Monoclonal antibody for detecting coxsackie virus a16 type virus solid particles and use thereof
A monoclonal antibody, Coxsackie virus technology, applied in the direction of antibodies, antiviral agents, antiviral immunoglobulin, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
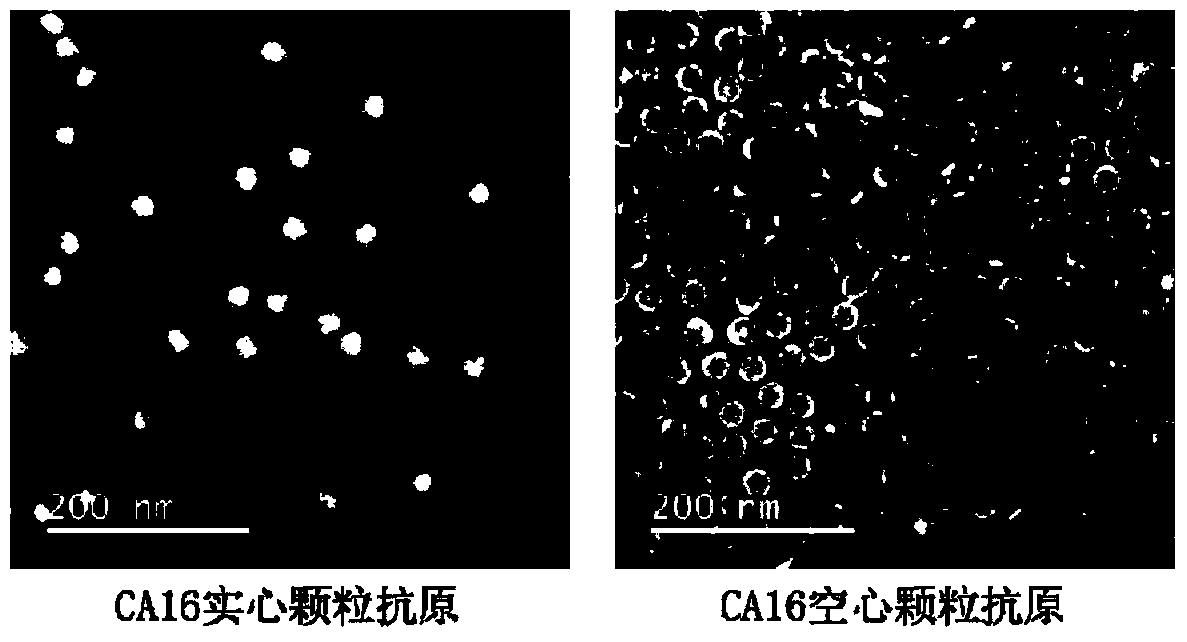
[0038] Example 1: Preparation of CA16 virus solid particles
[0039] The cultivation of virus: the CA16 strain used in the present invention is 2007-00190 (GenBank No. JF420555), and the cell used for cultivating virus is human rhabdoid cell RD ( CCL-136 TM ). First, RD cells were cultured in a 10 cm culture dish (NEST), and the medium was MEM medium (GIBCO) containing 10% fetal bovine serum (PAA). When the cell confluency reaches 80%, replace it with serum-free MEM medium, and inoculate the virus according to the amount of MOI=0.1. Culture at 37°C, and after 3 days, the virus was harvested after the cells were completely damaged. The virus harvesting method is as follows: the cells were scraped off and then frozen and thawed 3 times, the cell debris was removed by centrifugation, the cell lysed supernatant was collected, filtered through a 0.22 μm filter membrane to obtain the virus stock solution, and stored at -80°C for later use.
[0040] Sucrose density gradient cent...
Embodiment 2
[0042] Embodiment 2: Preparation of monoclonal antibody
[0043] The CA16 virus stock solution prepared in Example 1 was mixed and emulsified evenly with Freund's complete adjuvant, and multi-point injections were performed on 6-8 week-old BALB / c female mice, including back subcutaneous injection, groin subcutaneous injection, foot pad injection, limb muscle injection The immunogen was injected by injection and other ways, and the injection dose was 500 μL / time. After that, boost once every 2 weeks, and boost immunization in the same way. The immunogen is the mixture of the CA16 virus stock solution prepared in Example 1 and Freund's incomplete adjuvant. Before each immunization, 20 μL of tail vein blood or 200 μL of ocular vein blood were collected for titer determination. The serum titer was measured by indirect ELISA. When the serum titer of the mice reached a plateau, the mice were stopped from immunization and rested for two months before fusion. 72 hours before the fus...
