Patents
Literature
64 results about "Coxsackievirus A" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
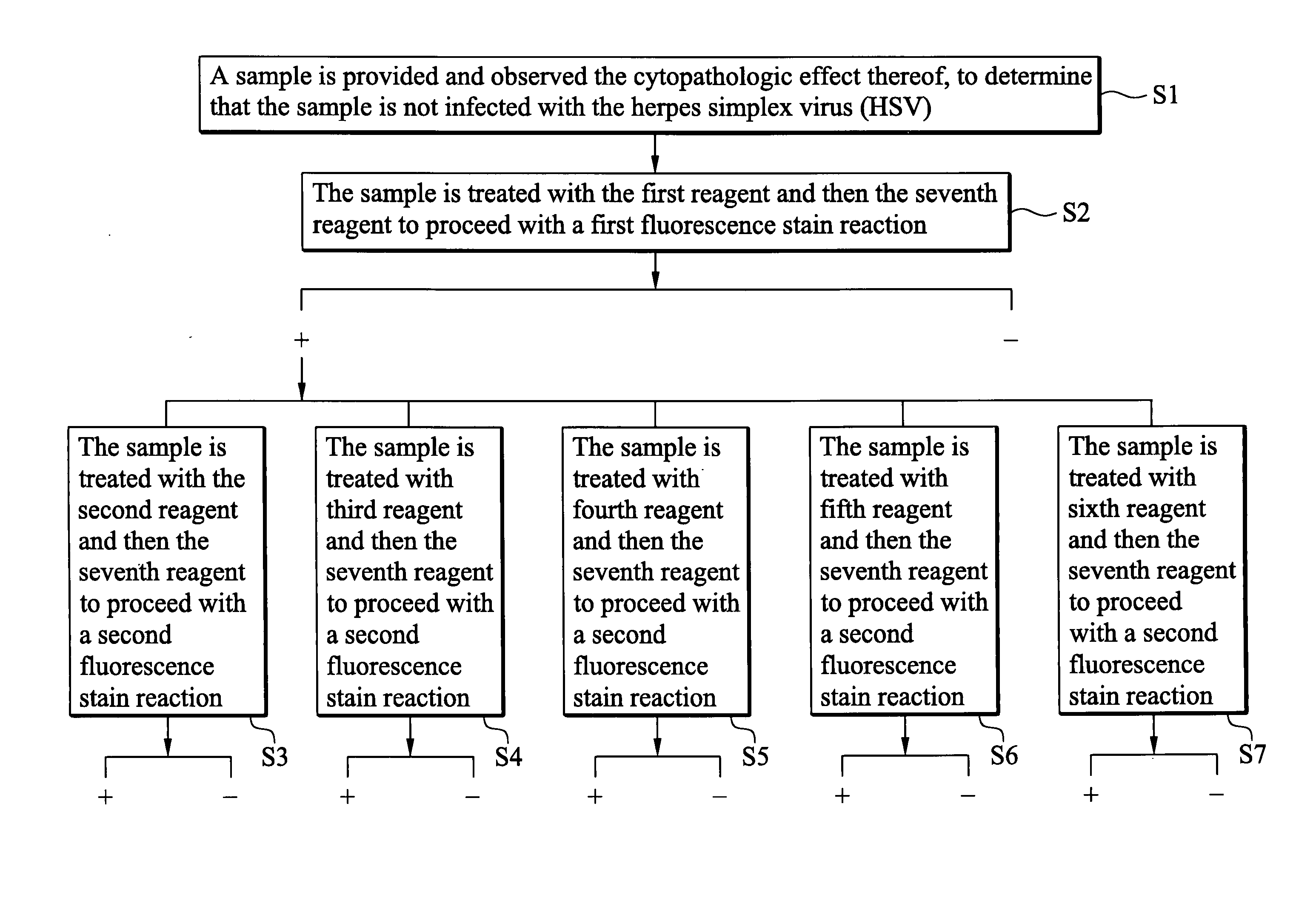
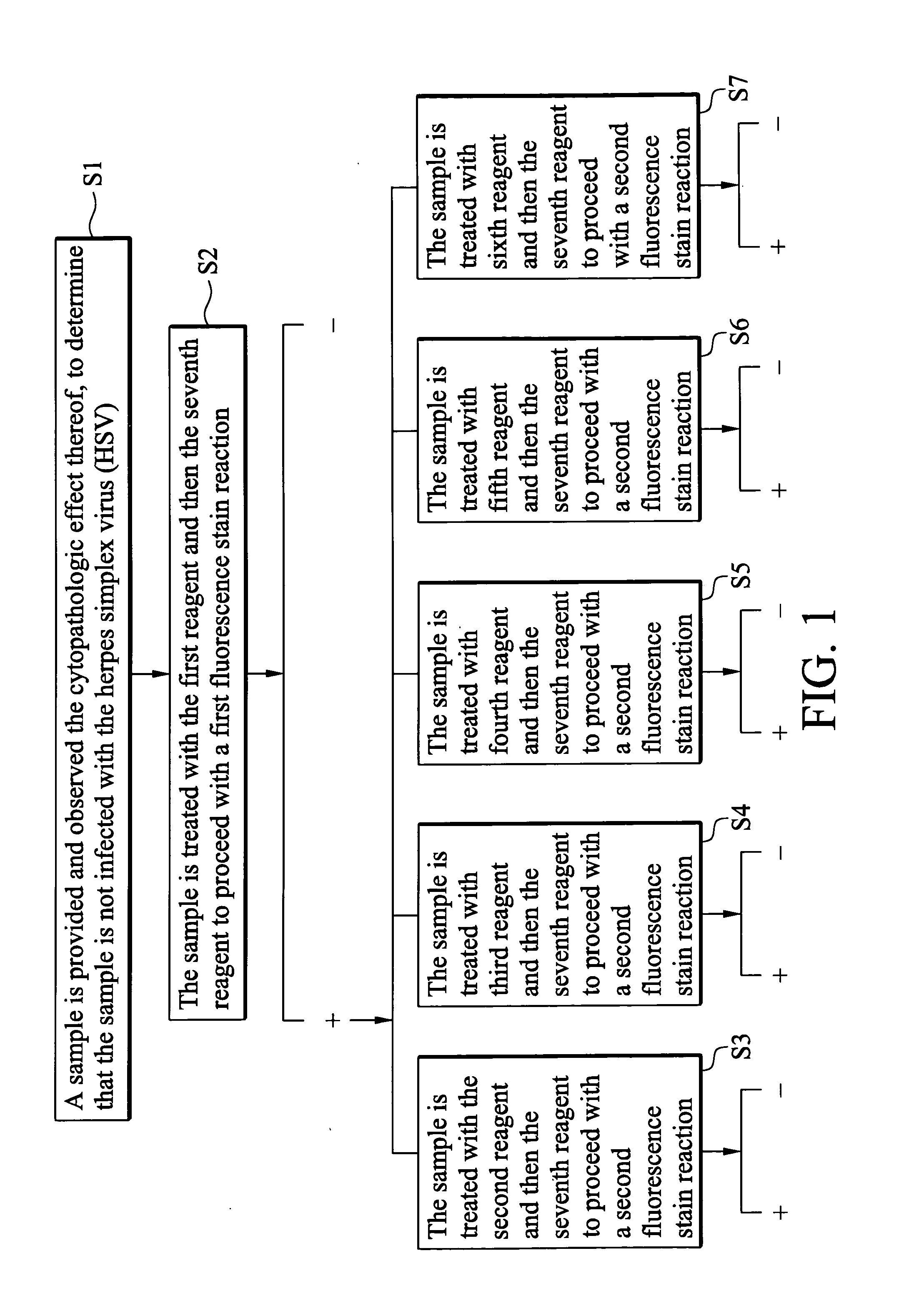
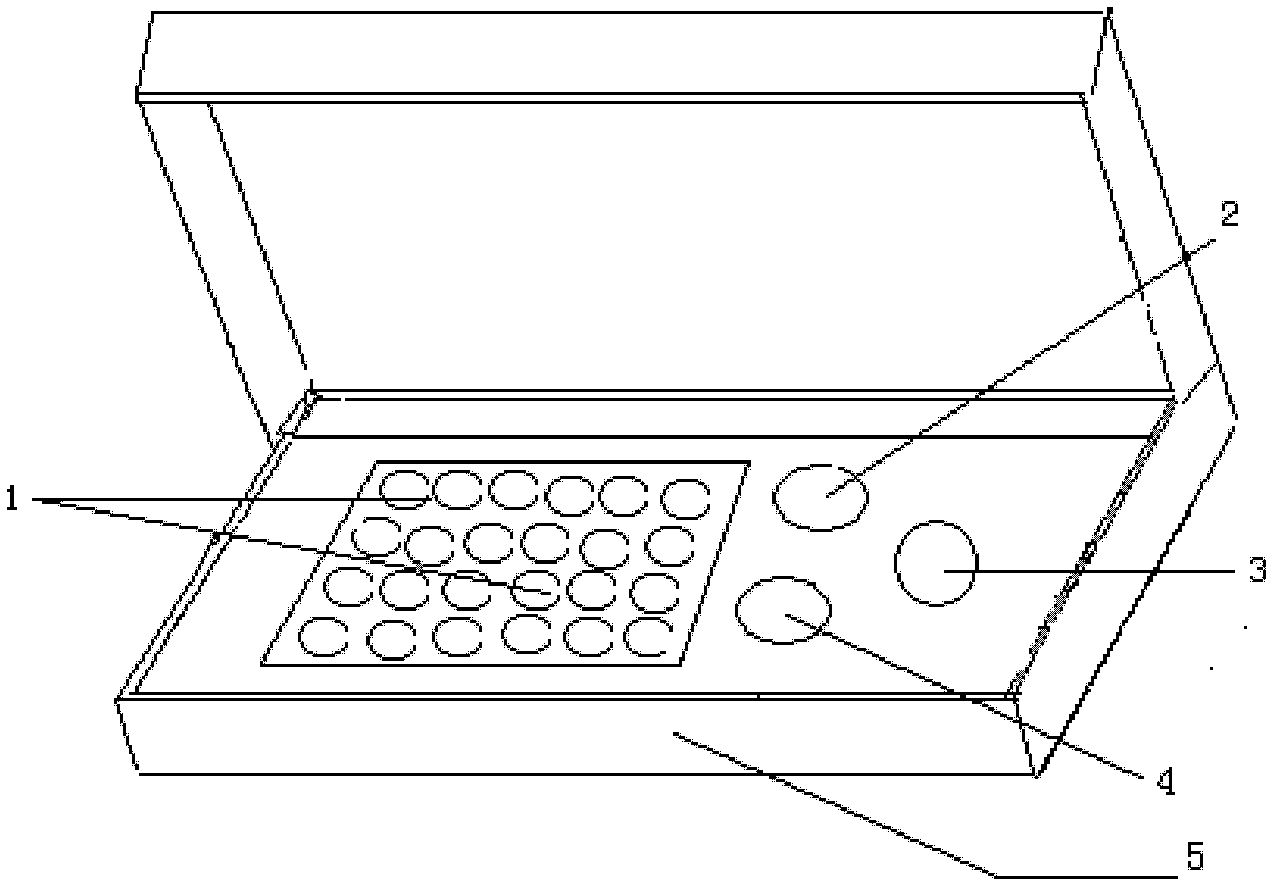
Indirect immunofluorescence assay typing kit for coxsackievirus A group and method for typing coxsackievirus A group
InactiveUS20090317796A1Microbiological testing/measurementBiological material analysisImmunofluorometric AssaysTyping
An indirect immunofluorescence assay typing kit for coxsackievirus, comprising: a first reagent comprising a mixture of an anti-coxsackievirus A2 polyclonal antibody, an anti-coxsackievirus A4 polyclonal antibody, an anti-coxsackievirus A5 polyclonal antibody, an anti-coxsackievirus A6 polyclonal antibody, and an anti-coxsackievirus A10 polyclonal antibody; a second reagent comprising the anti-coxsackievirus A2 polyclonal antibody; a third reagent comprising the anti-coxsackievirus A4 polyclonal antibody; a fourth reagent comprising the anti-coxsackievirus A5 polyclonal antibody; a fifth reagent comprising the anti-coxsackievirus A6 polyclonal antibody; a sixth reagent comprising the anti-coxsackievirus A10 polyclonal antibody; and a seventh reagent comprising a secondary antibody labeled with a fluorescence compound, wherein the secondary antibody is used for detecting the antibody anti-coxsackieviruses A2, A4, A5, A6 and A10 polyclonal antibodies and a titer of the anti-coxsackieviruses A2, A4, A5, A6 or A10 polyclonal antibody is about 1:5000-151:70000.
