Patents
Literature
38 results about "Particulate antigen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An antigen is anything that sets off an immune response. A particulate is something super super small. So a particulate antigen is a super super small particle that sets off an immune response.
Methods and compositions for increased priming of t-cells through cross-presentation of exogenous antigens
InactiveUS20080171059A1Easy to demonstrateEffective vaccineTissue cultureCancer antigen ingredientsDiseaseVaccination
Methods for eliciting in an animal in need thereof a cell-mediated immune response specific to an antigen, the method comprising providing an antigen preparation comprising particles on the surface of which the antigen is attached, and administering the antigen preparation to the animal, wherein the particles are taken up by antigen presenting cells (APC) of the animal via phagocytosis, forming a phagosome inside the APC, wherein the antigen is attached to the surface of the particle in such a way that the antigen is released in the phagosome before the phagosome fuses with a late endosome or a lysosome, and wherein the antigen is cross-presented on a Class I MHC molecule. Also provided are particulate antigen preparations or particulate vaccines that can be delivered to an animal in need thereof for vaccination against, for preventing or treating, a disease related to the antigen, such as cancer and a viral infection.
Owner:LUDWIG INST FOR CANCER RES +1
Particle-bound human immunodeficiency virus envelope glycoproteins and related compositions and methods
InactiveUS20060051373A1Inhibition of growth rateSlow growth rateBiocideOrganic active ingredientsParticulate antigenPharmaceutical medicine
This invention provides a first composition comprising a pharmaceutically acceptable particle and a stable HIV-1 pre-fusion envelope glycoprotein trimeric complex operably affixed thereto. This invention further provides a second composition comprising (a) a pharmaceutically acceptable particle, (b) an antigen, and (c) an agent which is operably affixed to the particle and is specifically bound to the antigen, whereby the antigen is operably bound to the particle. Finally, this invention provides related nucleic acids, vectors, cells, compositions, production methods, and prophylactic and therapeutic methods.
Owner:PROGENICS PHARMA INC
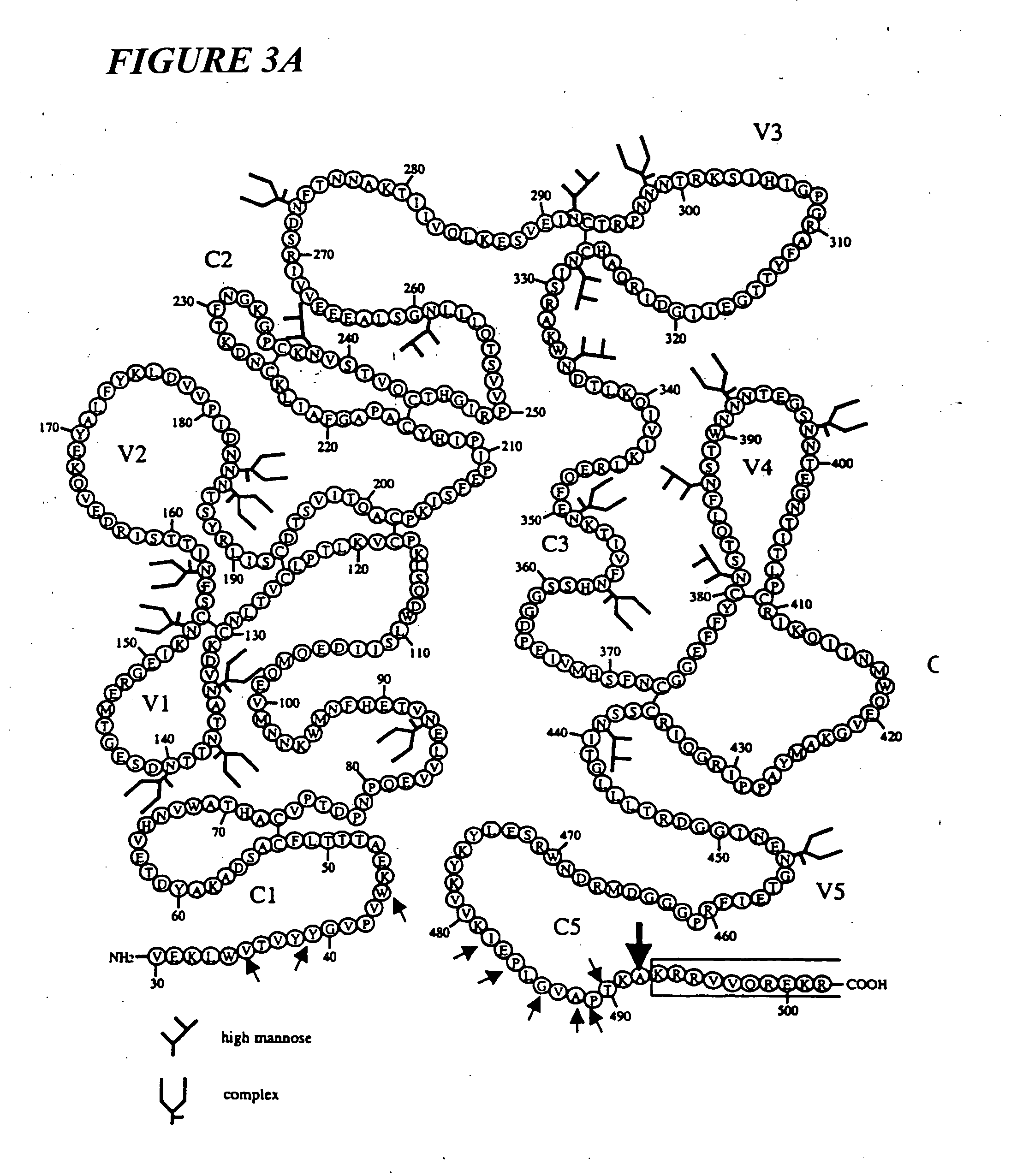
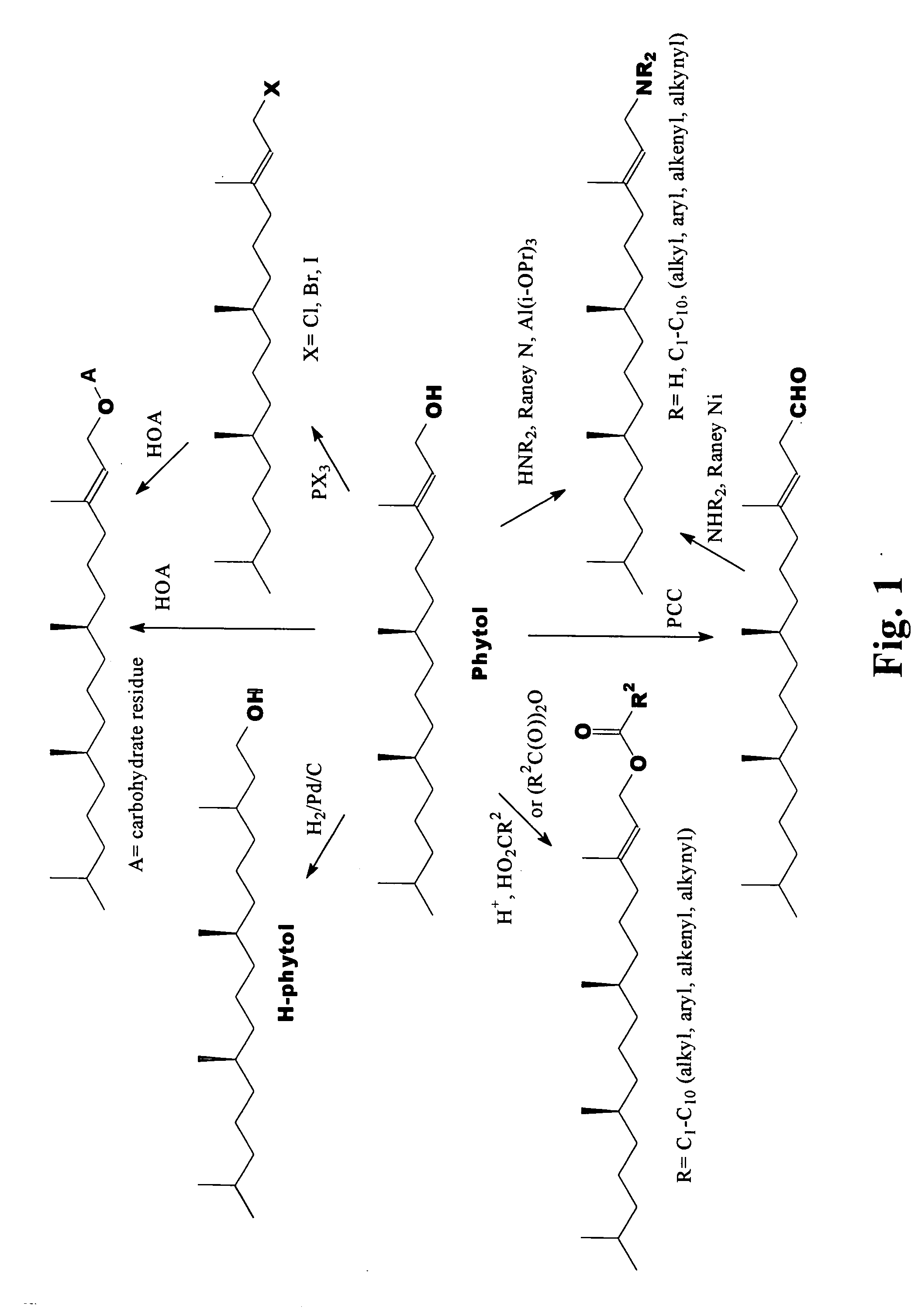
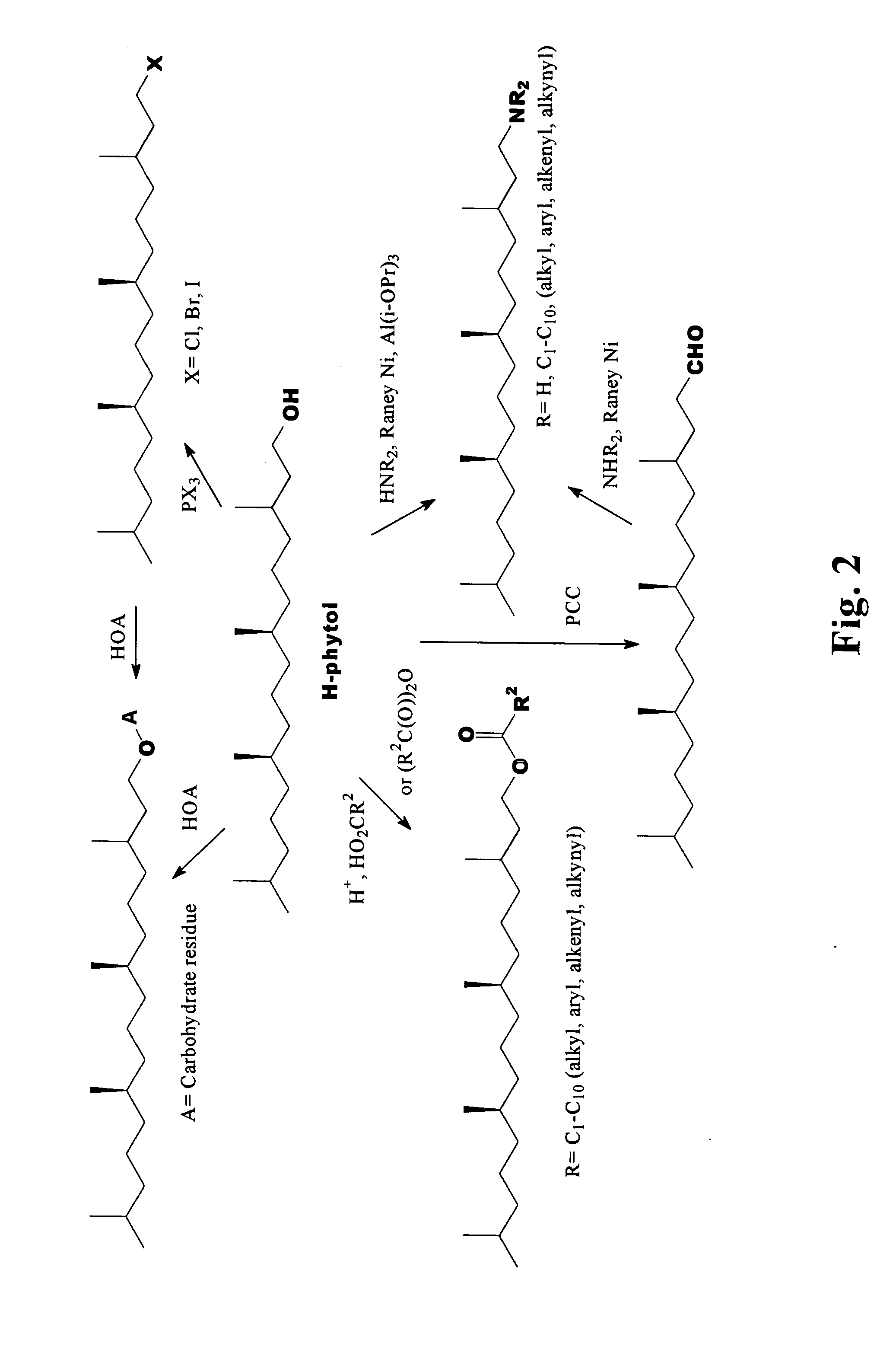
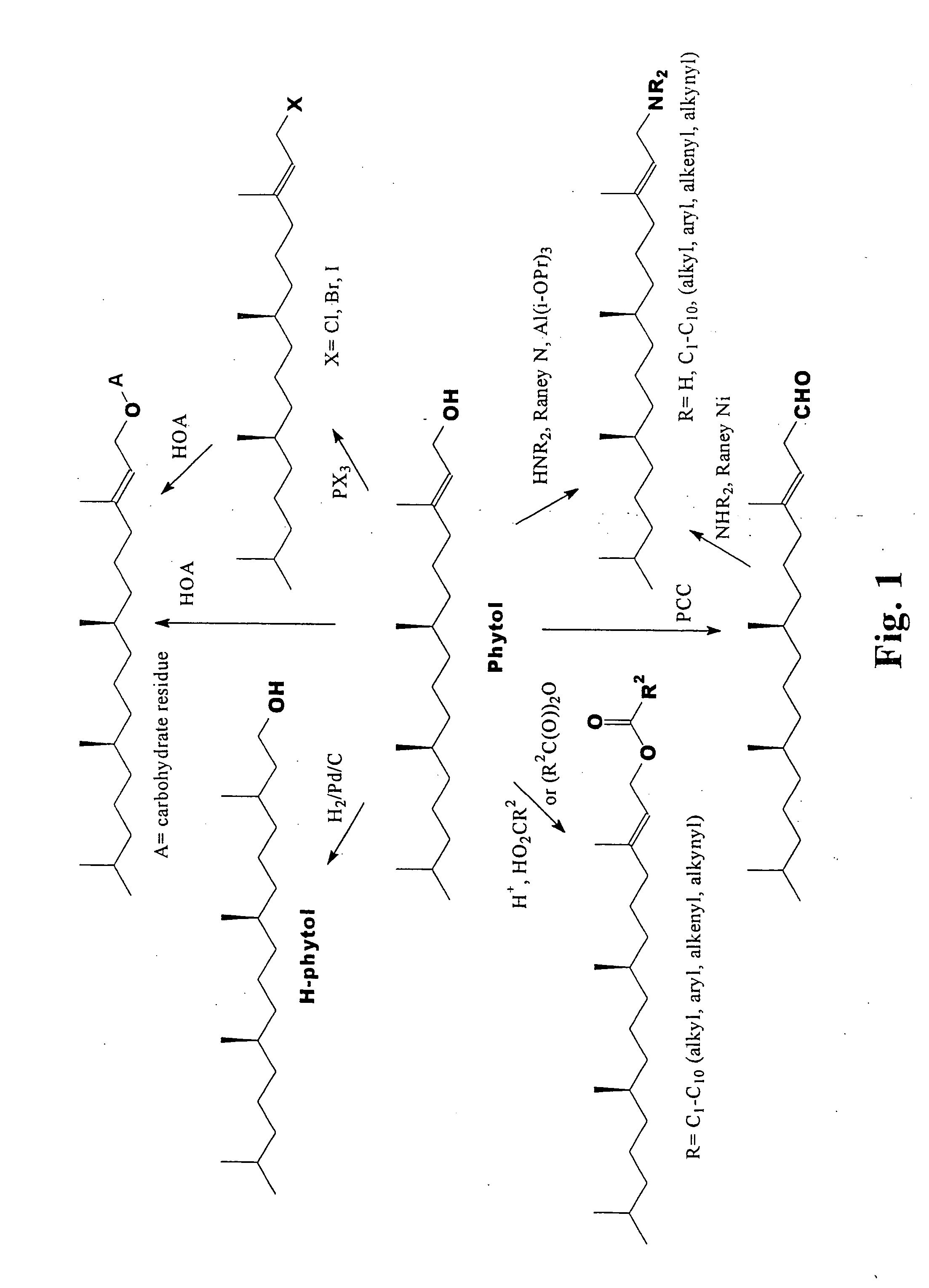
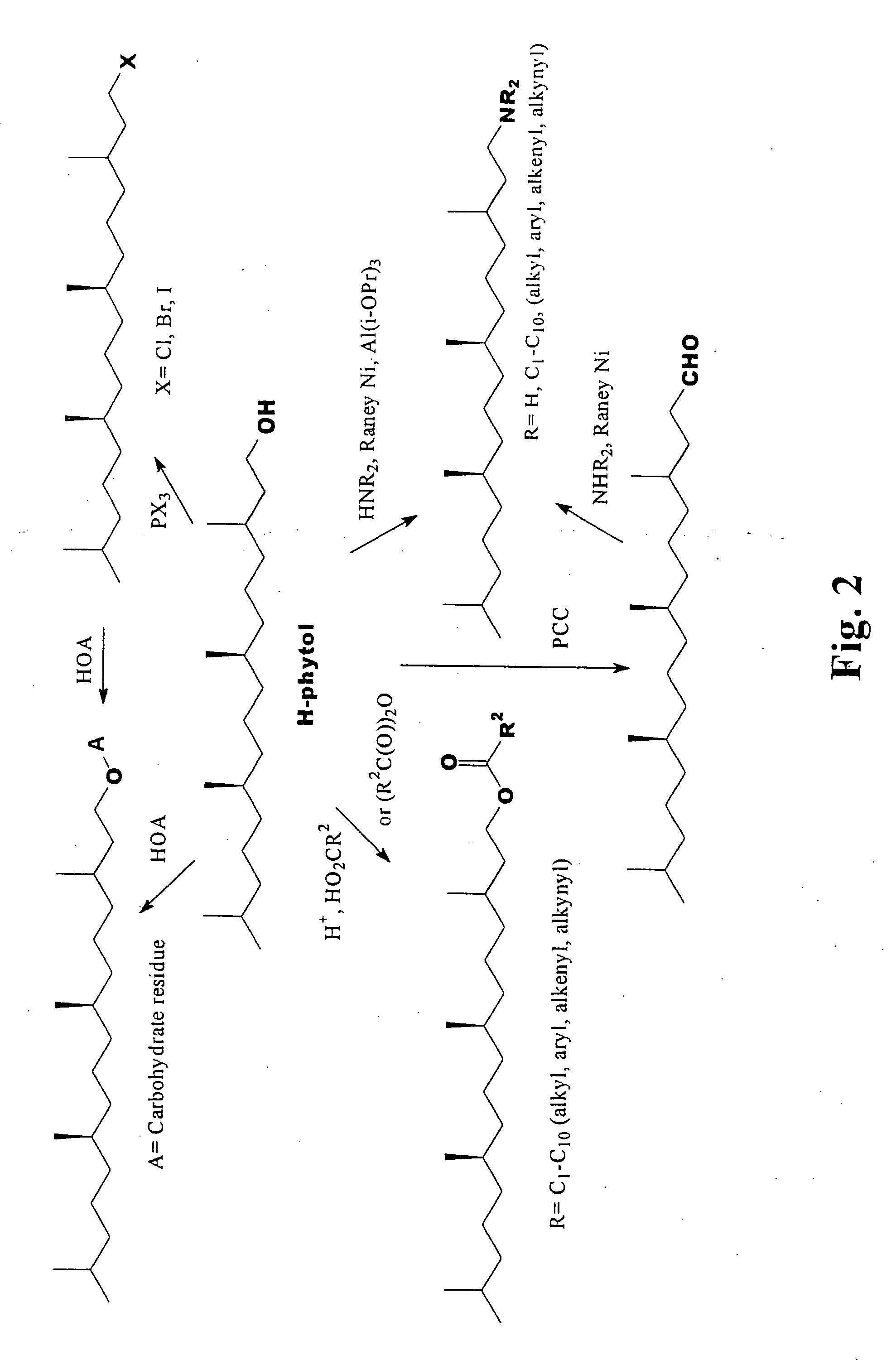
Novel phytol derived immunoadjuvants and their use in vaccine formulations
InactiveUS20050158329A1Improving immunogenicityInduce immunogenic responseBiocideHydroxy compound active ingredientsSide effectParticulate antigen
This invention relates to a novel immunoadjuvant, an adjuvant component, and vaccines containing the adjuvant component. The adjuvant includes phytol or a phytol derivative. The adjuvant component, when combined with a soluble or particulate antigen, provides a vaccine with an enhanced ability to induce both humoral and cytotoxic immune responses while displaying reduced toxicity and / or adverse side effects over vaccines that include the antigen but without the benefit of this adjuvant component.
