of antigen-presenting molecules (HLA-DR) and co-stimulatory molecules (CD80, CD40) of Langerhans cells by flow cytometry
[0053] step:
[0054] 1. Digest the Langerhans cells obtained in Example 2 with trypsin, centrifuge at 200 g for 10 min (1000 rpm, r=14.5 cm), discard the supernatant, and obtain the Langerhans cell pellet.
[0055] 2. Add 1mL ice-cold PBS buffer to resuspend the cell pellet, and count the cells.
[0056] 3. According to 10 5 Cells / 100 μL concentration was adjusted for cell concentration, and 100 μL of Langerhans cell suspension was taken from each tube.
[0057] 4. Label PE / CY5-HLA-DR, FITC-CD80, FITC-CD40 monoclonal antibodies and corresponding isotype controls respectively, mix well, and incubate at 4°C for 40 minutes in the dark.
[0058] 5. Add 100 μL PBS to each tube, and use flow cytometer for fluorescence detection on the same day. Or fix the cells and perform flow cytometry within 5 days: add 500 μL of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com