Owner:CENTS FOR DISEASE CONTROL DEPT OF HEALTH
Univalent and bivalent inactivated vaccine for hand-foot-and-mouth disease and preparation method thereof
InactiveCN101695570AInactivation/attenuationMicroorganism based processesHand-foot-and-mouth diseaseCoxsackievirus a16
The invention discloses a univalent and bivalent inactivated vaccine for preventing Enterovirus 71 (EV71) and Coxsackie virus A16 (Cox.A16) of hand-foot-and-mouth disease, and a preparation method thereof. The preparation method comprises the following steps: screening vaccine strains for the hand-foot-and-mouth disease; establishing and verifying a vaccine strain third-stage seed lot library; culturing cells; inoculating and propagating virus; collecting virus suspension; inactivating the virus; ultra-filtering, concentrating and purifying the virus suspension to obtain vaccine stock solution; and finally preparing the univalent and bivalent inactivated vaccine. The univalent and bivalent inactivated vaccine has good application prospect for preventing the hand-foot-and-mouth disease.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Univalent and bivalent gene engineered subunit vaccine for hand-foot-and-mouth disease and preparation method thereof
InactiveCN101695569AInactivation/attenuationMicroorganism based processesHand-foot-and-mouth diseaseCoxsackievirus a16
The invention discloses a univalent and bivalent gene engineered subunit vaccine for preventing Enterovirus 71 (EV71) and Coxsackie virus A16 (Cox.A16) of hand-foot-and-mouth disease, and a preparation method thereof. The preparation method comprises the following steps: respectively obtaining recombinant baculovirus Bac-EV71-P1-3CD and Bac-Cox.A16-P1-3CD by gene engineering means, respectively efficiently coexpressing similar SeQ ID No.1 EV71 P1 and Se Q ID No.2 Cox.A16 P1 and 3CD proteins in insect cells, and respectively self-assembling into EV71 VLP and Cox.A16 VLP; establishing and verifying a vaccine strain third-stage seed lot library; culturing cells; inoculating and propagating virus; lysing the cells, ultra-filtering and purifying virus suspension; and further preparing the univalent and bivalent vaccine. The vaccine has good application prospect for preventing the hand-foot-and-mouth disease.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