Embodiment 3
[0045] Example 3: Screening of antibodies that can specifically bind CA16 virus solid particle antigens
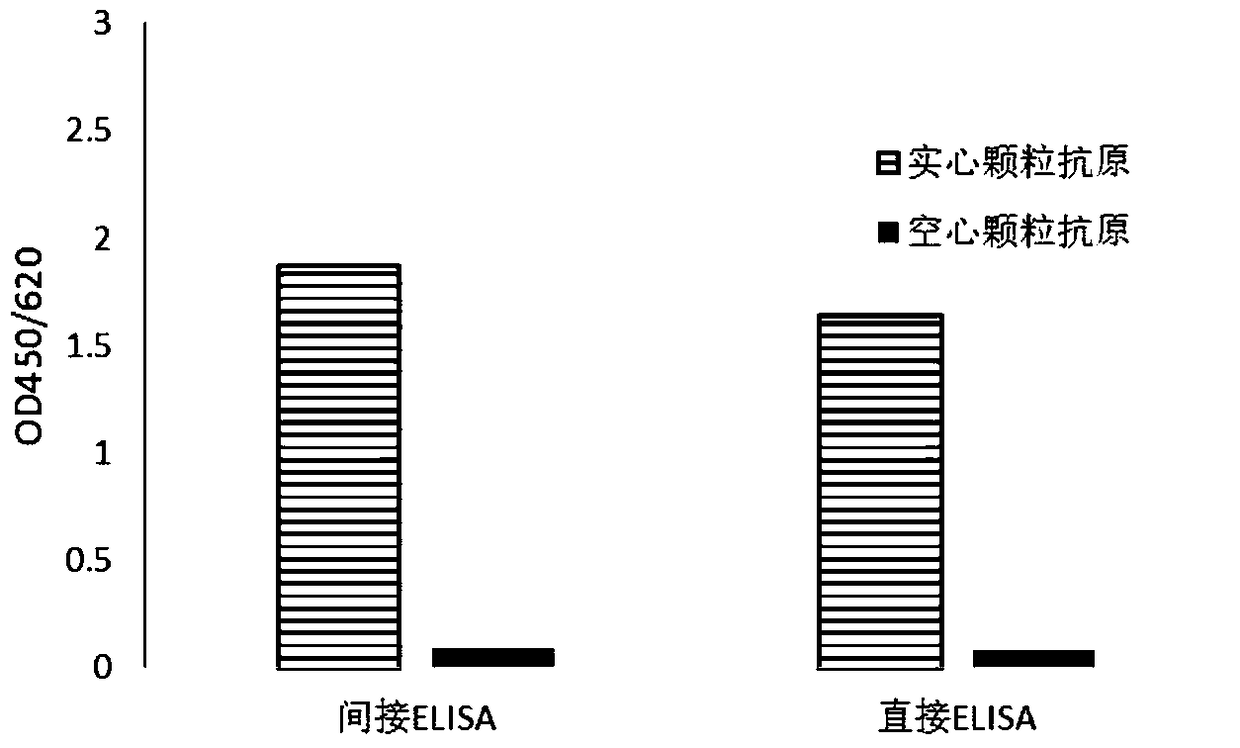
[0046] Indirect ELISA experiment: Dilute the different CA16 virus particle antigens prepared in Example 1 100 times with 20mM PB7.4, coat a 96-well microtiter plate, 100 μL / well, coat at 37°C for 2 hours, wash the plate once with PBST , with relevant blocking solution (20mM PB7.4 containing 150mM NaCL, 0.5% casein, 0.002% gelatin) to block non-specific binding sites, 200 μL / well, 4°C overnight. Dilute the monoclonal antibody sample to be tested (1 mg / mL) prepared in Example 2 by 200 times (the formula of the diluent is the same as that of the blocking solution), add 100 μL to the microtiter plate, and incubate at 37°C for 1 h. Wash the plate 5 times with PBST, add GAM-HRP (horseradish peroxidase-labeled goat anti-mouse antibody, purchased from bio-rad company in the United States, the diluent formula is the same as the blocking solution), and incubate at 37°C for 30min. Was...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com