Owner:GHOSH SWAPAN K
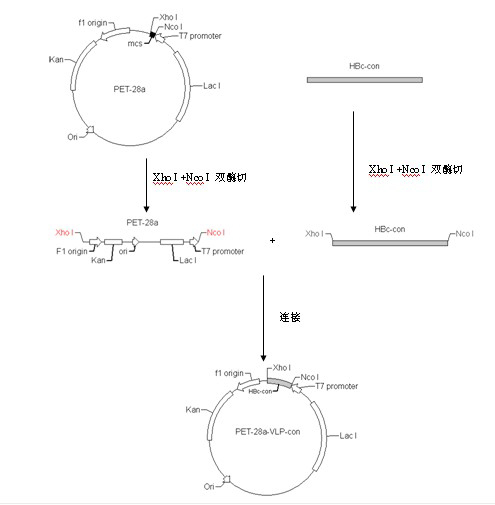
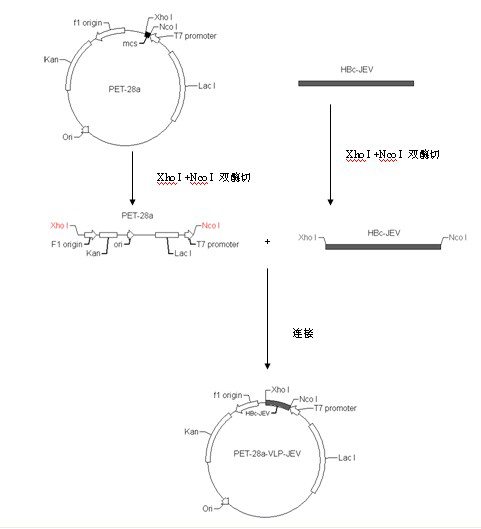
Japanese encephalitis particle vaccine and preparation method and application thereof
InactiveCN102127554AImprove the level ofStrong immune memoryViral antigen ingredientsVirus peptidesHepatitis B virus core AntigenEscherichia coli
The invention belongs to the field of biotechnology, and relates to a vaccine embedded with virus-like particles expressing multi-epitope antigen for Japanese encephalitis, and a preparation method and application thereof. The antigen of the vaccine is virus-like particles formed through spontaneous assembly of hepatitis B virus core antigen embedded with neutralizing antigen epitope expressing Japanese encephalitis virus and cytotoxic lymphocyte (CTL) antigen epitope, and is prepared through soluble expression of escherichia coli and purification. The Japanese encephalitis virus-like particle antigen is properly diluted with physiological saline, or is compatible with immunologic adjuvants to be prepared into the Japanese encephalitis particle vaccine. Animal experiments show that: the vaccine is safe and high-efficiency; mice inoculate with the vaccine generate high-level neutralizing antigen for the Japanese encephalitis virus to protect the mice against the attack of strong Japanese encephalitis virus by 100 percent.
Owner:NANJING AGRICULTURAL UNIVERSITY
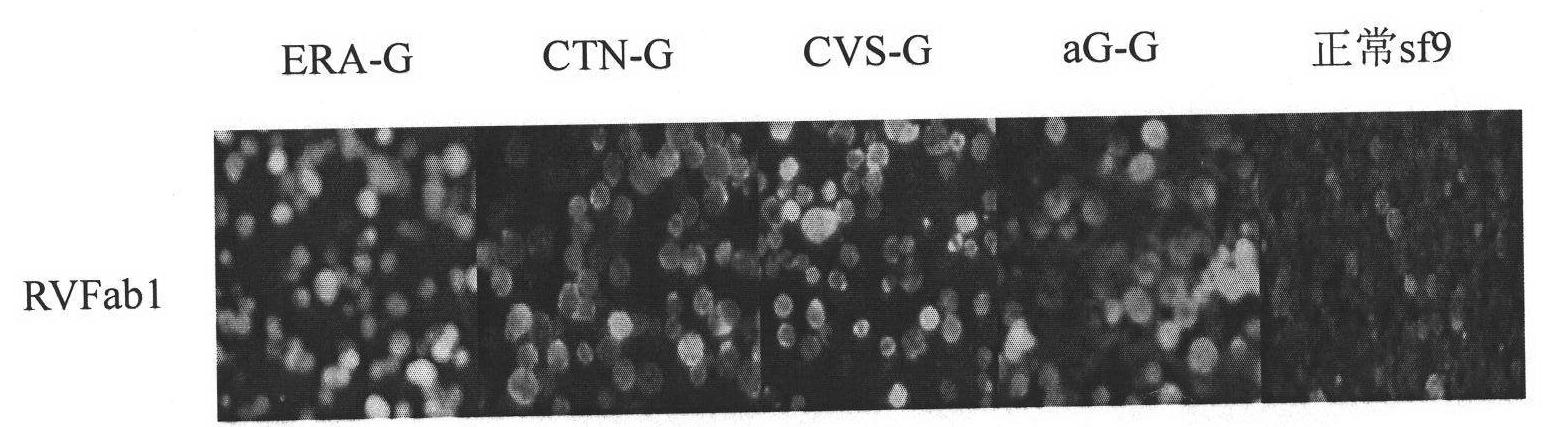
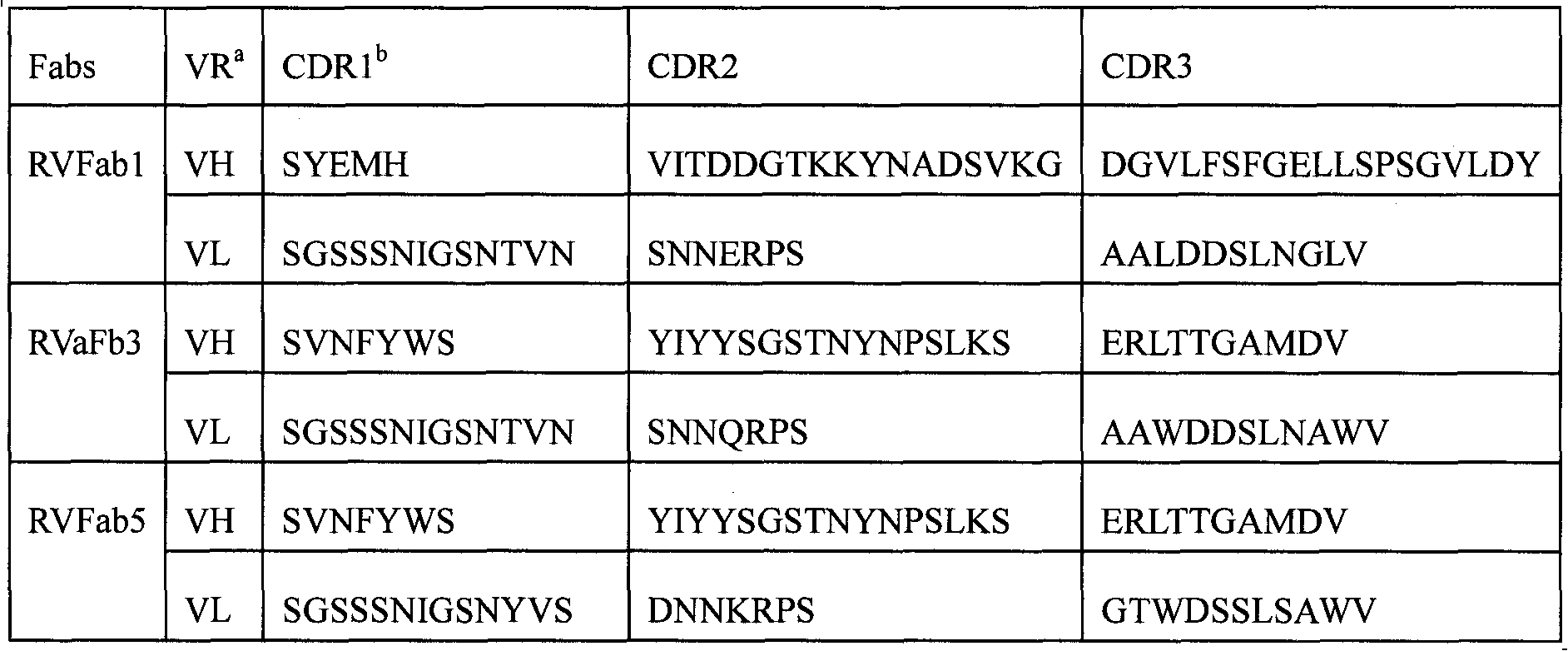
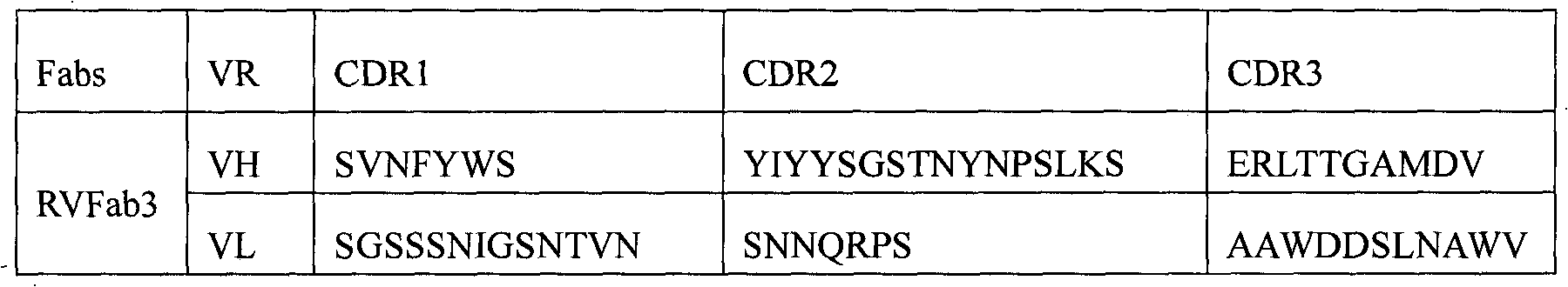
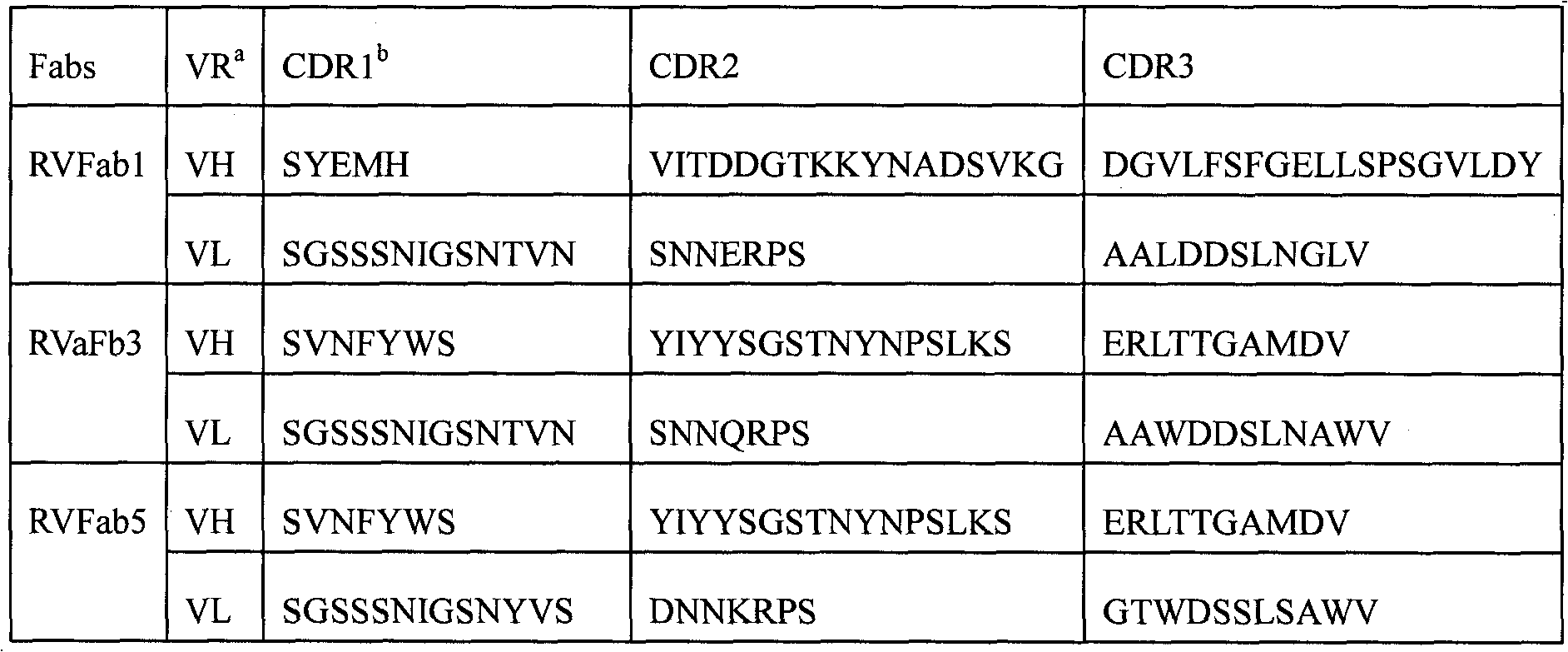
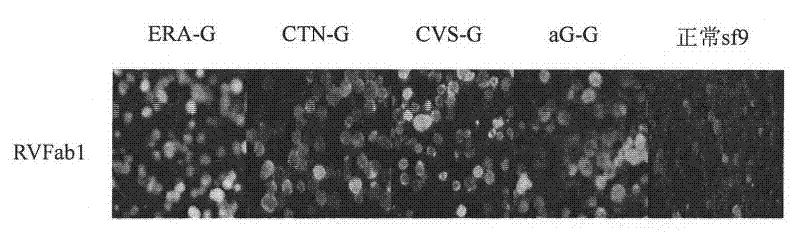
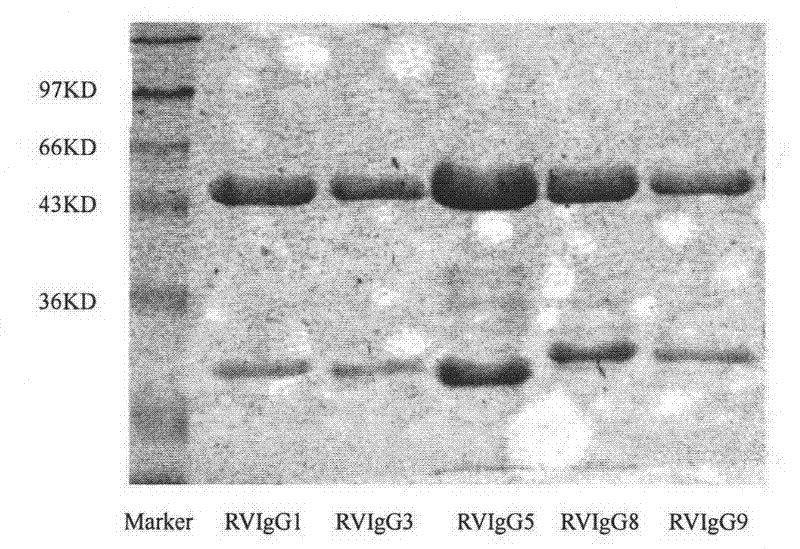
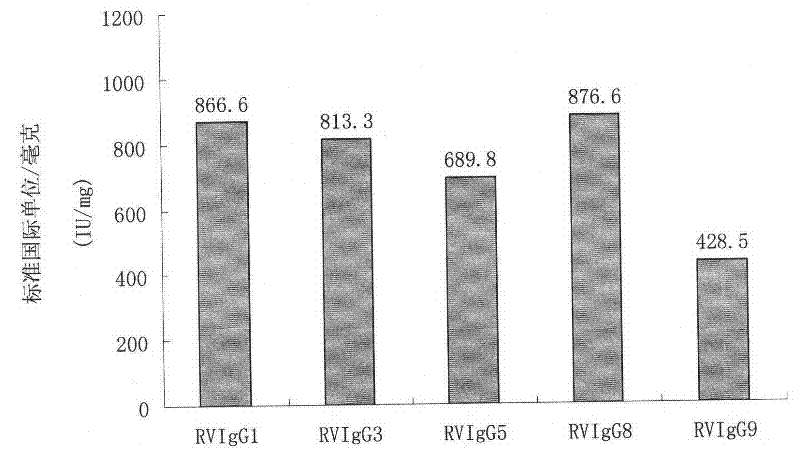
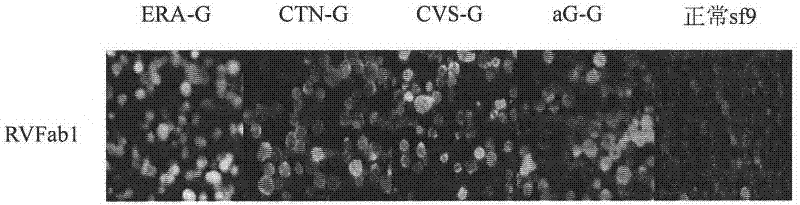
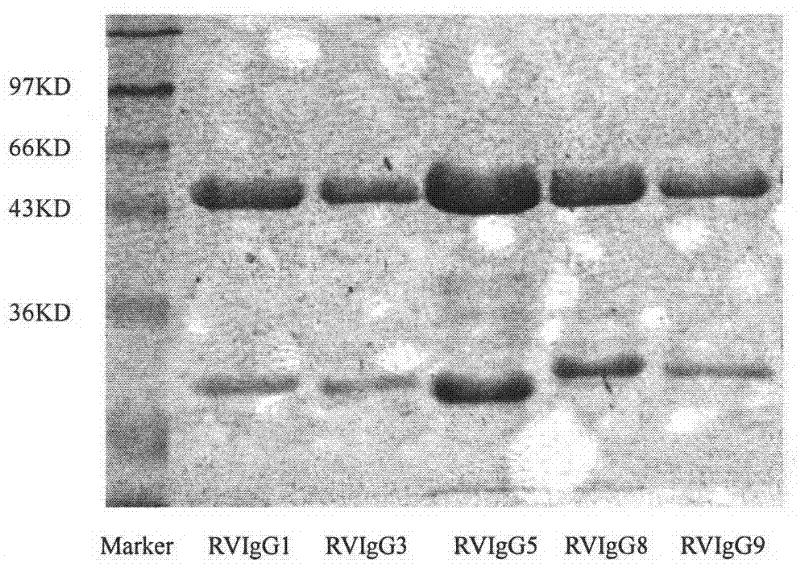
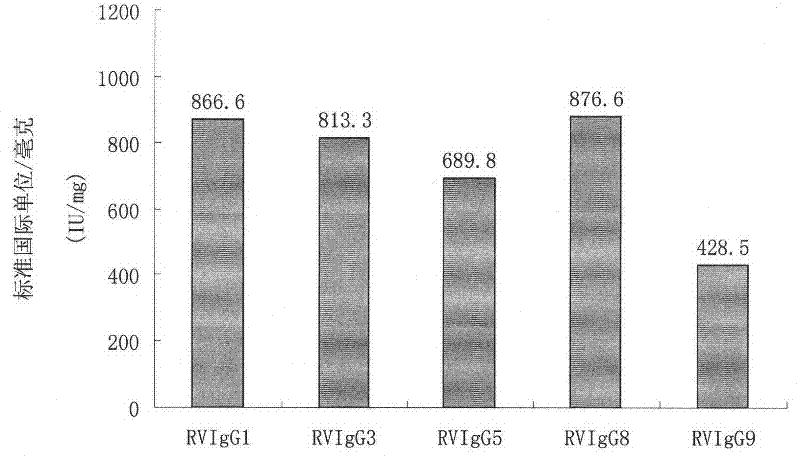
Humanized neutralizing antibody (RVFab8) against rabies virus glycoprotein
The invention discloses a humanized neutralizing antibody (RVFab8) against rabies virus glycoprotein, which is obtained through screening by utilizing phage display technology. The antibody specifically identifies the granule antigen of the rabies virus, is against the rabies virus glycoprotein G, has obvious immunofluorescence reaction and enzyme linked immunosorbent assay with the rabies virus and has the neutralizing activity function against rabies virus infection. The antibody can be prepared into the specific antibody drugs for preventing and treating rabies, thereby being clinically used for preventing and treating rabies caused by the rabies virus.