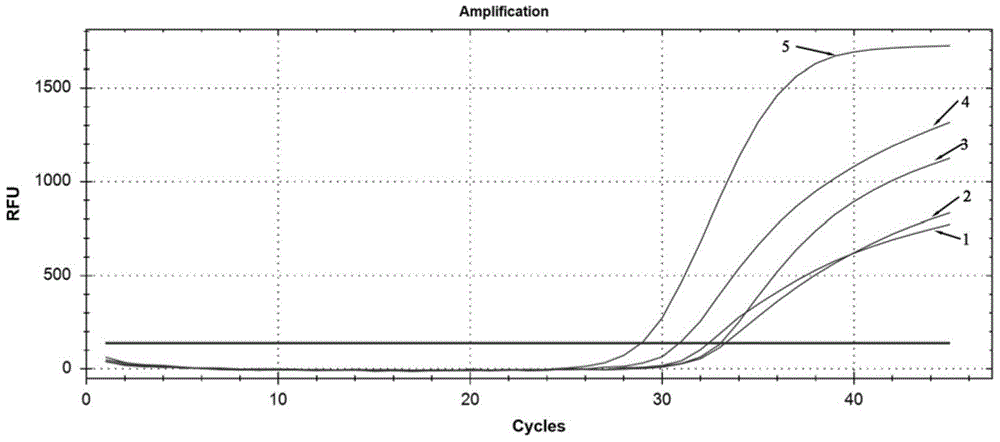
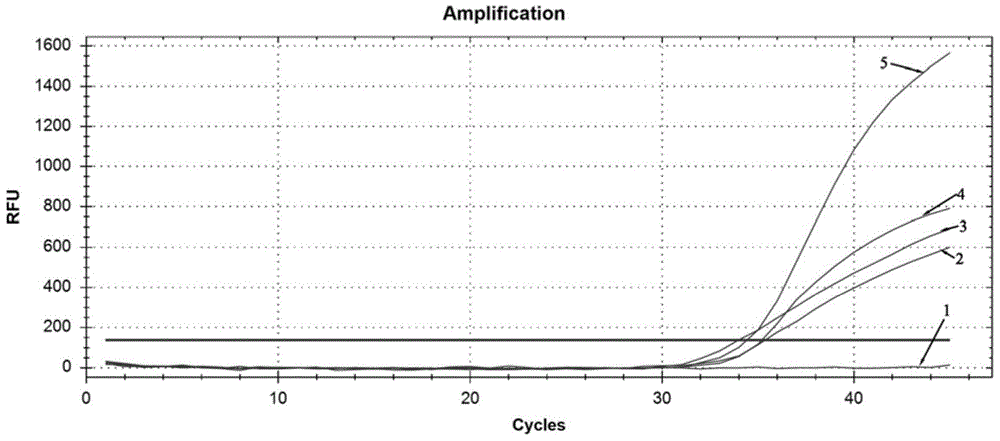
Three-color fluorescent RT-PCR (Reverse Transcription-Polymerase Chain Reaction) combined detection method of enterovirus 71, Coxsackie virus A16 and other subtypes of enterovirus as well as kit thereof
InactiveCN101886138AEasy to detectHigh sensitivityMicrobiological testing/measurementMicroorganism based processesCoxsackievirus a16Reverse transcription polymerase chain reaction
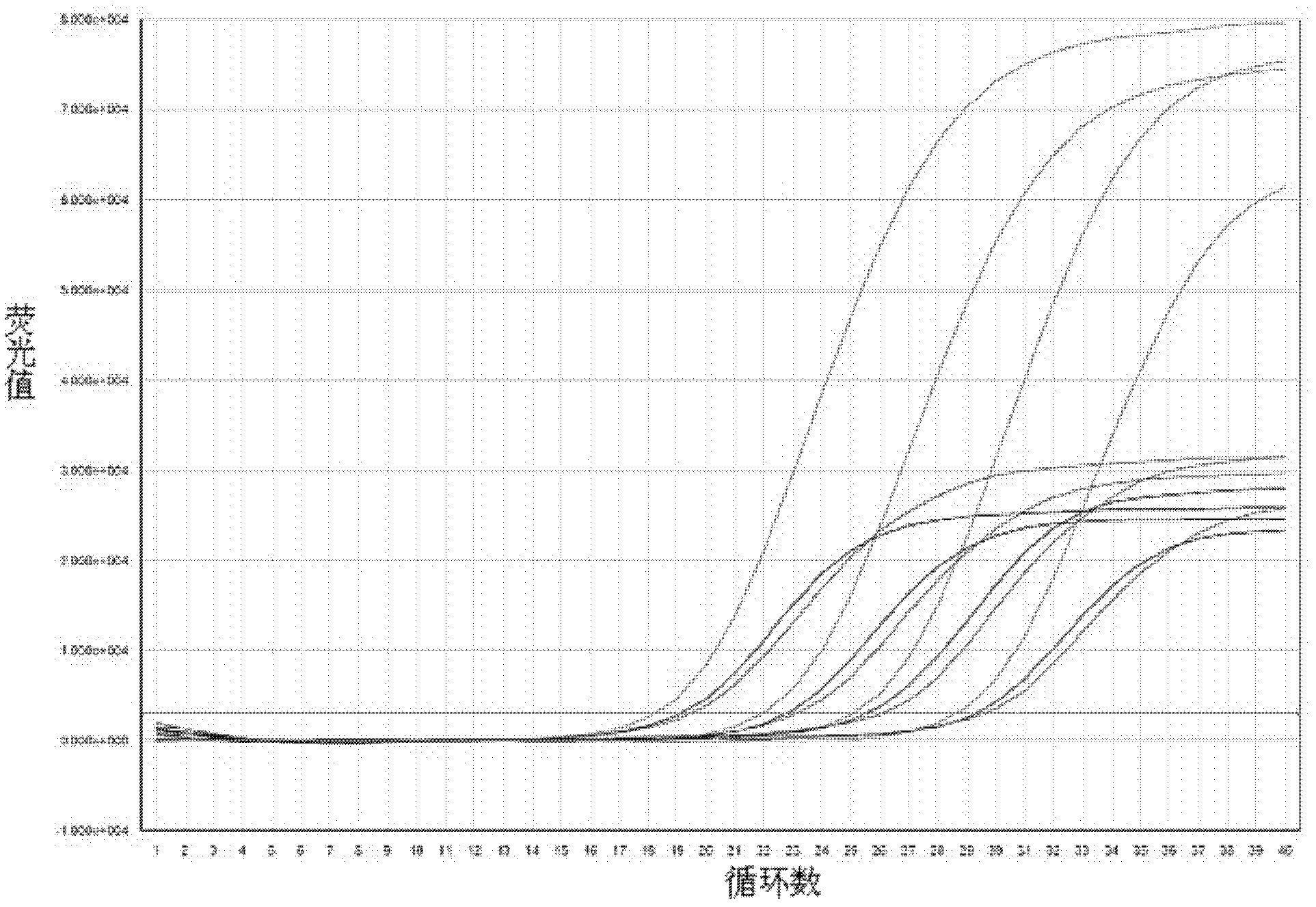
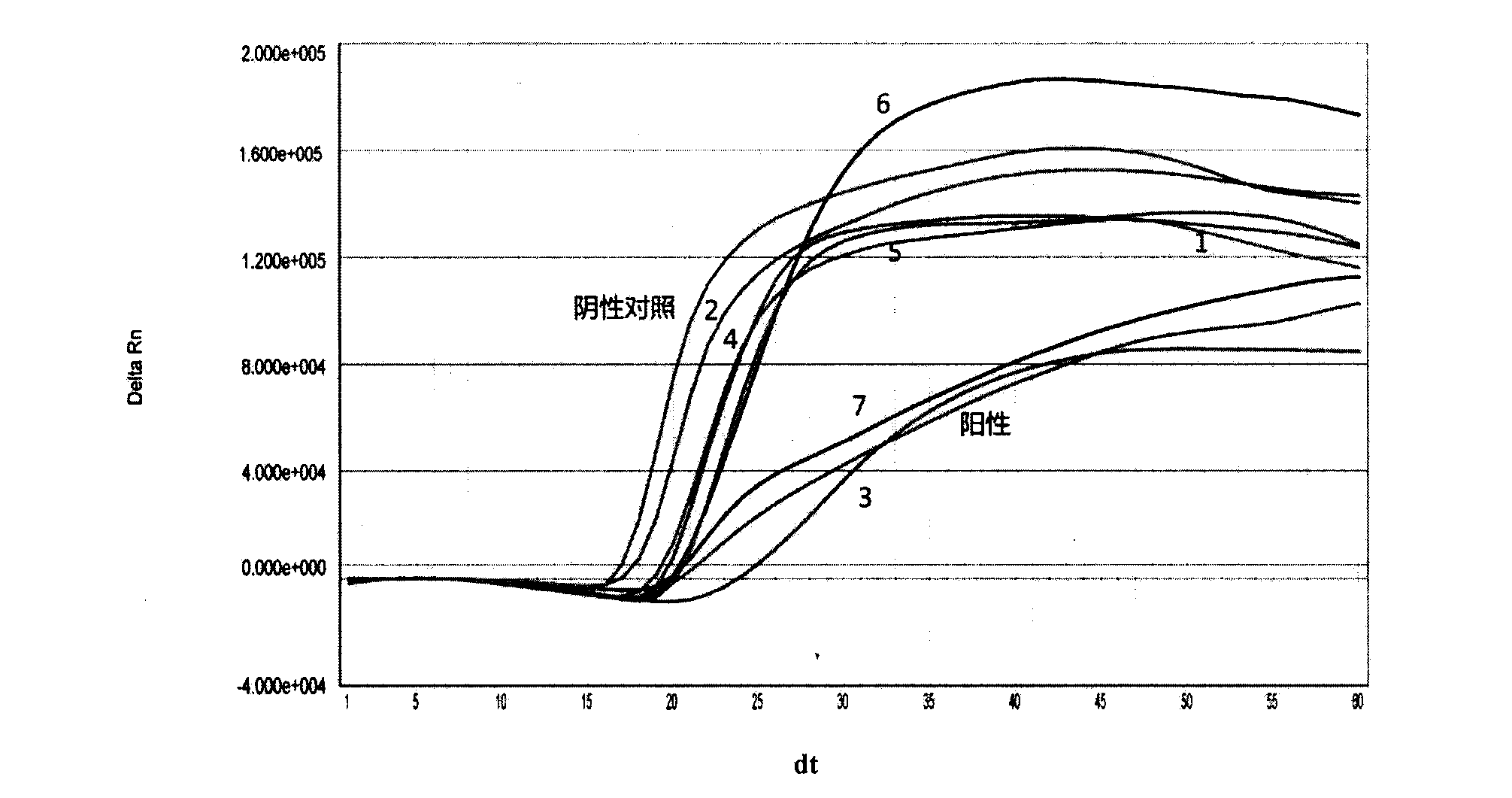
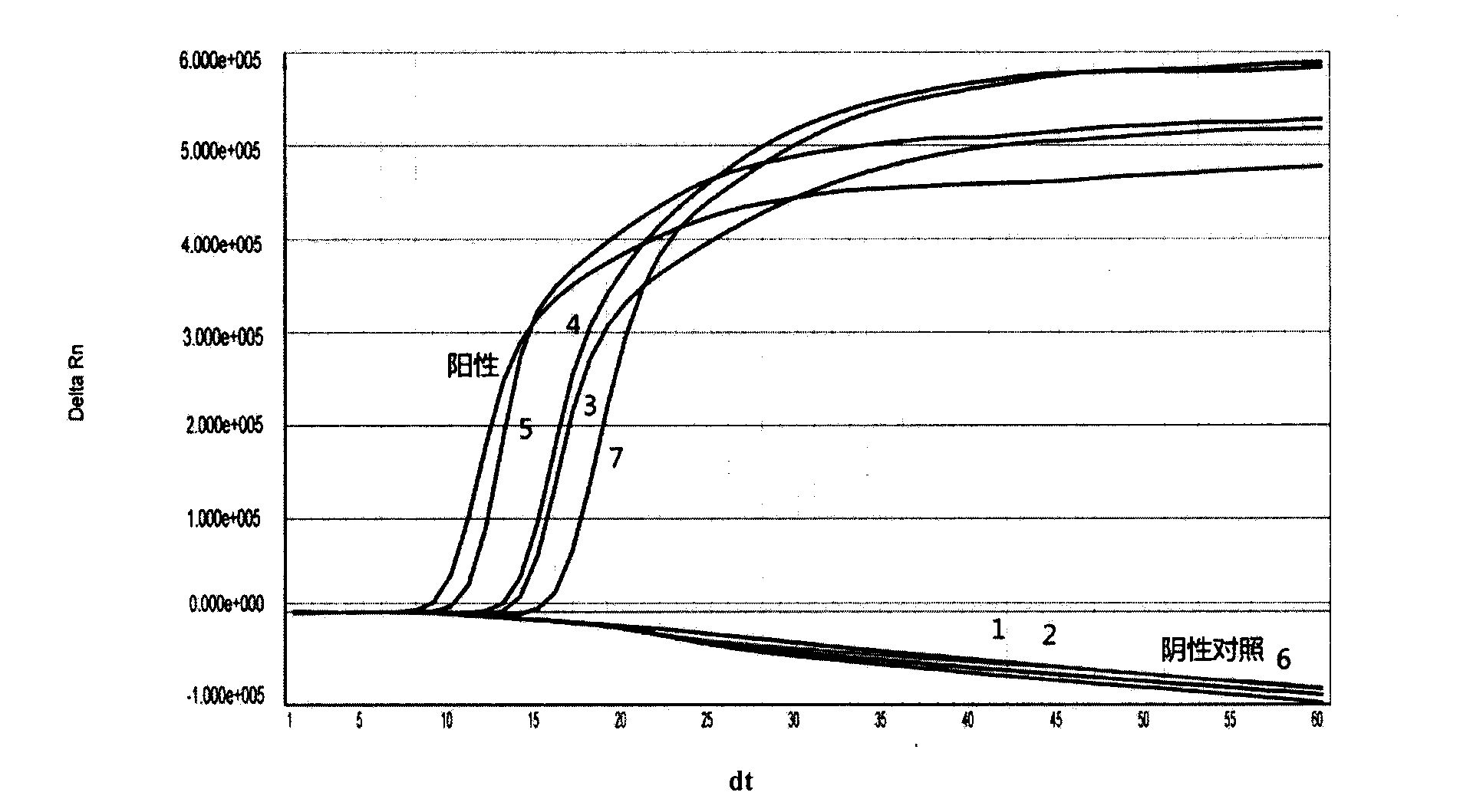
The invention provides a three-color fluorescent RT-PCR (Reverse Transcription-Polymerase Chain Reaction) combined detection method of enterovirus 71, Coxsackie virus A16 and other subtypes of enterovirus as well as a kit thereof. The method can rapidly and accurately detect the enterovirus 71, the Coxsackie virus A16 and the other subtypes of nucleic acids of enterovirus in a sample. The method comprises the following steps of: (1) acquiring and conveying a sample of an infected patient or a suspected patient; (2) preprocessing the sample and extracting RNA; (3) detecting the sample by adopting a one-step PCR-three-color fluorescent probe in-vitro amplification method; and (4) analyzing the corresponding sample according to the fluorescence intensity of each amplification reaction after the amplification reaction is finished, thereby judging the existence of the enterovirus 71, the Coxsackie virus A16 and the other subtypes of nucleic acids of the enterovirus in the acquired sample and being capable of carrying out accurate quantitation (a figure 3) on the enterovirus 71, the Coxsackie virus A16 and the other subtypes of nucleic acids of the enterovirus. The invention realizes the aim of carrying out rapid and accurate combined detection of the enterovirus 71, the Coxsackie virus A16 and the other subtypes of nucleic acids of the enterovirus.
