Conservative neutralizing epitope polypeptide of Coxsackievirus A16 and application thereof
A Coxsackie virus, A16 technology, applied in antiviral agents, antiviral immunoglobulins, medical preparations containing active ingredients, etc., can solve the problem of unidentified neutralizing epitopes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] 1 Materials and methods
[0041] 1.1 Cells, viruses and serum
[0042] For the cultivation of RD cells and VERO cells, please refer to: Liu Q, Ku Z, Cai Y, Sun B, Leng Q, Huang Z. Detection, characterization and quantitation of coxsackievirus A16 using polyclonal antibodies against recombinant capsid subunit proteins. J Virol Methods 2011 Apr;173(1):115-20.
[0043] For the strain CA16-SZ05 (GeneBank ID: EU262658), please refer to: (1) Wu Z, Gao Y, Sun L, Tien P, Jin Q. Quick identification of effective small interfering RNAs that inhibit the replication of coxsackievirus A16. Antiviral Res 2008 Dec;80(3):295-301 and (2) Yang F, Jin Q, He Y, Li L, Hou Y. The complete genome of Enterovirus 71 China strain. Sci China C Life Sci 2001 Apr;44( 2): 178-83.
[0044] Strain CA16-G08 was isolated from a fecal sample of a HFMD patient. For the isolation procedure, please refer to the literature: Li L, He Y, Yang H, Zhu J, Xu X, Dong J, Zhu Y, Jin Q. Genetic characterization of...
Embodiment 2
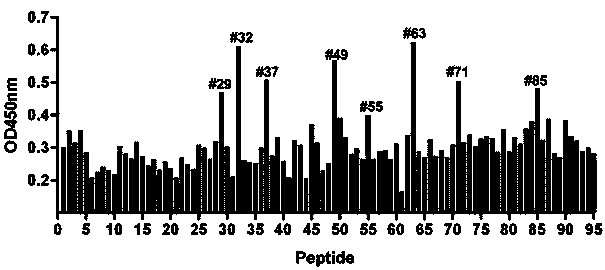
[0085] 1. Antigen: KLH-coupled synthetic polypeptide, the amino acid sequence of which is as described in SEQ ID NO.1-6.
[0086] 2. Immunization of mice: purebred BALB / C mice, aged 6-8 weeks. 50 μg KLH-conjugated synthetic peptide was injected intraperitoneally, and Freund's complete adjuvant was used for initial immunization. The same antigen dose was immunized with Freund's incomplete adjuvant every two weeks, and immunized three times in total. Mice were sacrificed two weeks after the last immunization for blood collection. All animal operations complied with the IACUC regulations of the Shanghai Pasteur Institute. Blood was collected from the mice during the immunization process, and ELISA was used to detect the antibody expression in the mouse serum, so as to judge the immune effect of the mice.
[0087] 3. Fusion with SP2 / 0 myeloma cell line: kill the immunized mice, and take splenocytes. Myeloma cells SP2 / 0 and mouse splenocytes were mixed at a ratio of 1:10, and ...
Embodiment 3
[0092] 1. Selection and acquisition of antigen: Synthesize the polypeptide with amino acid sequence as described in SEQ ID NO.1-6, add His tag to the nucleic acid sequence corresponding to the polypeptide, and construct it into the prokaryotic expression vector Pet28a. The expression vector of the polypeptide was transformed into Escherichia coli BL21 for expression, and the soluble protein supernatant was purified with a nickel column. The concentration of the peptide obtained after purification was measured, and the peptide was detected by western detection with His antibody. The western test is positive, ready to immunize animals;
[0093] 2. Animals to be immunized: Two New Zealand rabbits were selected, each time immunized with 200 μg of immunogen, Freund's complete adjuvant was used in the initial stage, and incomplete adjuvant was used in the later stage. Immunization was carried out at 0, 2, 4, 5, 6, 7, 8, and 9 weeks respectively, and from the 5th week, venous blood ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com