Owner:NCPC NEW DRUG RES & DEV
Rapid detection chromatography technology for pathogenic bacteria in water environment
The invention discloses an immunologic paper chromatographic strip for specifically and rapidly detecting common pathogenic bacteria in a water environment and a specific and rapid detection method thereof. The immunologic paper chromatographic strip of the invention which is used for detecting the common pathogenic bacteria in the water environment is new technology and application for rapidly detecting the common pathogenic bacteria in water. At present, a large number of reports on detection of soluble antigen by colloidal gold immunochromatographic test paper are available at home and abroad; and the bacteria serving as particulate antigen are extracted or processed by other methods and then detected abroad. In the technology, a special chromatographic film is selected and used and other conditions are optimized, so that the immunochromatographic strip can be directly used for detecting the bacteria. The technology can be used for rapidly detecting the common pathogenic bacteria in the water environment.
Owner:INST OF HYGIENE & ENVIRONMENTAL MEDICINE PLA ACAD OF MILITARY MEDICAL
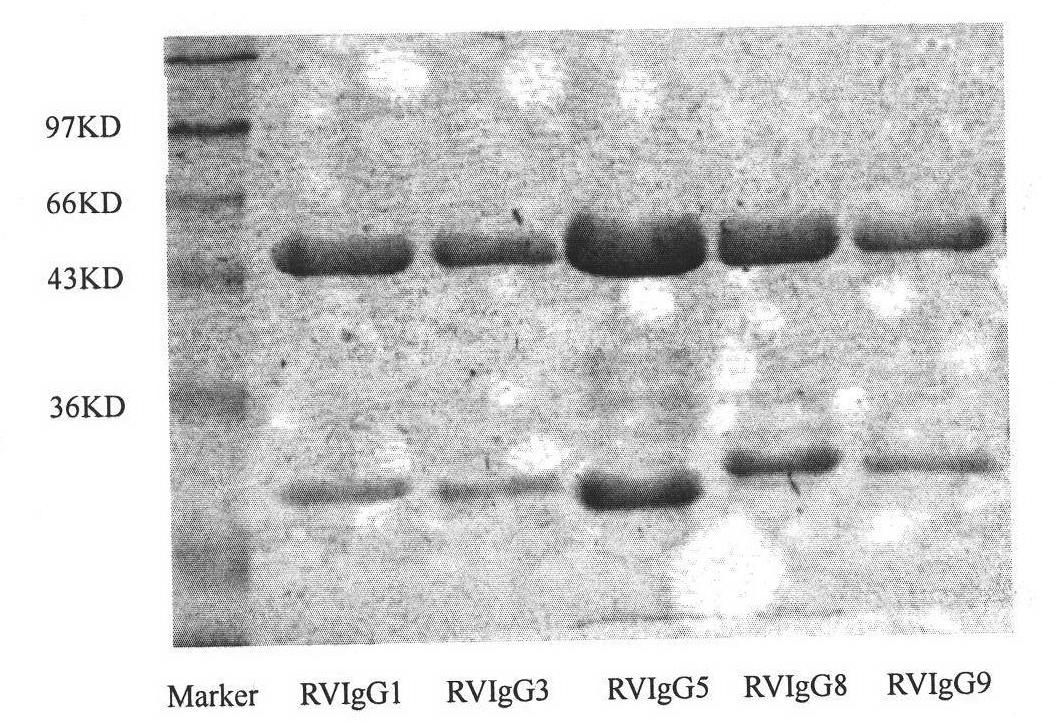
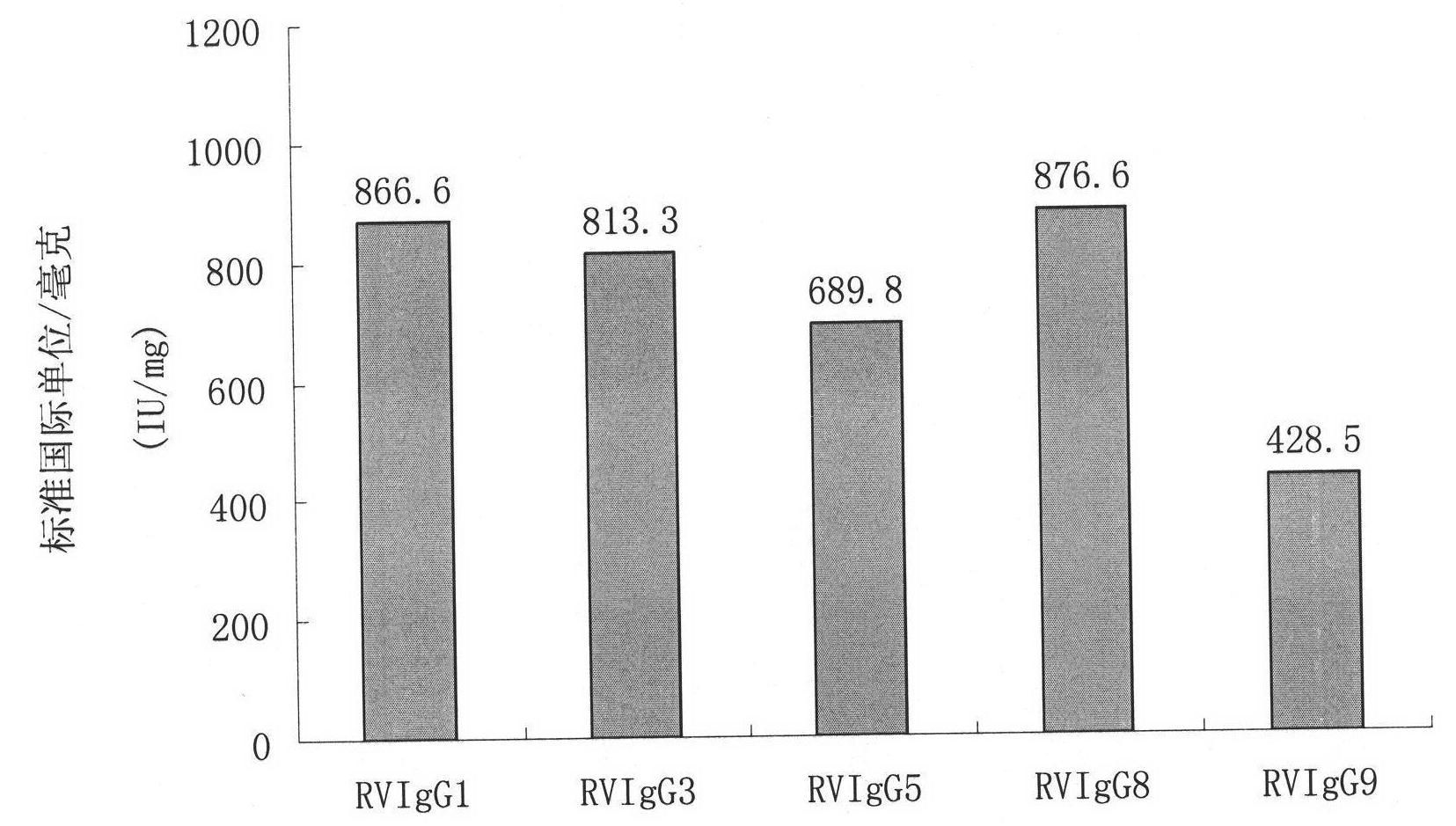
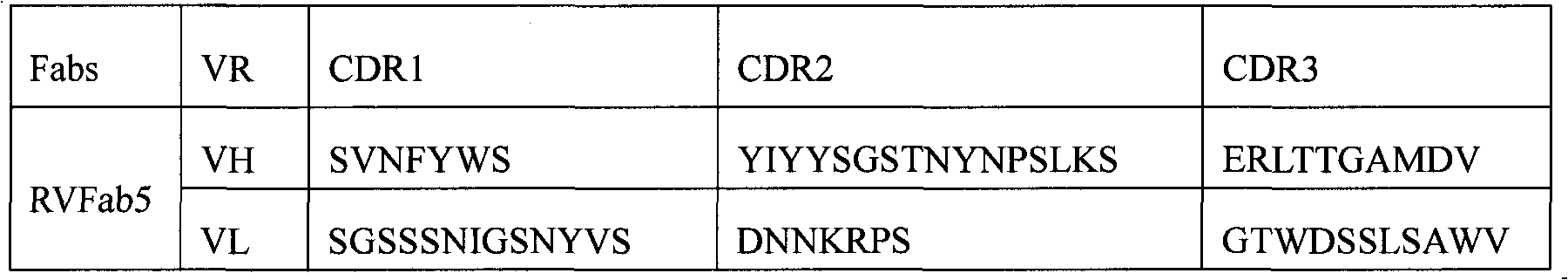
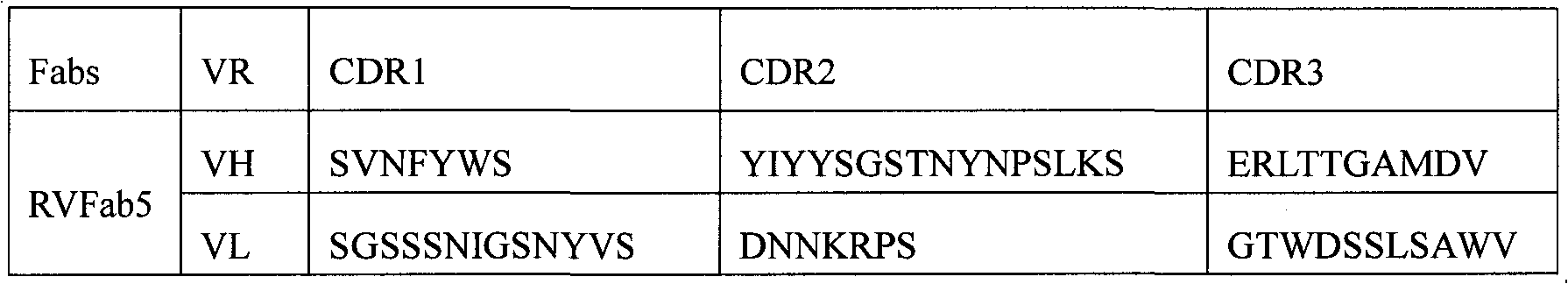
Humanized neutralizing antibody (RVFab5) against rabies virus glycoprotein
The invention discloses a humanized neutralizing antibody (RVFab5) against rabies virus glycoprotein, which is obtained through screening by utilizing phage display technology. The antibody specifically identifies the granule antigen of the rabies virus, is against the rabies virus glycoprotein G, has obvious immunofluorescence reaction and enzyme linked immunosorbent assay with the rabies virus and has the neutralizing activity function against rabies virus infection. The antibody can be prepared into the specific antibody drugs for preventing and treating rabies, thereby being clinically used for preventing and treating rabies caused by the rabies virus.