Owner:BEIJING SUOAO BIOTECH
Gene-modified coxsackievirus
ActiveUS20160143969A1Good effectImprove securityBiocideSsRNA viruses positive-senseNucleotideVirotherapy
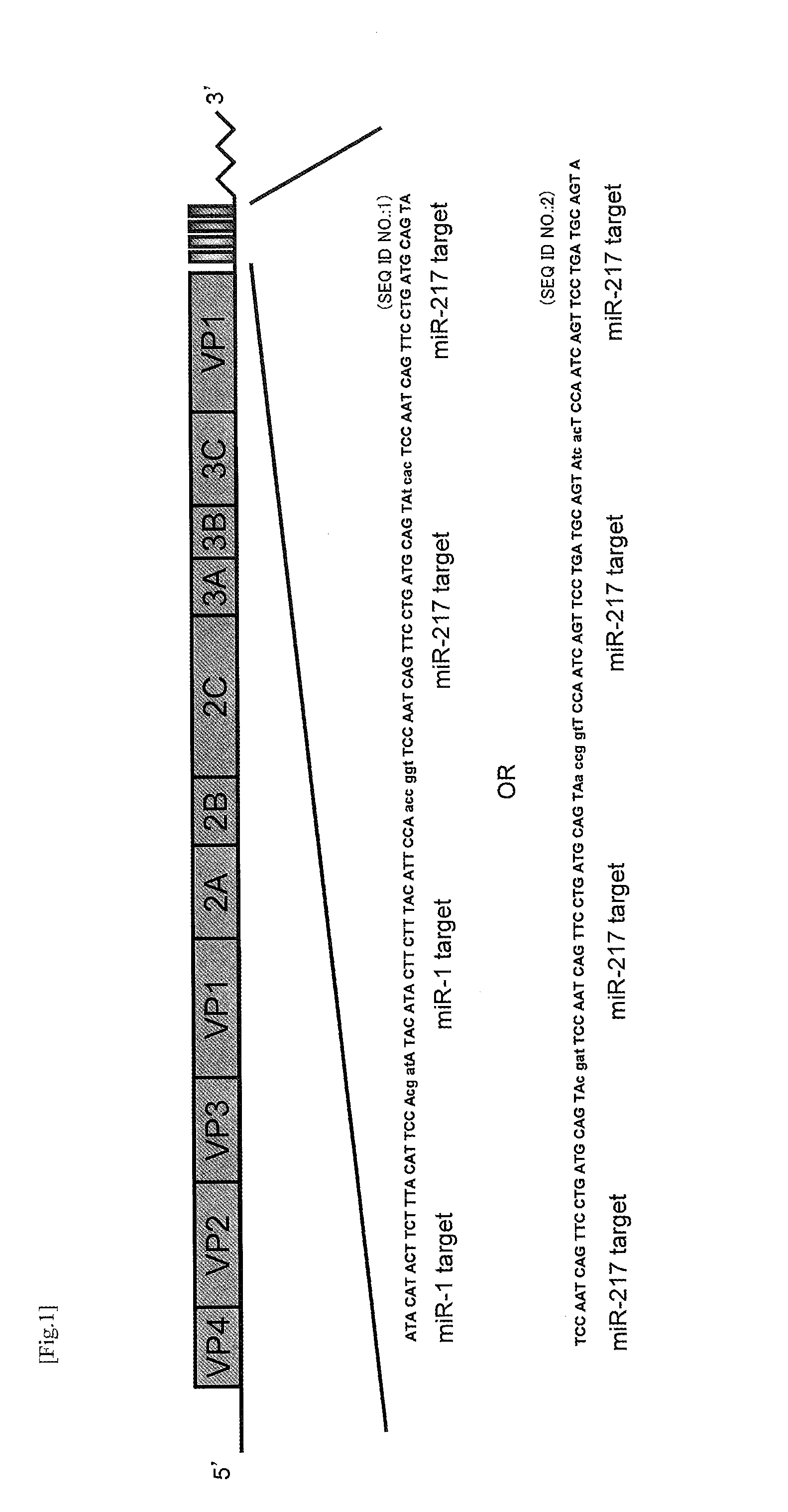
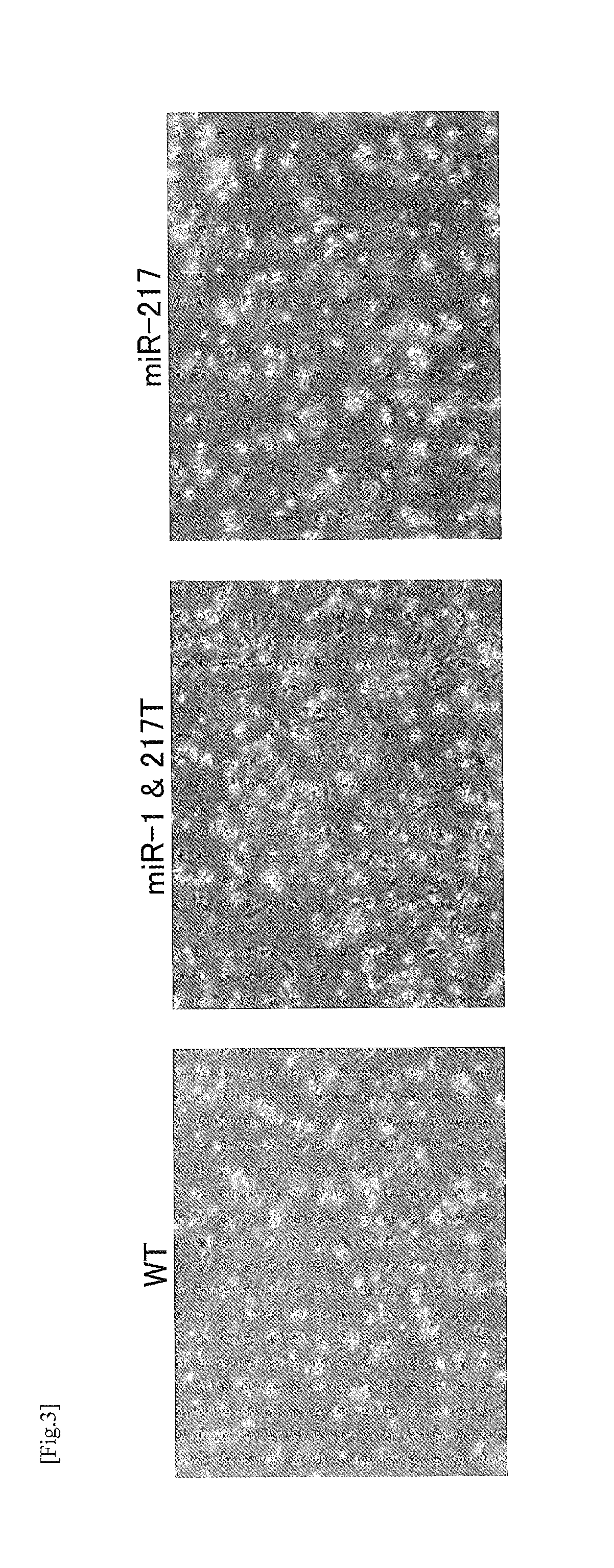
A modified coxsackievirus showing improved safety and / or aggressiveness to be used for oncolytic virotherapy is provided. A modified coxsackievirus showing tissue-specific suppression of proliferation and comprising a imitated genome consisting of the genome of coxsackievirus B3 wild-type (CVB3-WT) inserted with at least one polynucleotide consisting of a target sequence of tissue-specific microR NA (miRNA) is provided. The mutated genome is preferably further inserted with the region encoding GM-CSF in an expressible form.
Owner:NEOPRECISION THERAPEUTICS CO LTD +1
Preparation method for recombinant coxsackie virus A16 like particle and applications thereof
InactiveCN102533797AIncrease productionFungiInactivation/attenuationYeastHand-foot-and-mouth disease
The invention discloses a preparation method for a recombinant coxsackie virus A16 like particle, which comprises the following steps: (1) cloning a P1 gene and a 3CD gene of a coxsackie virus A16 to a target plasmid to obtain a recombinant expression vector; (2) transforming a target yeast cell by using the recombinant expression vector obtained in the step (1) to obtain a recombinant yeast cellfor expressing the P1 gene and the 3CD gene; and (3) cracking the recombinant yeast cell obtained in the step (2), and separating to obtain the recombinant coxsackie virus A16 like particle. The recombinant coxsackie virus A16 like particle can be prepared in a yeast expression system by the method provided by the invention. Compared with a wild-type P1 gene and a wild-type 3CD gene, the yield ofthe coxsackie virus A16 like particle in the yeast expression system is greatly increased through the optimization of codons of the P1 gene and the 3CD gene, and the recombinant coxsackie virus A16 like particle can be further used for producing candidate preventive vaccines and pharmaceutical compositions for infant hand-foot-and-mouth diseases.
Owner:BEIJING UNIV OF TECH
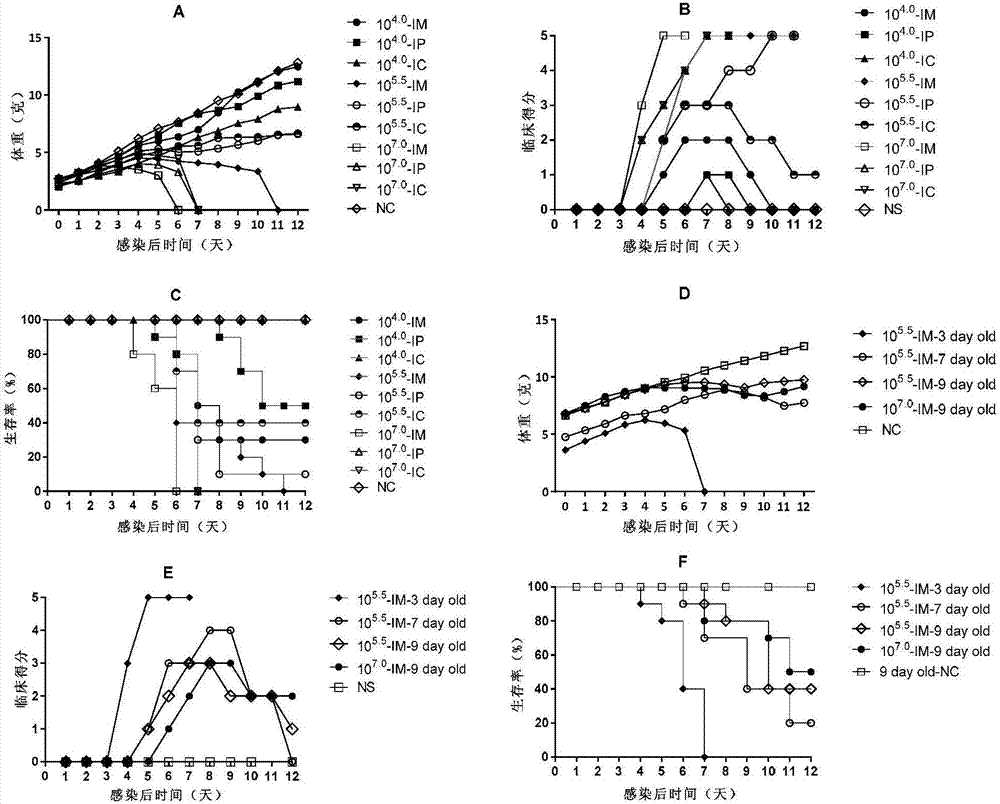
Coxsackie virus A16 virus strain, uses of strain, vaccine and preparation method of vaccine
ActiveCN104099301AEffective immune activityObvious paralysisSerum immunoglobulinsImmunoglobulins against virusesCoxsackievirus a16Antiviral drug
The invention provides a Coxsackie virus A16 virus strain, uses of the strain, a vaccine and a preparation method of the vaccine. The preservation number of the Coxsackie virus A16 virus strain is CGMCC No.6954. The CA16 virus strain has strong virulence, can be used for evaluating the CA16 vaccine, and can also be used for researching the CA16 virus infection mechanism. A method for establishing a Coxsackie virus A16 infected animal model provided by the invention can provide a stable animal model, and provides a foundation for the development of the Coxsackie virus A16 vaccine, the screening of antiviral drugs and the researches of the CA16 virus infection mechanism. The vaccine prepared by using the CA16 virus has effective immune activity.