Owner:STATION OF VIRUS PREVENTION & CONTROL CHINA DISEASES PREVENTION & CONTROL CENT
O-type foot-and-mouth disease virus-like particle antigen, vaccine composition containing O-type foot-and-mouth disease virus-like particle antigen, and preparation method and application of vaccine composition
ActiveCN111233984AImproving immunogenicityImprove stabilitySsRNA viruses positive-senseViral antigen ingredientsDiseaseParticulate antigen
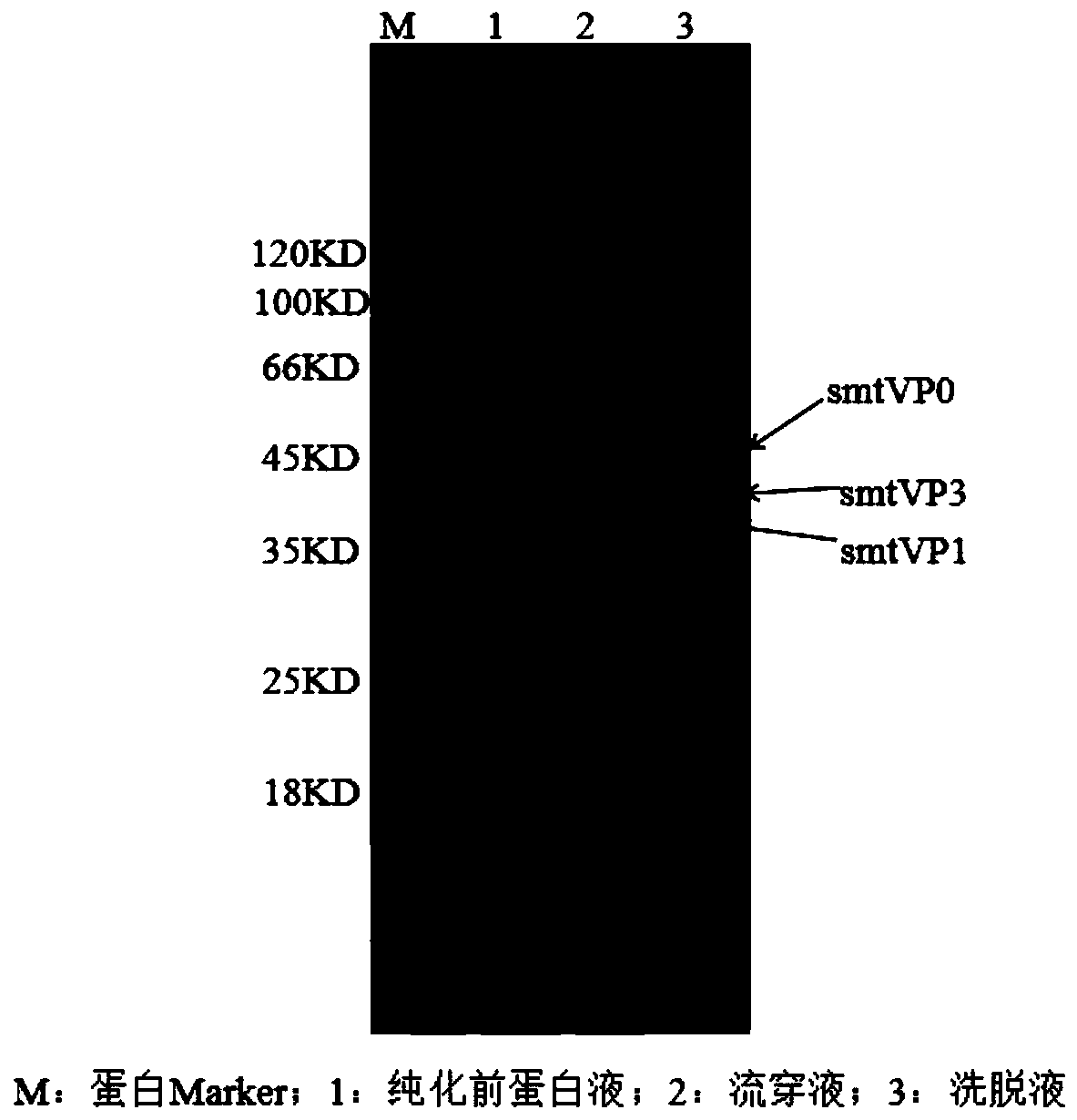
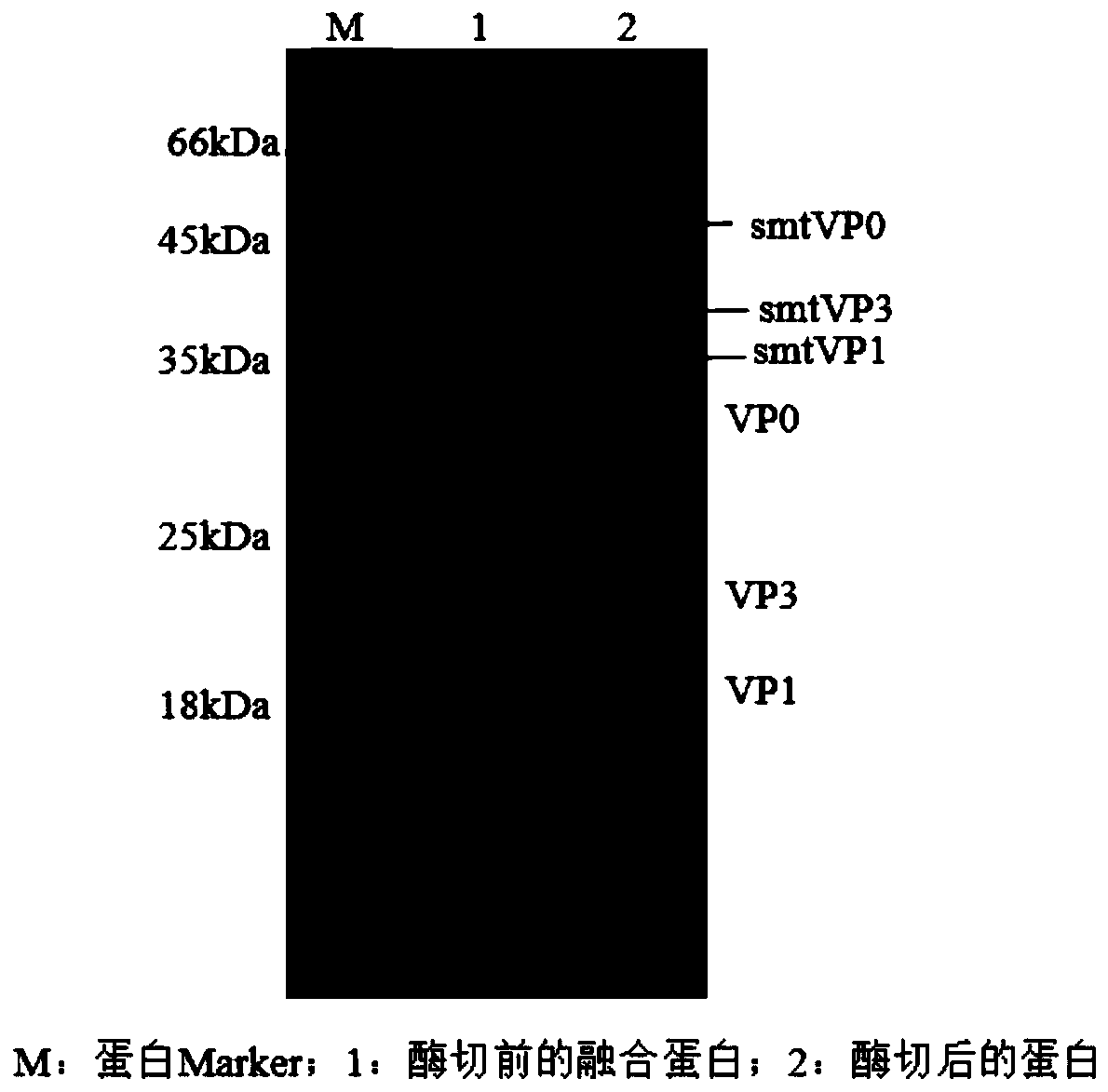
The invention provides a foot-and-mouth disease virus-like particle antigen. The foot-and-mouth disease virus-like particle antigen consists of a VP0 antigen protein, a VP1 antigen protein and a VP3 antigen protein through assembling, wherein the VP0 antigen protein is coded by a nucleotide sequence as shown in Seq ID No.1 or coded by a degenerate sequence of the nucleotide sequence as shown in Seq ID No.1, the VP1 antigen protein is coded by a nucleotide sequence as shown in Seq ID No.3 or coded by a degenerate sequence of the nucleotide sequence as shown in Seq ID No.3, and the VP3 antigen protein is coded by a nucleotide sequence as shown in Seq ID No.2 or coded by a degenerate sequence of the nucleotide sequence as shown in Seq ID No.2. The foot-and-mouth disease virus-like particle antigen has favorable immunogenicity, a vaccine composition prepared from the foot-and-mouth disease virus-like particle antigen can protect current epidemic O-type Ind strains through one-time immunization, and the immunoprotection period is long. The invention further provides the vaccine composition prepared from the foot-and-mouth disease virus-like particle antigen, and a preparation method andan application of the vaccine.
Owner:PU LIKE BIO ENG
Rapid one-step generation of antigen loaded dendritic cell vaccine from precursors
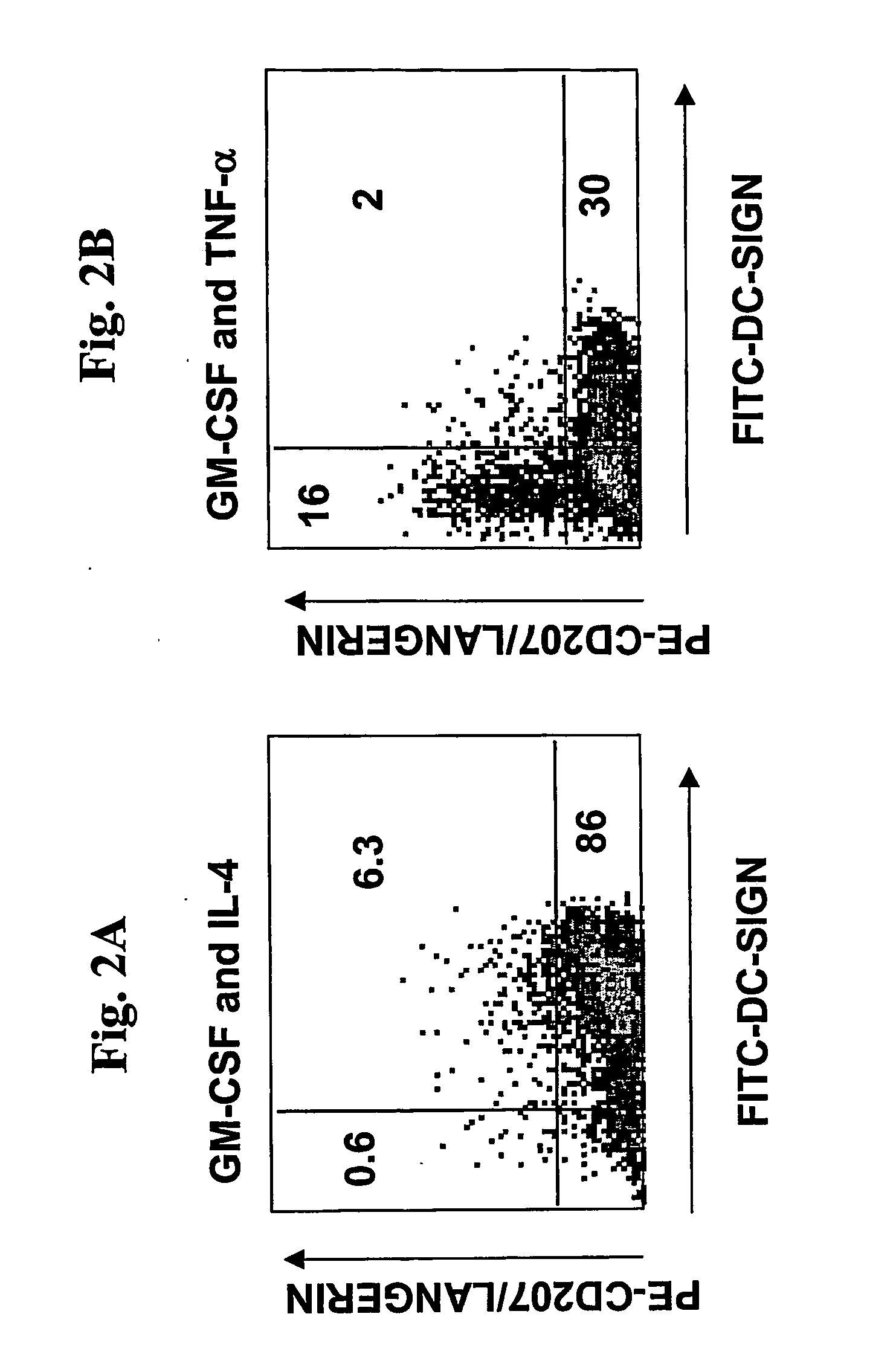
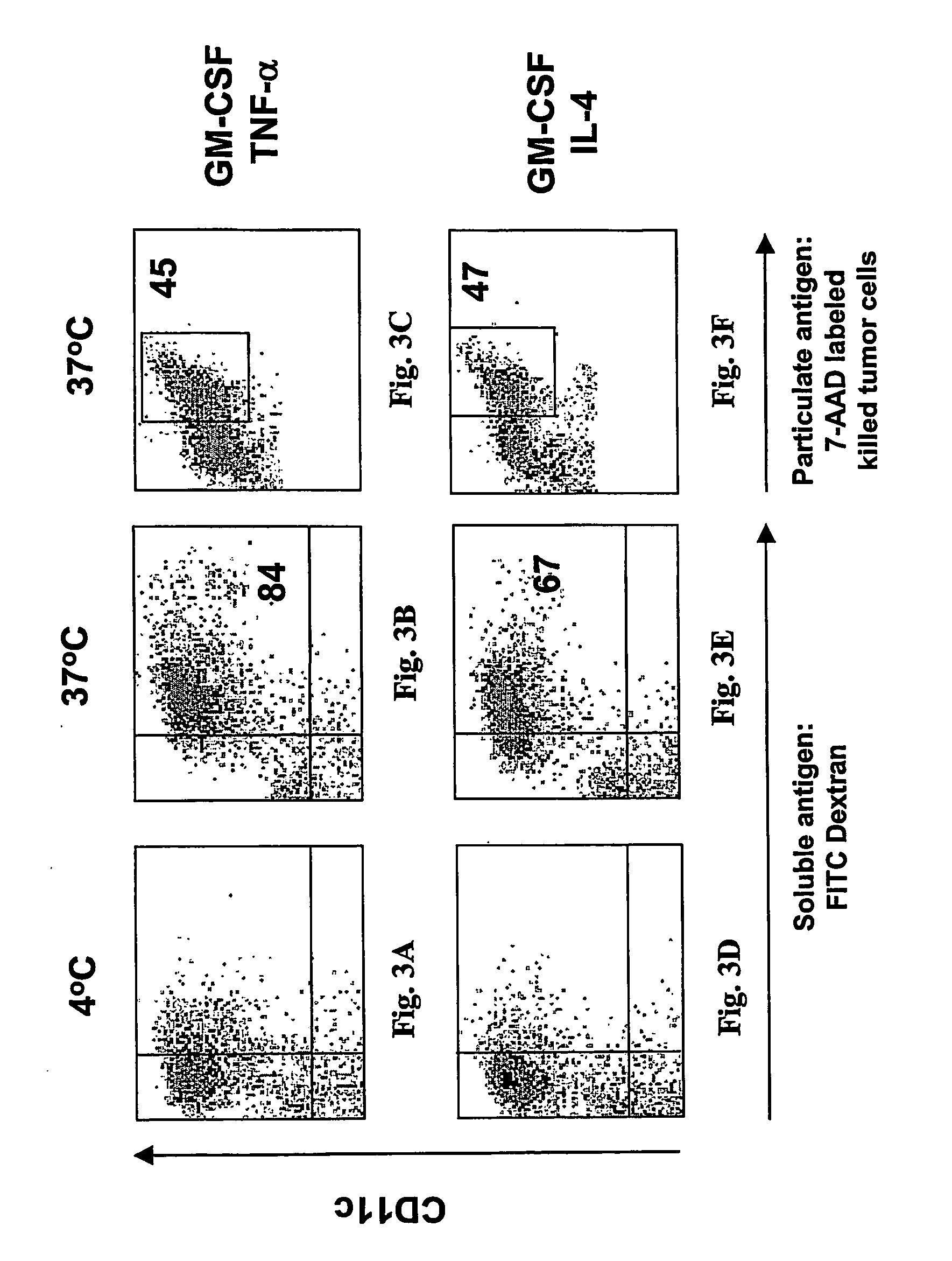
InactiveUS20060239962A1Peptide/protein ingredientsSnake antigen ingredientsLangerhan cellSurface marker
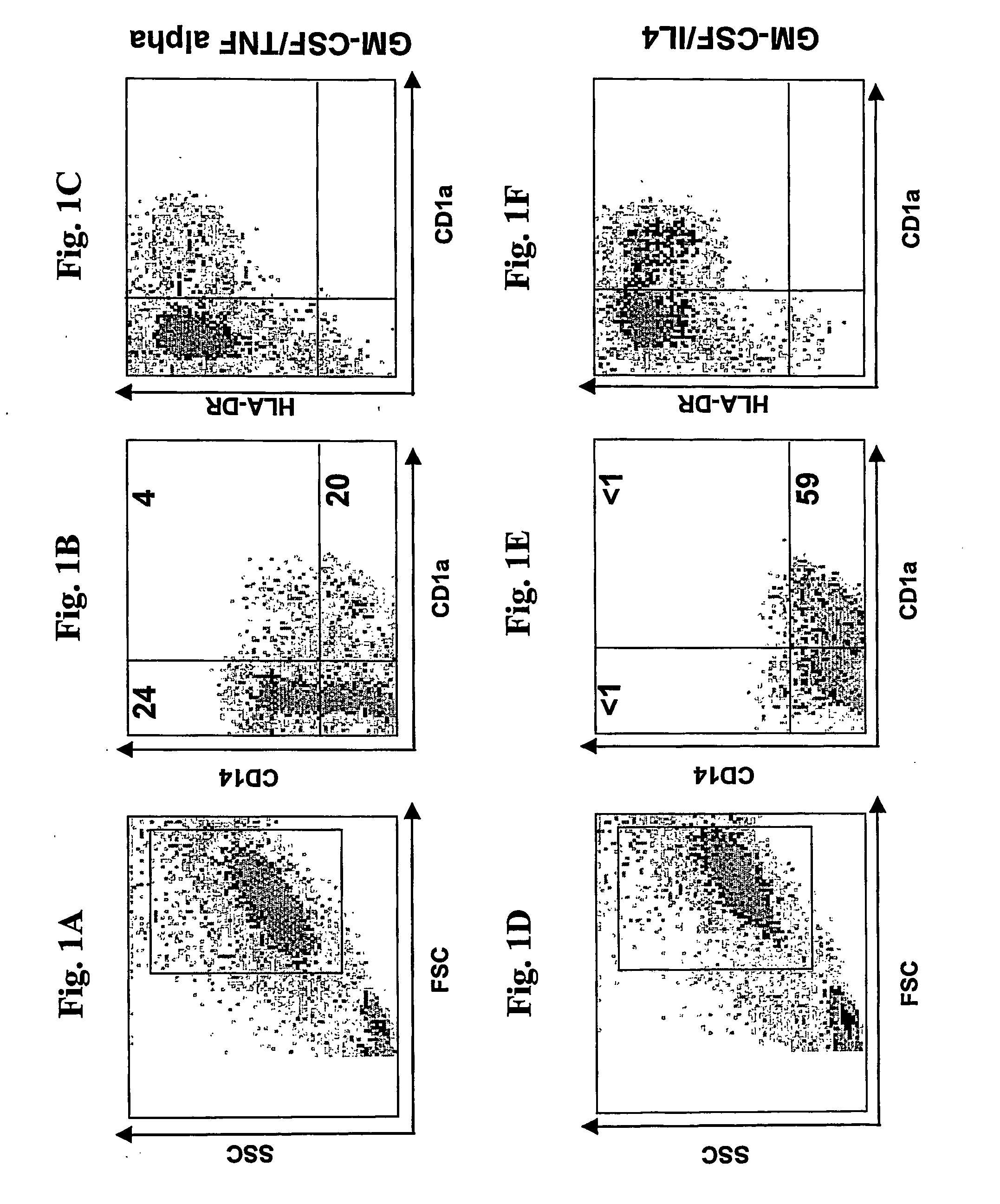
A one-step method for producing antigen loaded antigen-presenting cells from monocytes ex vivo has been found which comprises contacting the monocytes with a composition comprising an activator such as TNF alpha preferably in combination with at least one growth factor such as GM-CSF and at least one soluble or particulate antigen. According to the methods of the present invention, antigen-loaded dendritic cell vaccines can be generated within as little as three (3) days. In another method of the present invention, antigen loaded antigen-presenting cells are produced from monocytes ex vivo by contacting the monocytes with TNF alpha and granulocyte-macrophage colony stimulating factor at one time point to form antigen-presenting cells and then contacting antigen-presenting cells with soluble or particulate antigenic material antigen-presenting cells, wherein the antigen loaded antigen-presenting cells are produced in less than four days. The present invention also includes a vaccine which comprises monocyte-derived antigen loaded antigen-presenting cells, wherein the antigenpresenting cells are composed of two or more subsets selected from the group consisting of Langerhans cells with surface markers (CD 1 a+CD207+); interstitial dendritic cells with surface markers (CD 1a+CD207−); double negative dendritic cells with surface markers 20 (CD 1 a−CD 14−); and dendritic cells with surface markers (CD 14+CD 1 a−CD209+).
Owner:BAYLOR RES INST
Humanized neutralizing antibody (RVFab3) against rabies virus glycoprotein
The invention discloses a humanized neutralizing antibody (RVFab3) against rabies virus glycoprotein, which is obtained through screening by utilizing phage display technology. The antibody specifically identifies the granule antigen of the rabies virus, is against the rabies virus glycoprotein G, has obvious immunofluorescence reaction and enzyme linked immunosorbent assay with the rabies virus and has the neutralizing activity function against rabies virus infection. The antibody can be prepared into the specific antibody drugs for preventing and treating rabies, thereby being clinically used for preventing and treating rabies caused by the rabies virus.
Owner:STATION OF VIRUS PREVENTION & CONTROL CHINA DISEASES PREVENTION & CONTROL CENT
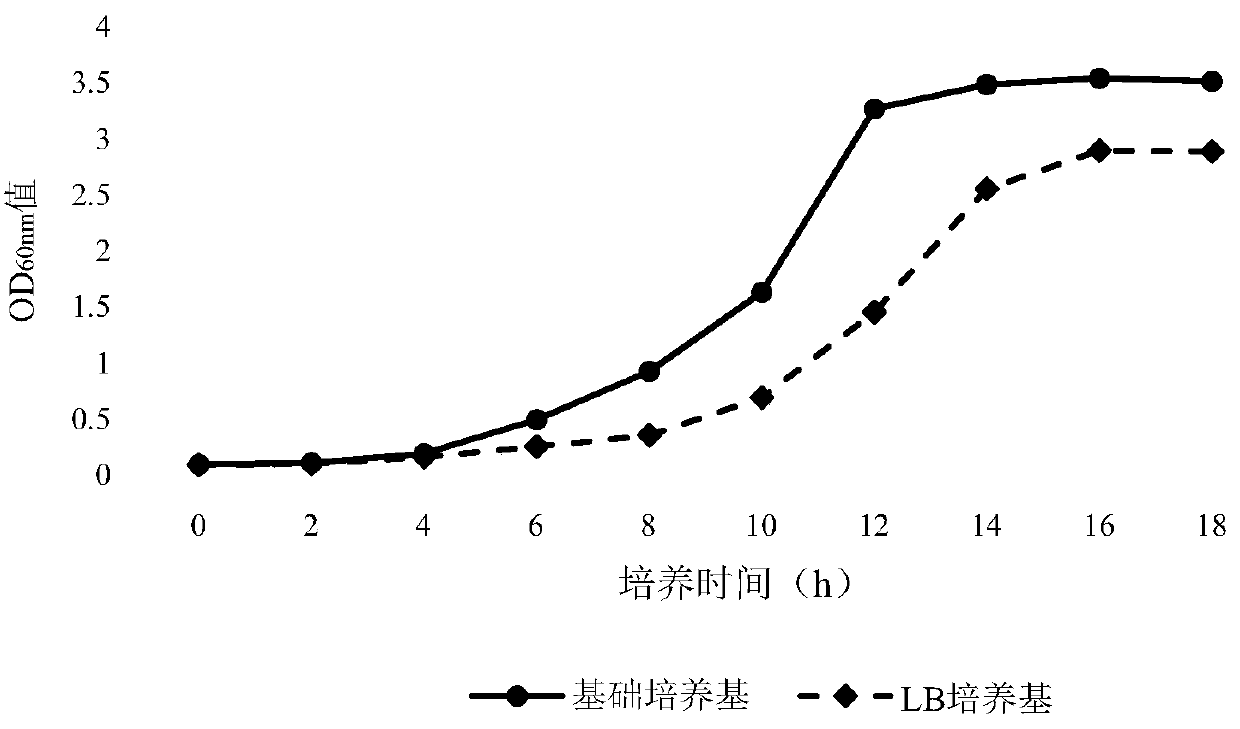
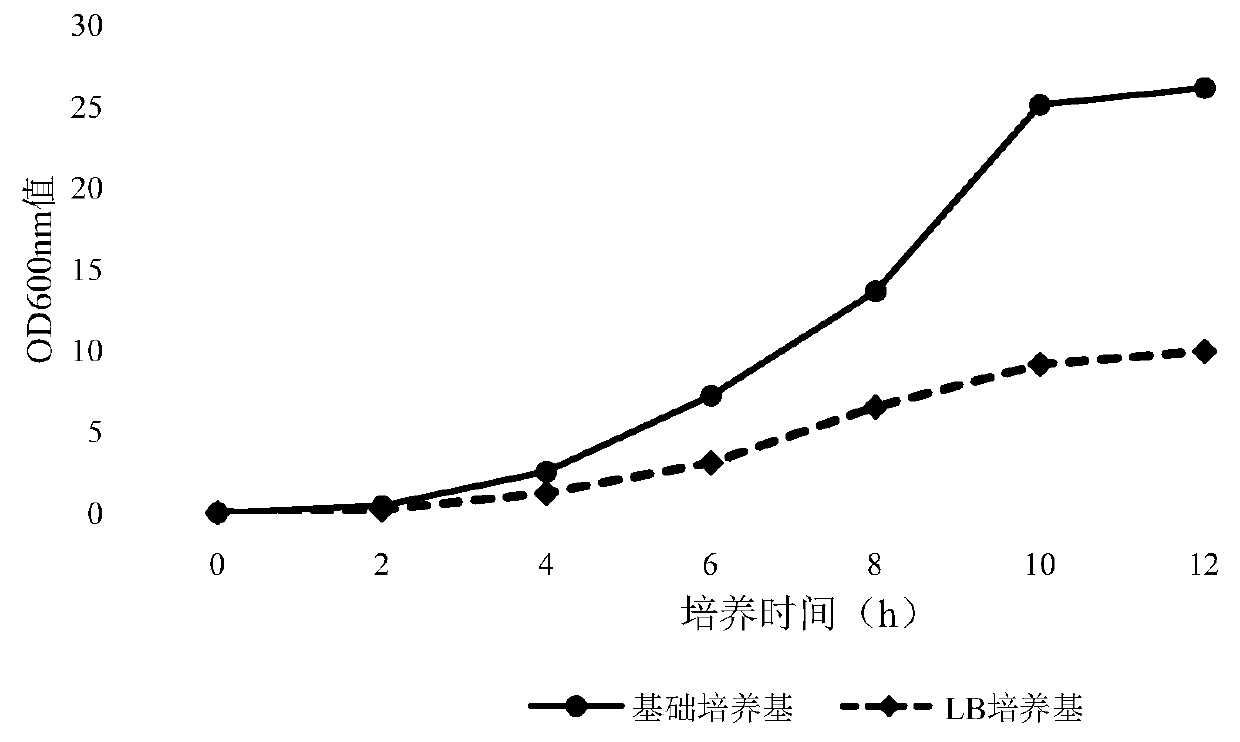
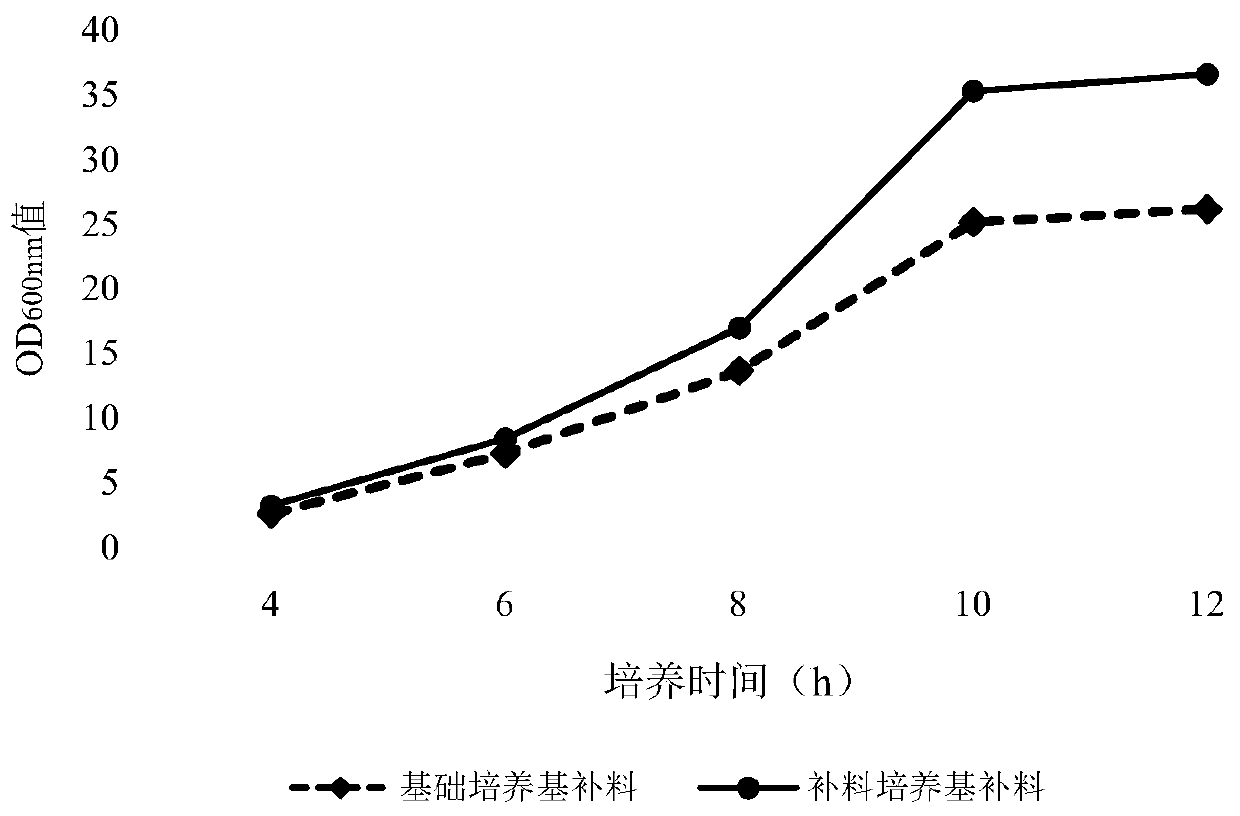
Fermentation culture medium for antigen expression of foot-and-mouth disease virus-like particles through Escherichia coli and culture method using fermentation culture medium
ActiveCN110819582AHigh expressionHigh proportion of soluble antigenSsRNA viruses positive-senseBacteriaEscherichia coliParticulate antigen
The invention discloses a fermentation culture medium for antigen expression of foot-and-mouth disease virus-like particles through Escherichia coli and a culture method using the fermentation culturemedium. The fermentation culture medium comprises a basic medium, a material supplementing medium and an induced expression medium, wherein the basic medium can be used for shortening the lag phase of Escherichia coli strains for production so that the strains enter a logarithmic growth phase as soon as possible, the material supplementing medium can be used for increasing the multiplication capacity of Escherichia coli in the logarithmic growth phase and ensuring that a higher microbial content of Escherichia coli is achieved in a high-density fermentation process, and the induced expressionmedium is used for maintaining the plateau phase of Escherichia coli so as to increase the expression amount of antigens of the virus-like particles; and meanwhile expression of the antigens in a soluble form is promoted by components during culture. The fermentation culture method includes culture of the production strains, expansion culture of the strains, induction expression and other steps.Through the fermentation culture medium and culture method, the expression amount of the antigens of the virus-like particles can be increased greatly, and at the same time expression of the antigensin a soluble form can be achieved to a maximum extent.
Owner:SICHUAN HUAPAI BIO PHARMA
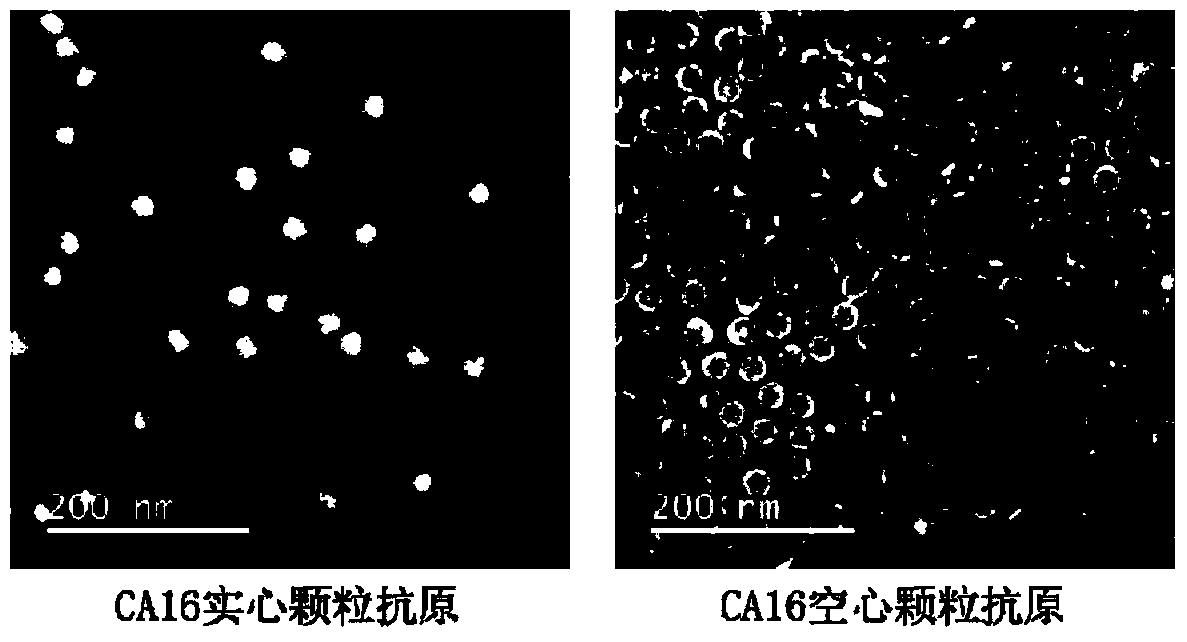
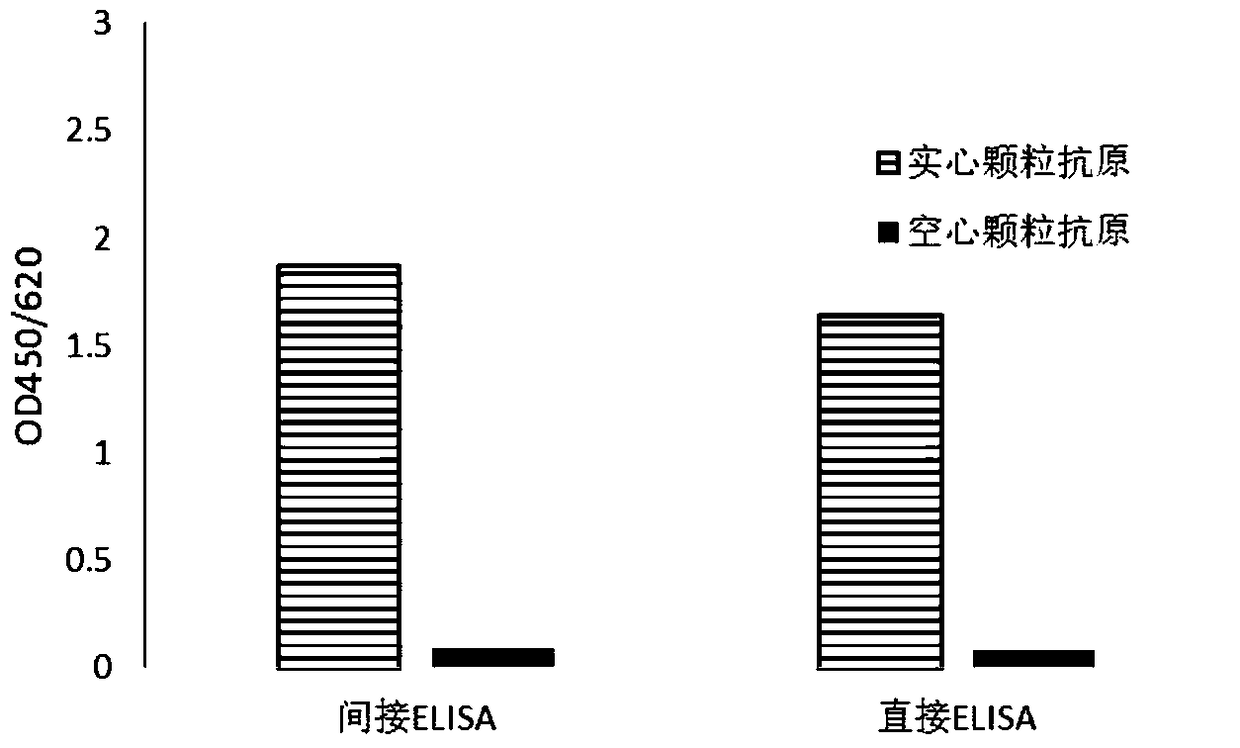
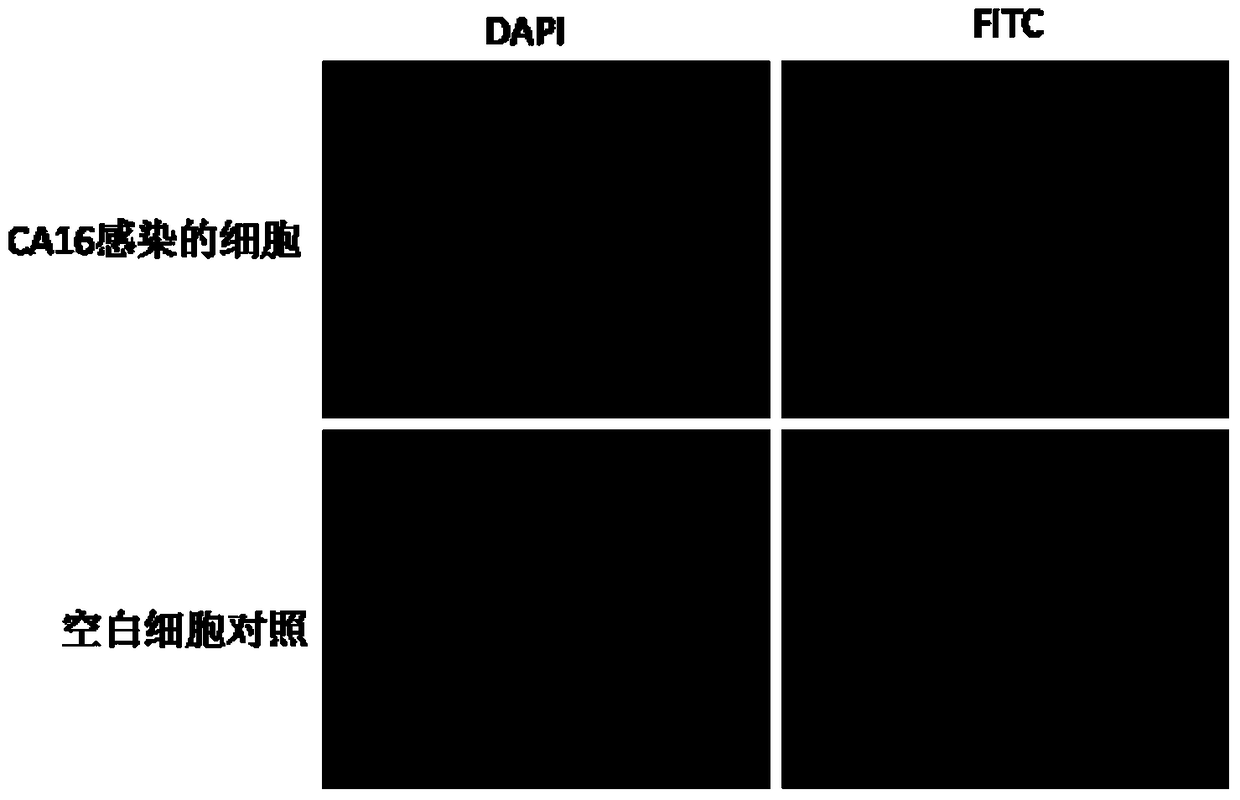
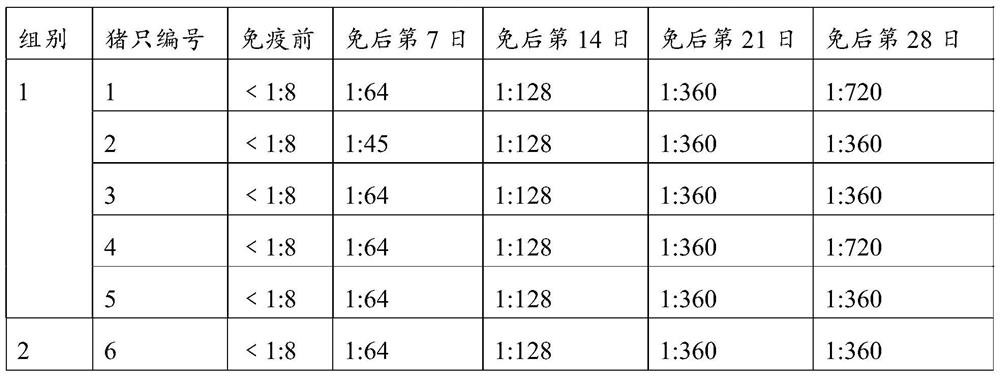
Monoclonal antibody for detecting coxsackie virus a16 type virus solid particles and use thereof
The invention relates to a monoclonal antibody of solid particles of specific binding coxsackievirus A16 (CA16), and a conservative variant or an active fragment of the monoclonal antibody. The invention further relates to a method for detecting a CA16 solid particle antigen by using the monoclonal antibody and use of the monoclonal antibody for preparing drugs for preventing or detecting or treating CA16.