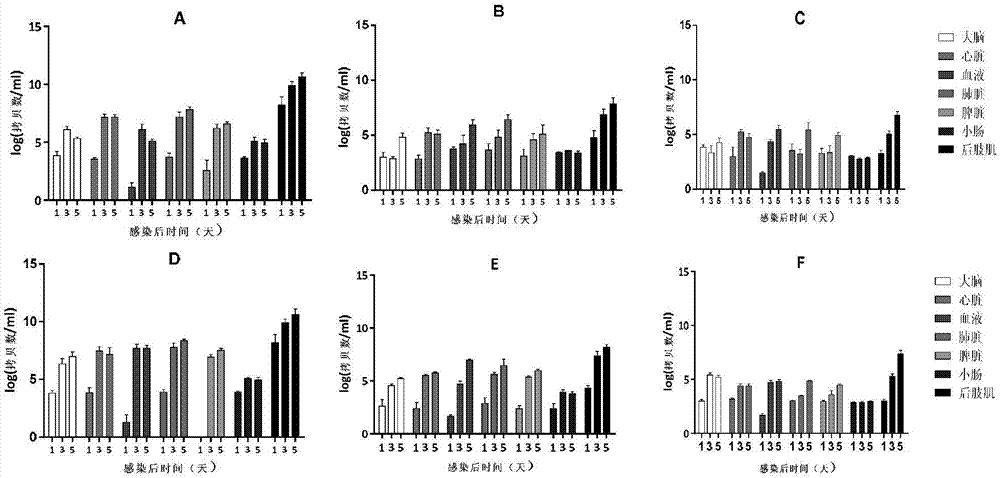
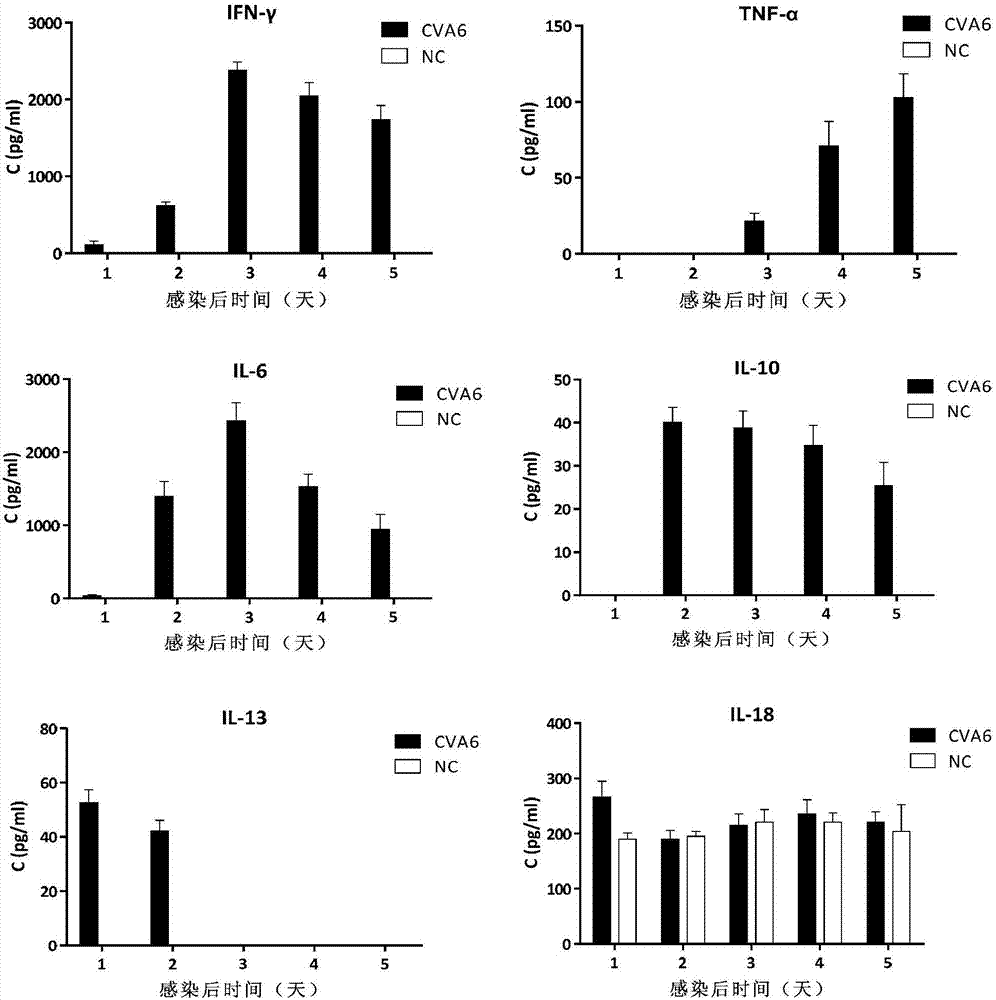
Coxsackievirus A6 type infected animal model as well as preparation method and application thereof
InactiveCN106867974AGood repeatabilitySsRNA viruses positive-senseMicroorganism based processesMicroorganismViral Vaccine
The invention discloses a coxsackievirus A6 type infected animal model as well as a preparation method and an application thereof. The preparation method of the coxsackievirus A6 type infected animal model provided by the invention comprises a step of infecting an animal with an inactivated coxsackievirus A6 type strain, so that the coxsackievirus A6 type infected animal model is obtained, wherein the coxsackievirus A6 type strain is preserved in China General Microbiological Culture Collection Center with preservation number of CGMCC No.13393. The coxsackievirus A6 type infected animal model, which is stable and is good in repeatability, is constructed on the basis of the CVA6 strain WF057R; and with the application of the model, a research tool is provided for next medicine antiviral therapy and assessment of an immune protective effect of an inactivated viral vaccine.
Owner:TAISHAN MEDICAL UNIV
Human embryo lung fibroblast strain and method for using human embryo lung fibroblast strain for producing hand-foot-mouth viral vaccine
InactiveCN102911910AAvoid Residual EffectsReduced purification stepsAntiviralsEmbryonic cellsEmbryoViral Vaccine
The invention provides a novel human embryo lung diploid fibroblast strain Walvax-2, CCTCCC201055. The cell strain is sensitive to main epidemic disease viral strains-coxsackievirus 16-type COX.A16 in a group A and enterovirus 71-type EV71 second-strain virus of a hand-foot-mouth disease, and virus output is high. The invention further provides a method for preparing a divalent hand-foot-mouth virus inactivated vaccine and application of the human embryo lung diploid fibroblast strain in preparation of the hand-foot-mouth virus inactivated vaccine. The divalent inactivated vaccine produced by the human embryo lung diploid fibroblast strain can effectively prevent hand-foot-mouth disease.