Owner:XIAMEN UNIV +1
Foot and mouth disease virus-like particulate antigen, and vaccine composition, preparation method and application thereof
ActiveCN113563432AProduced fastProduce high levelsSsRNA viruses positive-senseViral antigen ingredientsDiseaseNucleotide
Owner:PU LIKE BIO ENG
Preparation method of O-type foot-and-mouth disease virus-like particle antigen, prepared O-type foot-and-mouth disease virus-like particle antigen and application thereof
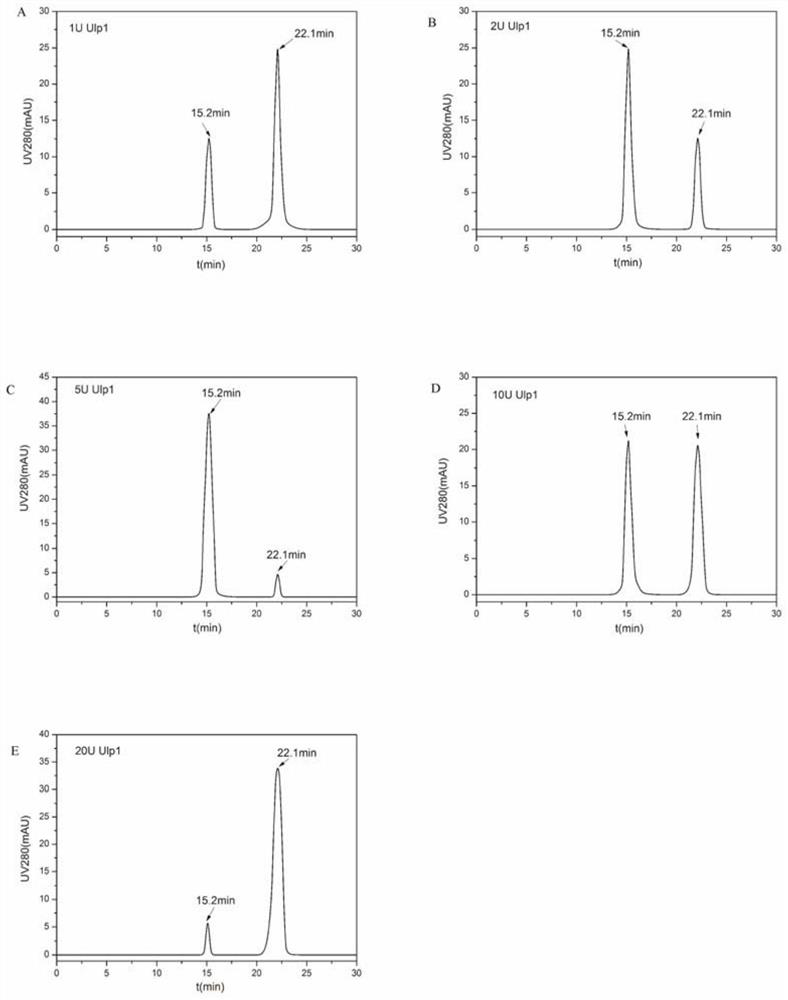
PendingCN113956335AImprove assembly efficiencyHigh yield of soluble expressionSsRNA viruses positive-senseViral antigen ingredientsEscherichia coliDisease
The invention relates to a preparation method of O-type foot-and-mouth disease virus-like particles, which comprises the following steps: recombining a VP0 gene, a VP3 gene and a VP1 gene of an O-type foot-and-mouth disease virus to a pETSUMO vector, forming fusion protein genes with SUMO, amplifying the three fusion protein genes, cloning into the same pET28a expression vector to obtain a recombinant expression vector pET28a-SUMO-VP0-SUMO-VP3-SUMO-VP1, transforming the expression vector into escherichia coli to express fusion protein, and removing SUMO, VP0, VP3 and VP1 proteins from the expressed fusion protein by enzyme digestion under the condition of 10U / mL Ulp1 protease concentration to form the O-type foot-and-mouth disease virus-like particles through self-assembly. The method can be used to efficiently prepares the O-type foot-and-mouth disease virus-like particles, and is suitable for large-scale industrial production.
Owner:PU LIKE BIO ENG
Mesoporous silicon nano material, preparation method and application thereof
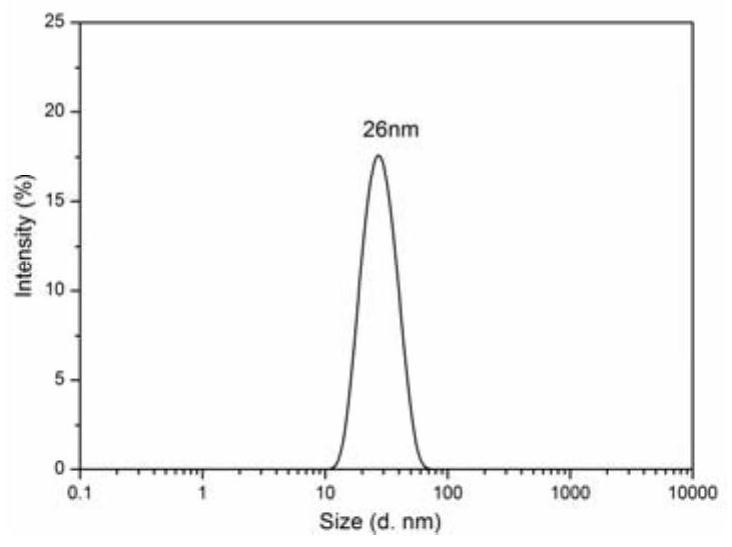
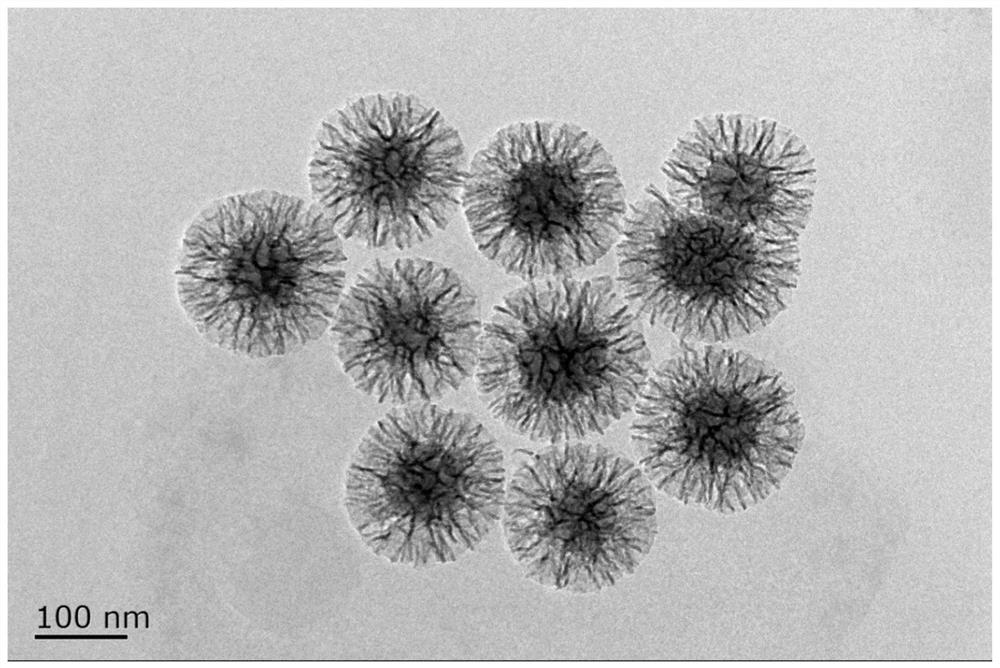
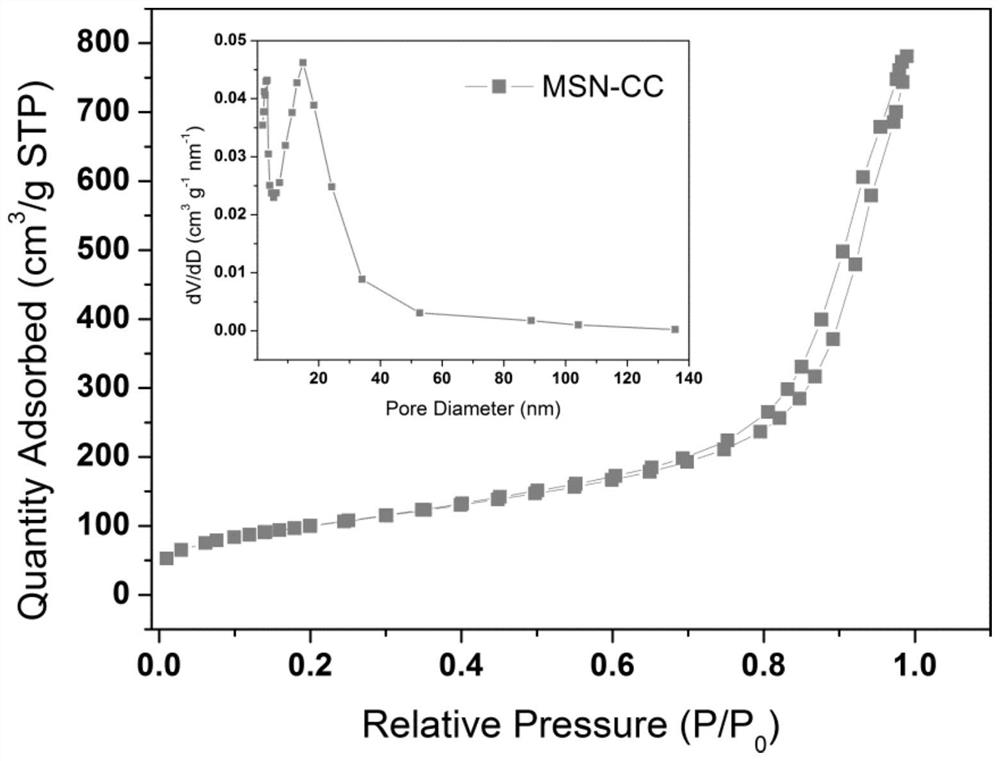
ActiveCN113955762AUniform particle sizeConducive to loadMaterial nanotechnologySilicaAdjuvantFoot mouth disease virus
The invention belongs to the technical field of materials, and particularly relates to a mesoporous silicon nano material, a preparation method and application thereof. The mesoporous silicon nano material is of a core-cone structure; the interior of the material is a silicon dioxide solid core, and the exterior of the material is a dendritic mesoporous silicon shell layer radiating outwards from the core. The size of the silicon dioxide core is 108+ / -10nm; and the thickness of the mesoporous silicon shell layer is 66+ / -10nm. The mesoporous silicon nano-material is spherical, and the particle size of the mesoporous silicon nano-material is 237+ / -10nm. The mesoporous silicon nano material has the advantages of large specific surface area and pore volume, uniform particle size, stable structure and high biological safety. According to the present invention, the foot and mouth disease virus-like particle antigen can be loaded and slowly released, and advantages of high protein loading capacity, low cytotoxicity, good blood compatibility and low tissue and organ toxicity are provided. As an adjuvant, the mesoporous silicon nano material can enhance the immune response level and improve the challenge protection rate.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
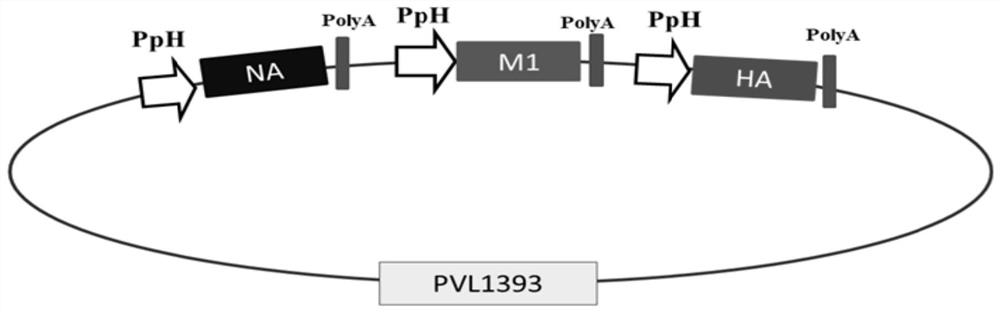
Avian influenza virus-like particle vaccine as well as preparation method and application thereof
ActiveCN113461786AIncrease productionReduce manufacturing costSsRNA viruses negative-senseViral antigen ingredientsParticulate antigenImmunogenicity
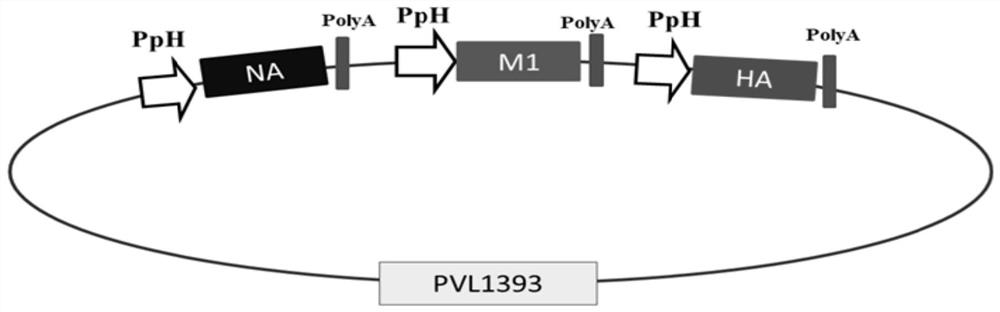
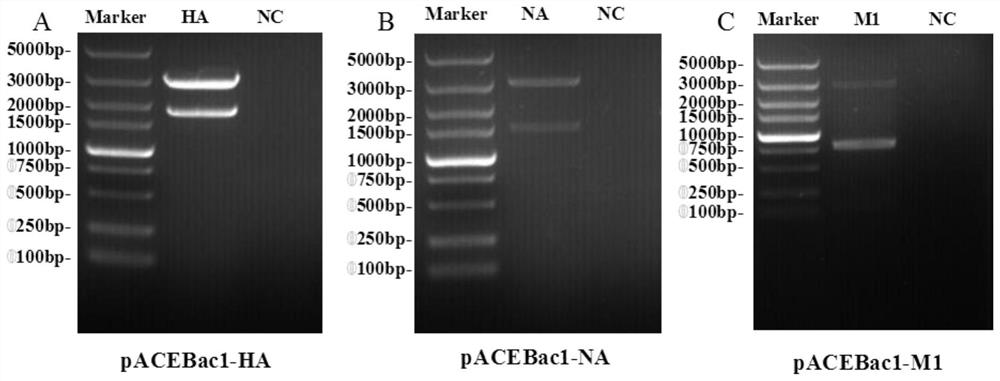
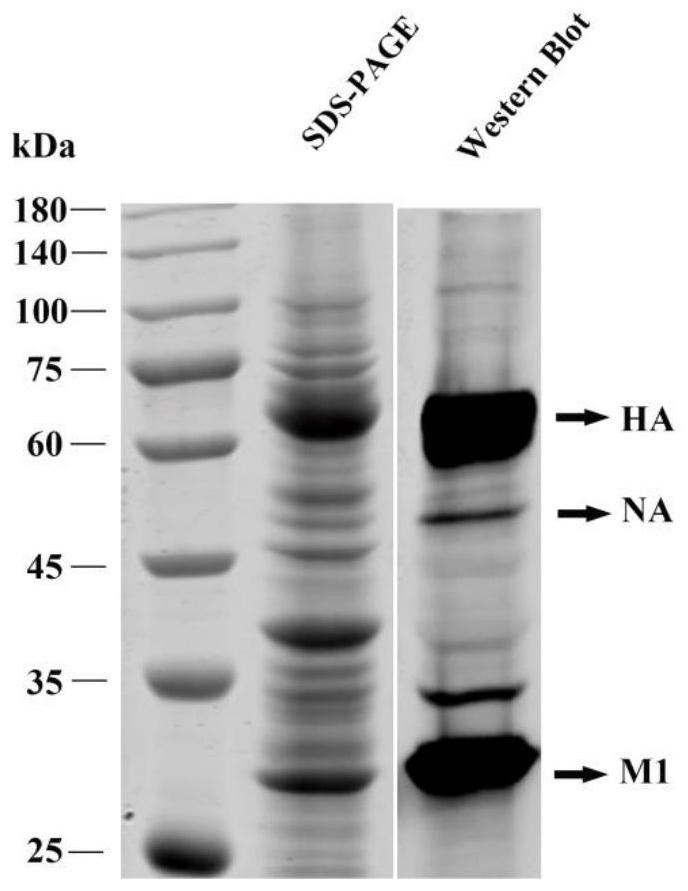
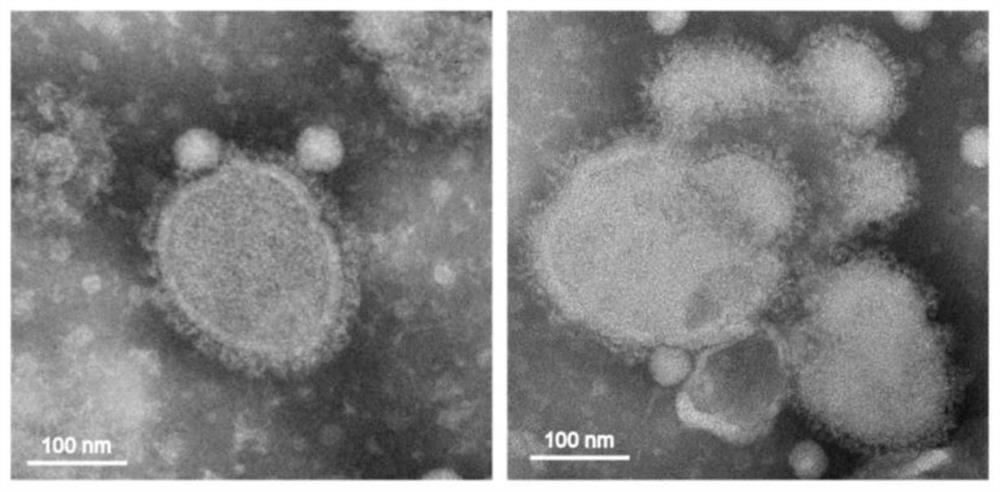
The invention relates to an avian influenza virus-like particle vaccine, the avian influenza virus-like particle vaccine comprises an immune dose of H5 subtype and / or H7 subtype avian influenza virus-like particle antigen and a pharmaceutically acceptable carrier, and the avian influenza virus-like particle antigen is formed by assembling HA, NA and M1 antigen proteins of H5 subtype and / or H7 subtype avian influenza viruses. The avian influenza virus-like particle vaccine disclosed by the invention has good immunogenicity, and the immune effect is better than that of a commercial inactivated vaccine with higher antigen content when the dosage is low.
Owner:PU LIKE BIO ENG +2
Solution for large-scale production of foot-and-mouth disease virus-like particle antigens, and purification and assembly method
InactiveCN110981946AHigh purityHigh assembly rateSsRNA viruses positive-senseVirus peptidesEscherichia coliDisease
The invention discloses a solution for large-scale production of foot-and-mouth disease virus-like particle antigens and a purification and assembly method, belonging to the technical field of biological medicines. With the method, foot-and-mouth disease virus-like particles with high purity and high assembly rate is purified and prepared from foot-and-mouth disease virus recombinant protein fermentation liquor expressed by escherichia coli. The method is simple in technological process, convenient to operate and high in production efficiency; the purification yield of the foot-and-mouth disease virus-like particles reaches 90% or above, and the in-vitro assembly rate of the particles reaches 67%; and the method is particularly suitable for industrial production of foot-and-mouth disease virus-like particle antigens expressed by escherichia coli.
Owner:SICHUAN HUAPAI BIO PHARMA
Recombinant PRRSV virus-like particle antigen-antibody complex and preparation method thereof
ActiveCN113425838AImprove impactHigh titerSsRNA viruses positive-senseViral antigen ingredientsParticulate antigenImmune complex deposition
According to the invention, a recombinant baculovirus capable of being efficiently replicated is obtained by modifying a GP5-M protein, the culture titer of the recombinant baculovirus is relatively high, the prepared virus-like particles similar to PRRSV virions are further compounded with IgM to prepare an IgM-RPPSV VLPs immune complex, and the IgM-RPPSV VLPs immune complex can stimulate an organism to generate a high-concentration antibody earlier.
Owner:NORTHWEST A & F UNIV +1
Foot and mouth disease virus-like particle antigen, vaccine composition, preparation method and application
ActiveCN111434677AHigh Titer Antibody TiterImproving immunogenicitySsRNA viruses positive-senseViral antigen ingredientsParticulate antigenFoot mouth disease virus
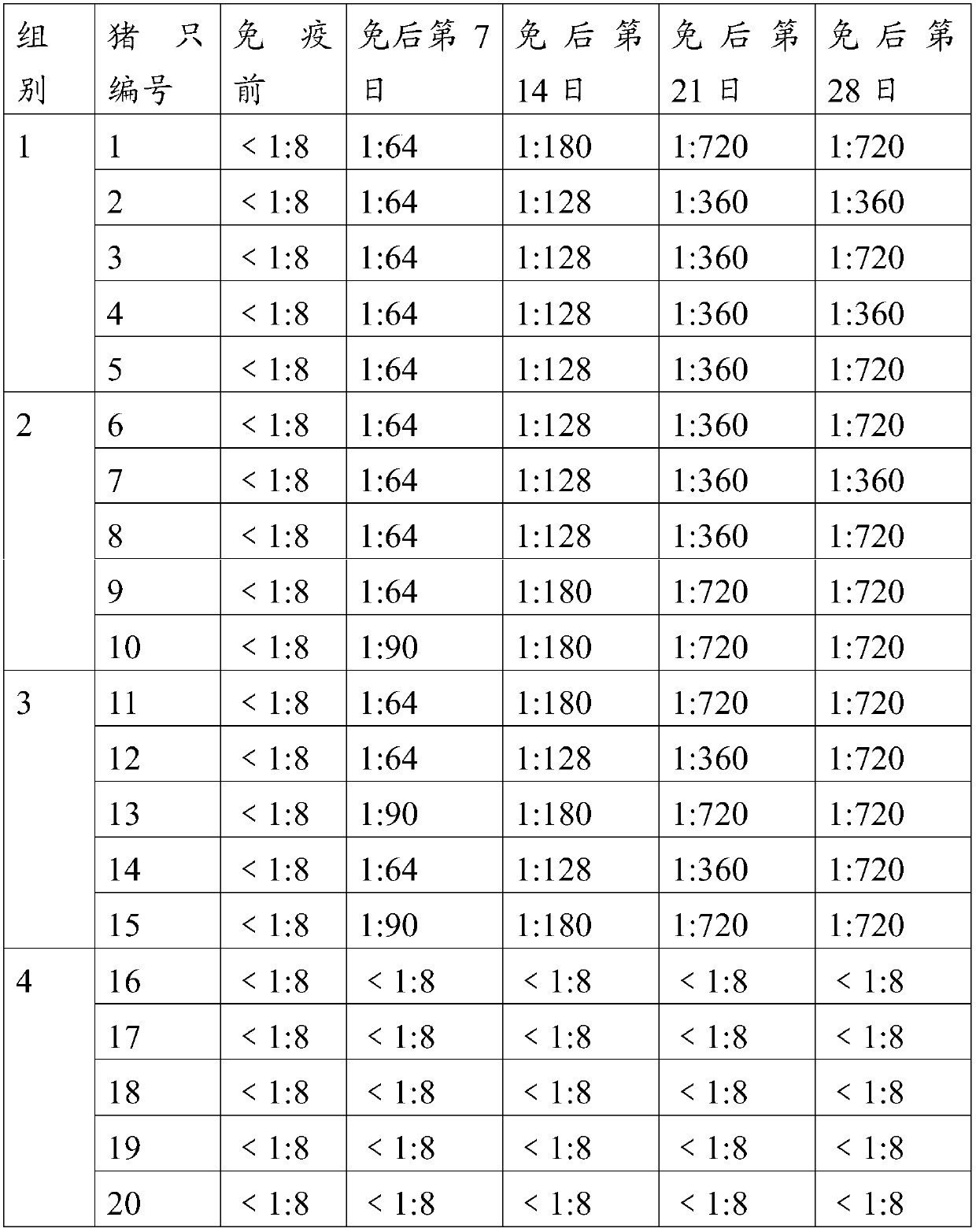
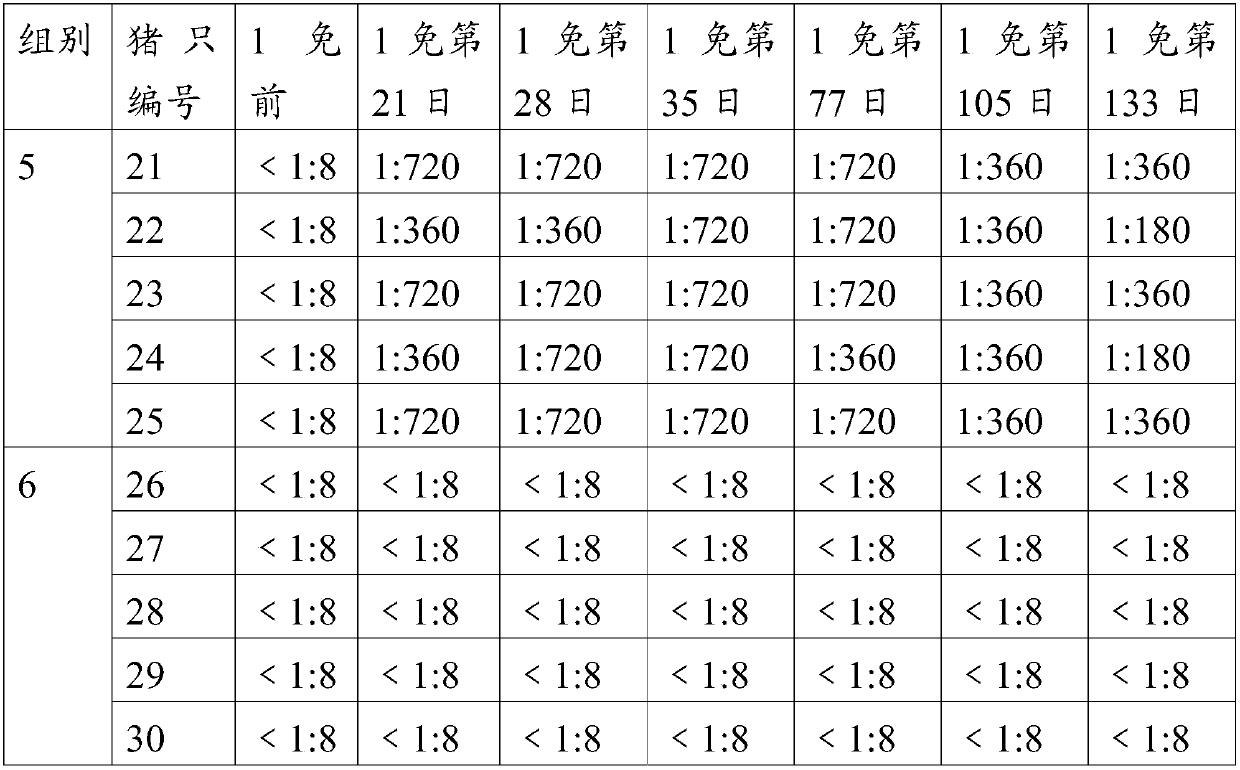
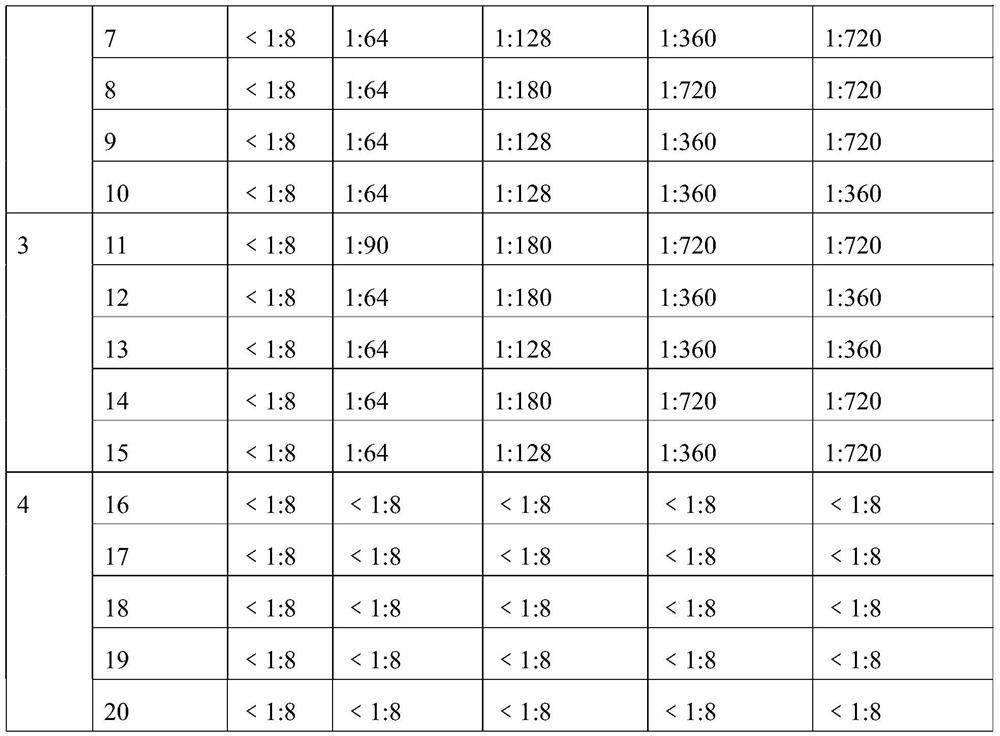
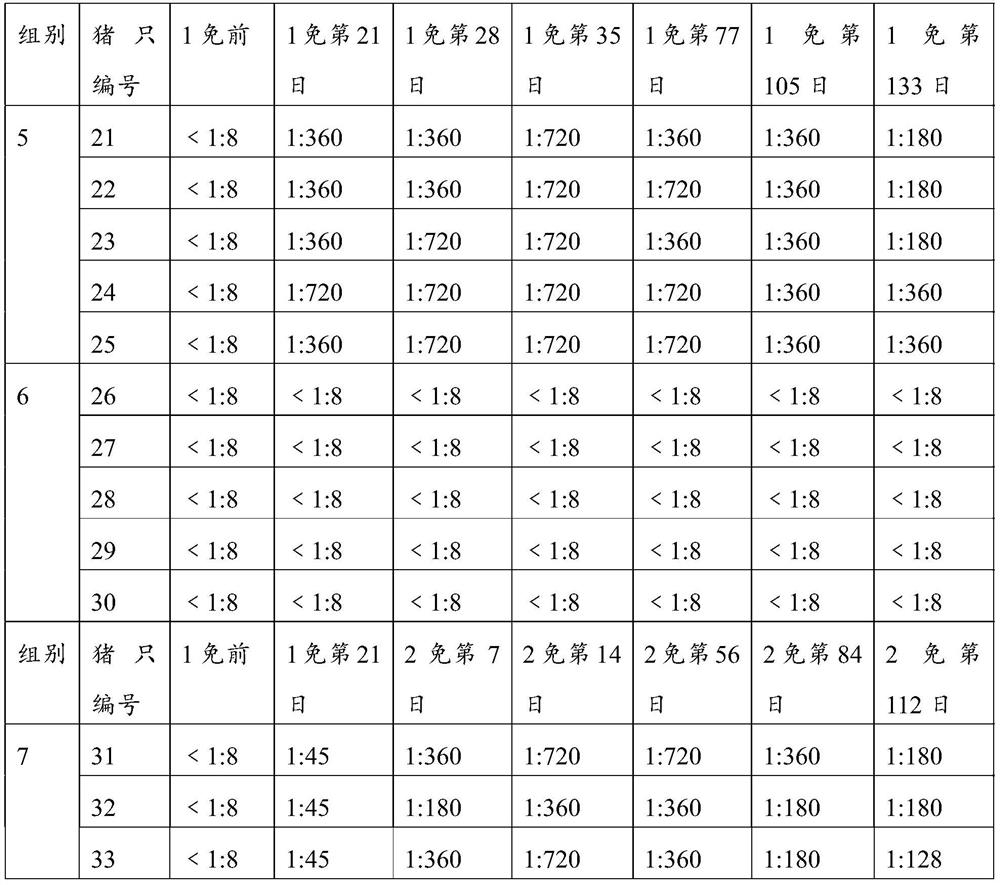
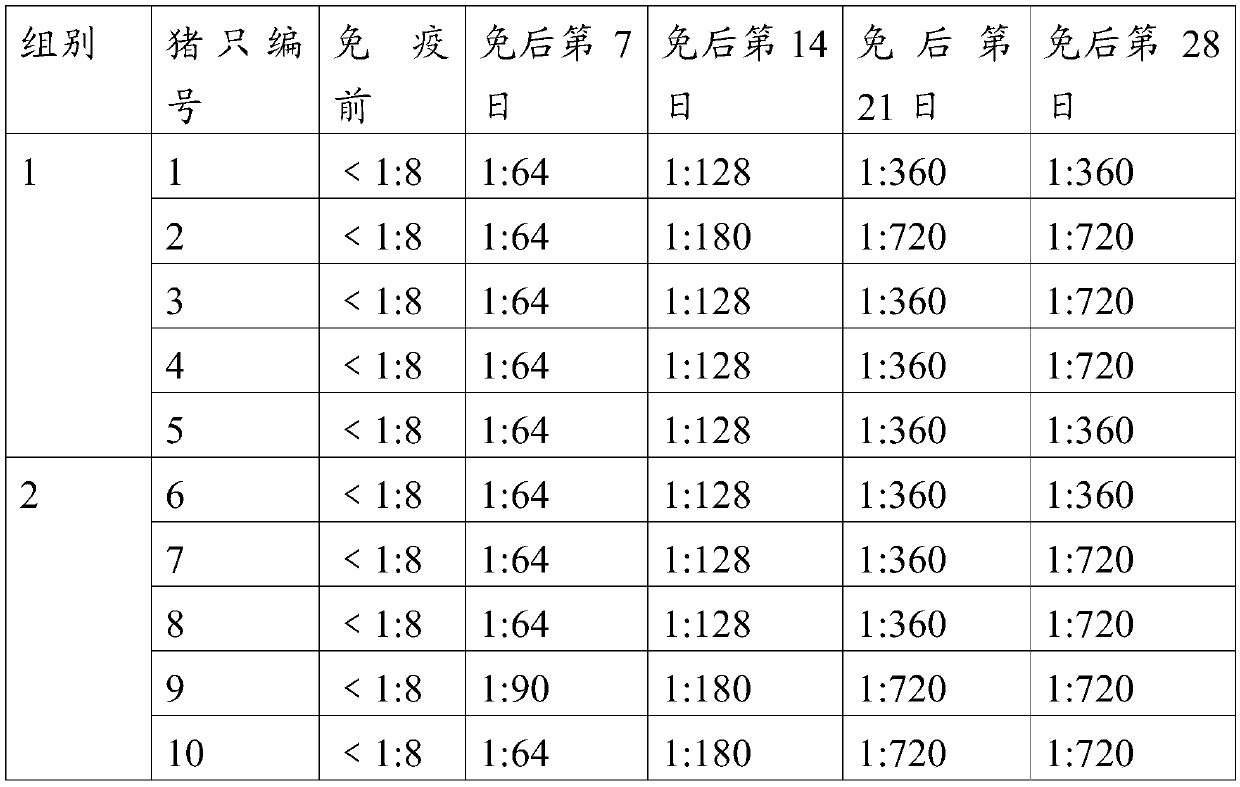
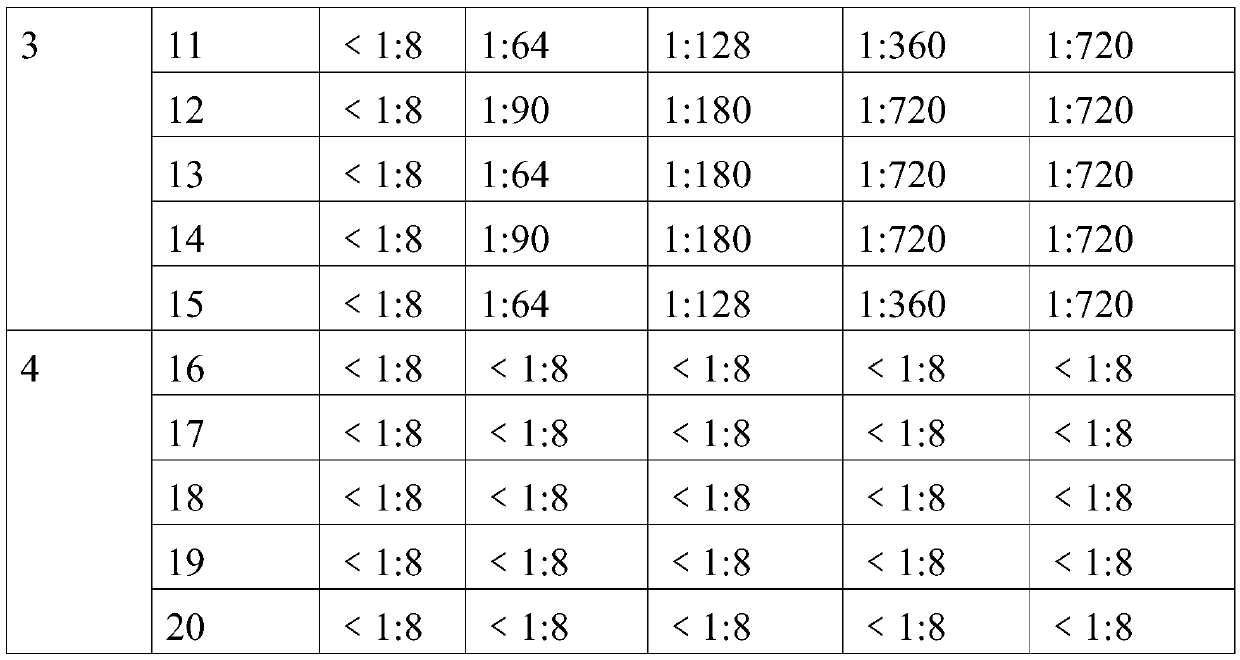
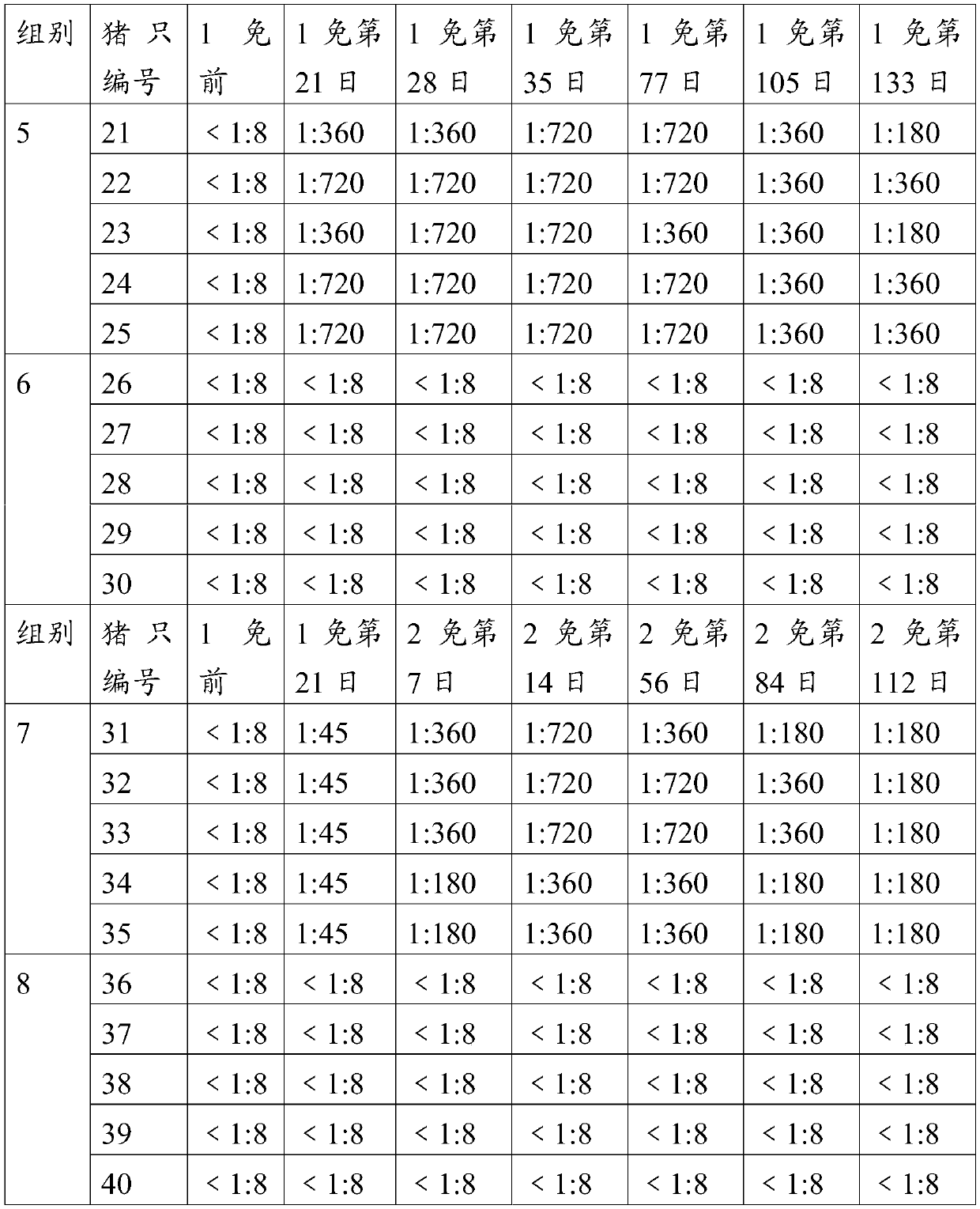
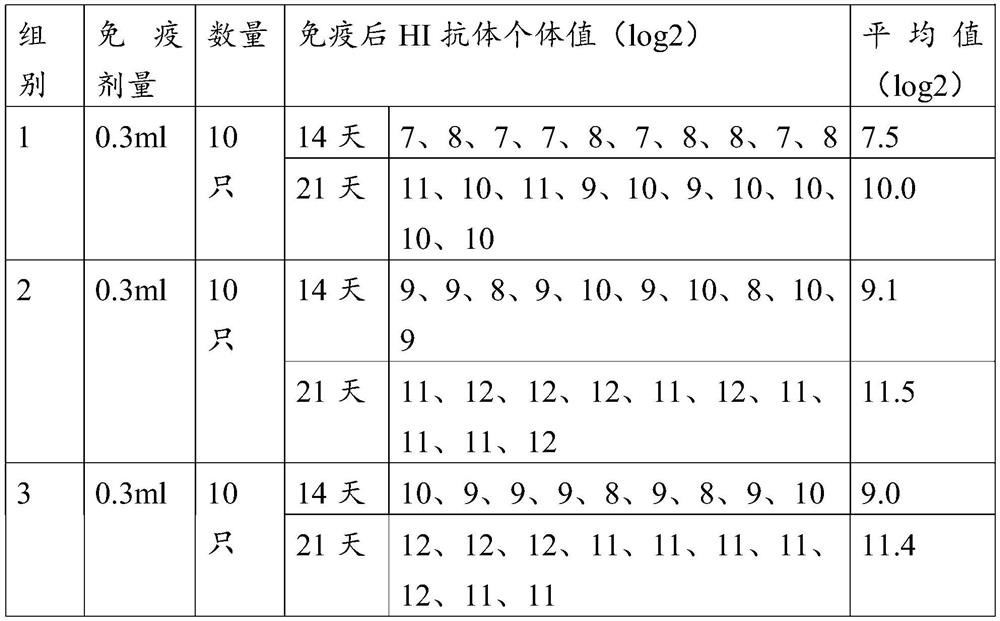
The invention provides an O-type foot and mouth disease virus-like particle antigen. The O-type foot and mouth disease virus-like particle antigen is a CATHAY-type O-type foot and mouth disease virus-like particle antigen and the CATHAY-type O-type foot and mouth disease virus-like particle antigen is composed from the composition of CATHAY-type O-type foot and mouth disease virus VP0, VP3 and VP1antigen proteins. The O-type foot and mouth disease virus-like particle antigen has good immunogenicity, a prepared vaccine can realize primary immunization, complete protection against O-type foot and mouth disease virus can be realized on the 14th day after immunization, the titer of a generated antibody is higher than the titer of a commercial inactivated vaccine, and the protective immune period can last for at least 133 days. The invention further relates to a prepared vaccine composition and a preparation method thereof and application.
Owner:PU LIKE BIO ENG
Vaccine composition for resisting H7 subtype avian influenza virus as well as preparation method and application of vaccine composition
PendingCN113827713AImprove stabilityIncrease productionSsRNA viruses negative-senseViral antigen ingredientsImmune effectsParticulate antigen
The invention relates to a vaccine composition for resisting H7 subtype avian influenza virus, the vaccine composition comprises an immune dose of H7 subtype avian influenza virus-like particle antigen and a pharmaceutically acceptable carrier, and the H7 subtype avian influenza virus-like particle antigen is assembled by H7 subtype avian influenza virus HA, NA and M1 antigen proteins. The vaccine composition for resisting the H7 subtype avian influenza virus can achieve the immune effect of an inactivated vaccine with a higher immune dose when the immune dose is low, the high-titer antibody is generated in a shorter time, the poultry are protected, the vaccine composition is good in stability, and the stability and durability of immunogenicity of the vaccine composition are advantageously guaranteed.
Owner:PU LIKE BIO ENG
Recombinant prrsv virus-like particle antigen-antibody complex and preparation method thereof
ActiveCN113425838BImprove impactHigh titerSsRNA viruses positive-senseViral antigen ingredientsParticulate antigenImmune complex deposition
In the present invention, by modifying the GP5-M protein, a recombinant baculovirus capable of efficiently replicating is obtained, and its culture titer is relatively higher, and the virus-like particle similar to the prepared PRRSV virion is further compounded with IgM to obtain IgM ‑RPPSV VLPs immune complex, the IgM‑RPPSV VLPs immune complex can stimulate the body to produce a high concentration of antibodies earlier.
Owner:NORTHWEST A & F UNIV +1
Avian influenza virus-like particle antigen, vaccine, preparation method of avian influenza virus-like particle antigen and application of avian influenza virus-like particle antigen or vaccine
ActiveCN112079905AGood cross reactivityInhibition of replicationSsRNA viruses negative-senseAntibody mimetics/scaffoldsEmbryoNeutralizing antibody
The invention discloses an avian influenza virus-like particle antigen, a vaccine, a preparation method of the avian influenza virus-like particle antigen and application of the avian influenza virus-like particle antigen or the vaccine. A strain of recombinant baculovirus based on tandem expression of HA, NA and M1 genes of an H7N9 subtype avian influenza virus transfects insect cells and performs self-assembly to form the avian influenza virus-like particle antigen; and the virus-like particle antigen prepared according to the invention is used for preparing the vaccine. After a chicken group is immunized by the vaccine prepared according to the invention, HI antibodies, neutralizing antibodies and IgY antibodies with the higher titer can be generated, 100-percent clinical protection onthe H7N9 subtype highly pathogenic avian influenza virus can be provided, and virus expelling can be effectively inhibited. The rely on the chicken embryos is avoided; the production cost is low; theperiod is short; and higher protection capability can be induced only by once low-dose immunization. A foundation is laid for industrial production of VLP vaccines.
Owner:YANGZHOU UNIV
H7N9 subtype avian influenza virus-like particle vaccine preparation as well as preparation and application thereof
ActiveCN113827714AImprove cross-protection effectivenessEase of mass productionSsRNA viruses negative-senseVirus peptidesVariant strainTGE VACCINE
The invention belongs to the technical field of biology and particularly relates to an H7N9 subtype avian influenza virus-like particle vaccine preparation as well as preparation and application thereof. The H7N9 subtype avian influenza virus-like particle vaccine preparation provided by the invention comprises a recombinant chimeric protein HMN and H7N9 subtype avian influenza virus-like particles, wherein the recombinant chimeric protein HMN is formed by chimeric combination of avian influenza virus conservative epitopes, bee venom signal peptides and 6x-His tag proteins. The recombinant chimeric protein HMN is combined with an H7N9 subtype avian influenza virus-like particle antigen, so that the defect that an avian influenza virus-like particle vaccine is insufficient in cross protection effect on variant strains is overcome, complete protection is provided for attack of the H7N9 subtype variant strains, and thus, the avian influenza epidemic situation is more effectively prevented and controlled.
Owner:SOUTH CHINA AGRI UNIV
Humanized neutralizing antibody (RVFab8) against rabies virus glycoprotein
Owner:NCPC NEW DRUG RES & DEV
Phytol derived immunoadjuvants and their use in vaccine formulations
ActiveUS20060292163A1Improving immunogenicityInduce immunogenic responseBiocideHydroxy compound active ingredientsParticulate antigenSide effect
This invention relates to a novel immunoadjuvant, an adjuvant component, and vaccines containing the adjuvant component. The adjuvant includes phytol or a phytol derivative. The adjuvant component, when combined with a soluble or particulate antigen, provides a vaccine with an enhanced ability to induce both humoral and cytotoxic immune responses while displaying reduced toxicity and / or adverse side effects over vaccines that include the antigen but without the benefit of this adjuvant component.
Owner:INDIANA STATE UNIVERSITY
A kind of water-based gel chromatography medium and method for detection
ActiveCN103706340BLow priceLow technical requirements for productionOther chemical processesBiological testingFicollParticulate antigen
Owner:SUZHOU INST OF BIOMEDICAL ENG & TECH CHINESE ACADEMY OF SCI
Humanized neutralizing antibody (RVFab5) against rabies virus glycoprotein
The invention discloses a humanized neutralizing antibody (RVFab5) against rabies virus glycoprotein, which is obtained through screening by utilizing phage display technology. The antibody specifically identifies the granule antigen of the rabies virus, is against the rabies virus glycoprotein G, has obvious immunofluorescence reaction and enzyme linked immunosorbent assay with the rabies virus and has the neutralizing activity function against rabies virus infection. The antibody can be prepared into the specific antibody drugs for preventing and treating rabies, thereby being clinically used for preventing and treating rabies caused by the rabies virus.
Owner:STATION OF VIRUS PREVENTION & CONTROL CHINA DISEASES PREVENTION & CONTROL CENT
A kind of avian influenza virus-like particle antigen, vaccine and its preparation method and application
ActiveCN112079905BGood cross reactivityInhibition of replicationSsRNA viruses negative-senseAntibody mimetics/scaffoldsBird fluEmbryo
The invention discloses an avian influenza virus-like particle antigen, a vaccine and its preparation method and application. It is formed by self-assembly of a recombinant baculovirus transfected insect cells based on serial expression of H7N9 subtype avian influenza virus HA, NA, and M1 genes Bird flu virus-like particle antigen; the virus-like particle antigen prepared by the present invention is used to prepare vaccine. The vaccine prepared by the invention can produce higher titers of HI antibody, neutralizing antibody and IgY antibody after immunizing chickens, can provide 100% clinical protection against H7N9 subtype highly pathogenic avian influenza virus, and can effectively inhibit shedding . The invention does not rely on chicken embryos, has low production cost and short cycle; only one immunization with a low dose can induce strong protective ability. The invention provides a basis for the industrialized production of VLP vaccines.
Owner:YANGZHOU UNIV
Celine parvovirus-like particle as well as preparation method and application thereof
PendingCN114573666AImprove securityStimulate immunityViral antigen ingredientsVirus peptidesFeline parvovirusEngineered genetic
The invention discloses a cat parvovirus-like particle and a preparation method and application thereof.A VP2 sequence of an autonomously separated FPV JL-125 strain is selected for preparing a recombinant baculovirus FPV-VP2 strain, the FPV JL-125 strain and a current Chinese prevalent strain have high homology, after the VP2 sequence is optimized according to insect cell codons, the VP2 sequence is converted into a recombinant baculovirus FPV-VP2 strain, and the recombinant baculovirus FPV-VP2 strain is converted into a recombinant baculovirus FPV-VP2 strain. The prepared recombinant baculovirus FPV-VP2 strain can be used for correctly expressing the FPVVP2 protein. According to the invention, a full suspension culture process is adopted for culture, the expression quantity is greatly improved, and the HA titer can reach 220-221, which is 256-512 times of the culture titer of wild viruses. The expressed protein can be autonomously assembled into complete FPV virus-like particles, the space structure of the protein is similar to that of an original virus, and the virus-like particles are high in titer and higher in safety and have the advantages of stimulating humoral immunity and cellular immunity at the same time. According to the invention, the virus-like particle antigen is prepared by using a genetic engineering means, the novel cat parvovirus-like particle vaccine is prepared, and the vaccine has higher safety and effectiveness.
Owner:CHANGCHUN SR BIOLOGICAL TECH
Design of broad-spectrum rabies virus-like particle antigen and stable expression cell strain HEK-293 thereof
PendingCN114262365ACorrect glycosylation structureMaintain immunogenicityAntiviralsDepsipeptidesProtein targetBinding site
The preparation method comprises the following steps: firstly, designing a broad-spectrum rabies virus-like particle antigen by using RVLPs, which is self-assembled by glycoprotein RVGP and matrix protein RVMP of a CVS strain rabies virus RABV, as an antigen; then, the RVGP, the RVMP and the EGFP are jointly constructed into a eukaryotic expression vector pcDNA3.1 (+), and the eukaryotic expression vector is named as pcDNA3.1 (+)-RVLPs-EGFP. Each protein has an independent promoter (CMV), a ribosome binding site (Kozak sequence) and a PloyA tail (PA) so as to ensure the surface level of each protein, and meanwhile, EGFP (enhanced green fluorescent protein) is used for monitoring the expression of the target protein in real time so as to construct a eukaryotic recombinant expression plasmid; and then transfecting HEK-293 cells with the eukaryotic recombinant expression plasmids, carrying out amplification and passage on the cells, screening and identifying, and screening to obtain the positive monoclonal cell strain HEK-293 / RVLPs capable of stably and efficiently expressing RVLPs. The obtained antigen has post-translational modification of mammalian cells, the defect that RVLPs derived from bacteria, yeast, insects and plant cells show low immunogenicity due to incorrect glycosylation modification is overcome, and a foundation is laid for large-scale production of the RVLPs antigen.
Owner:EAST CHINA UNIV OF SCI & TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com