Owner:云南沃森生物技术股份有限公司
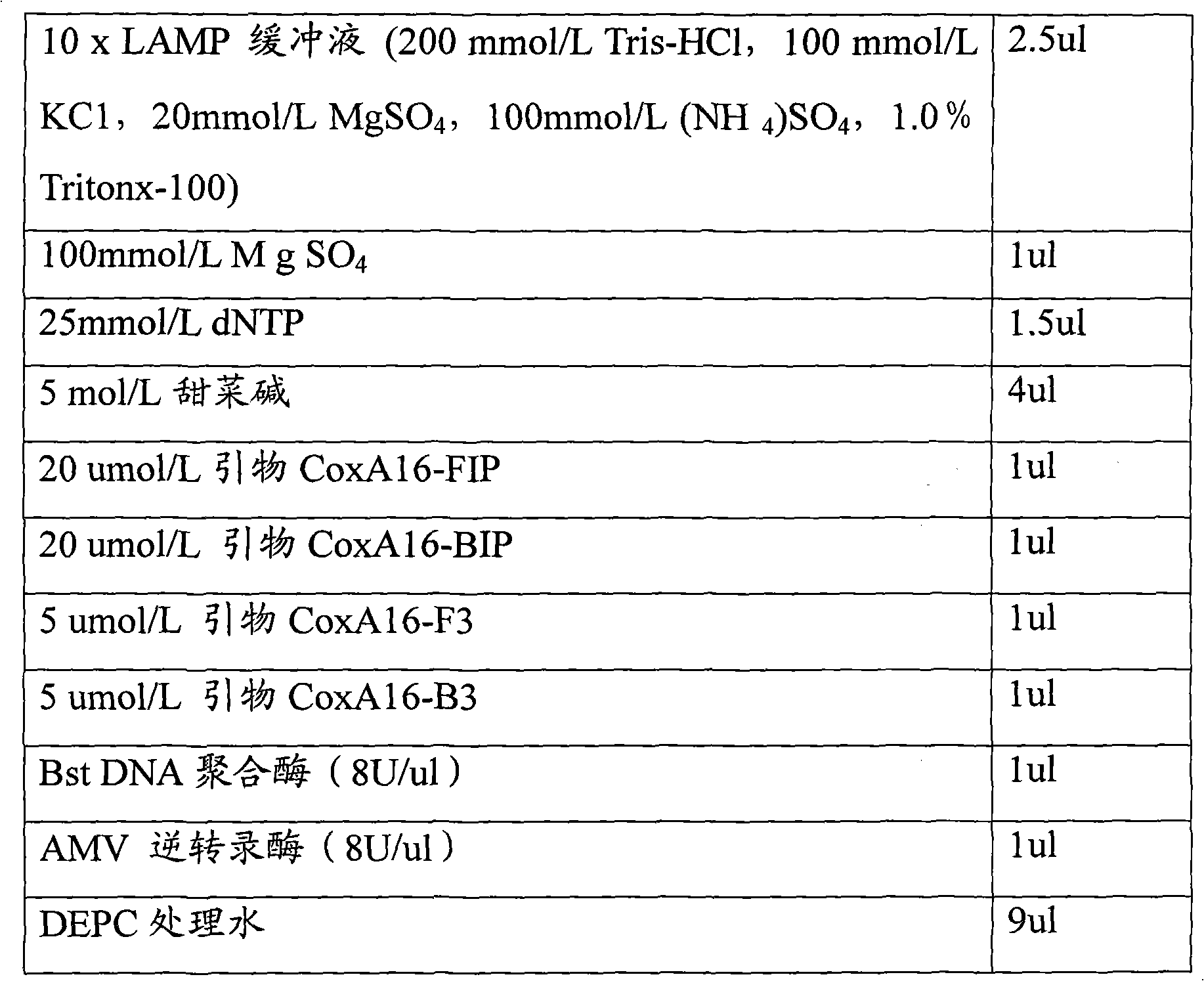
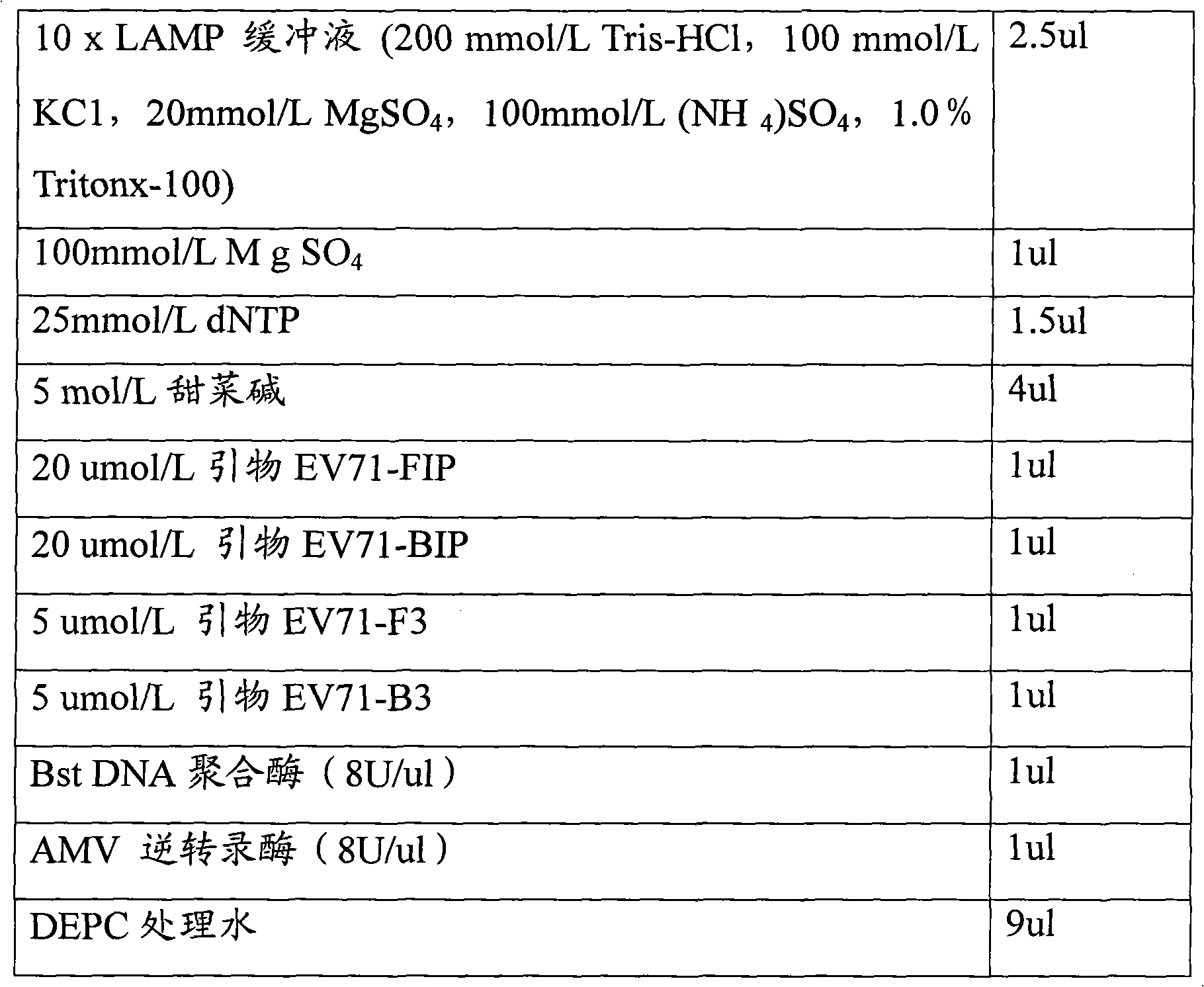
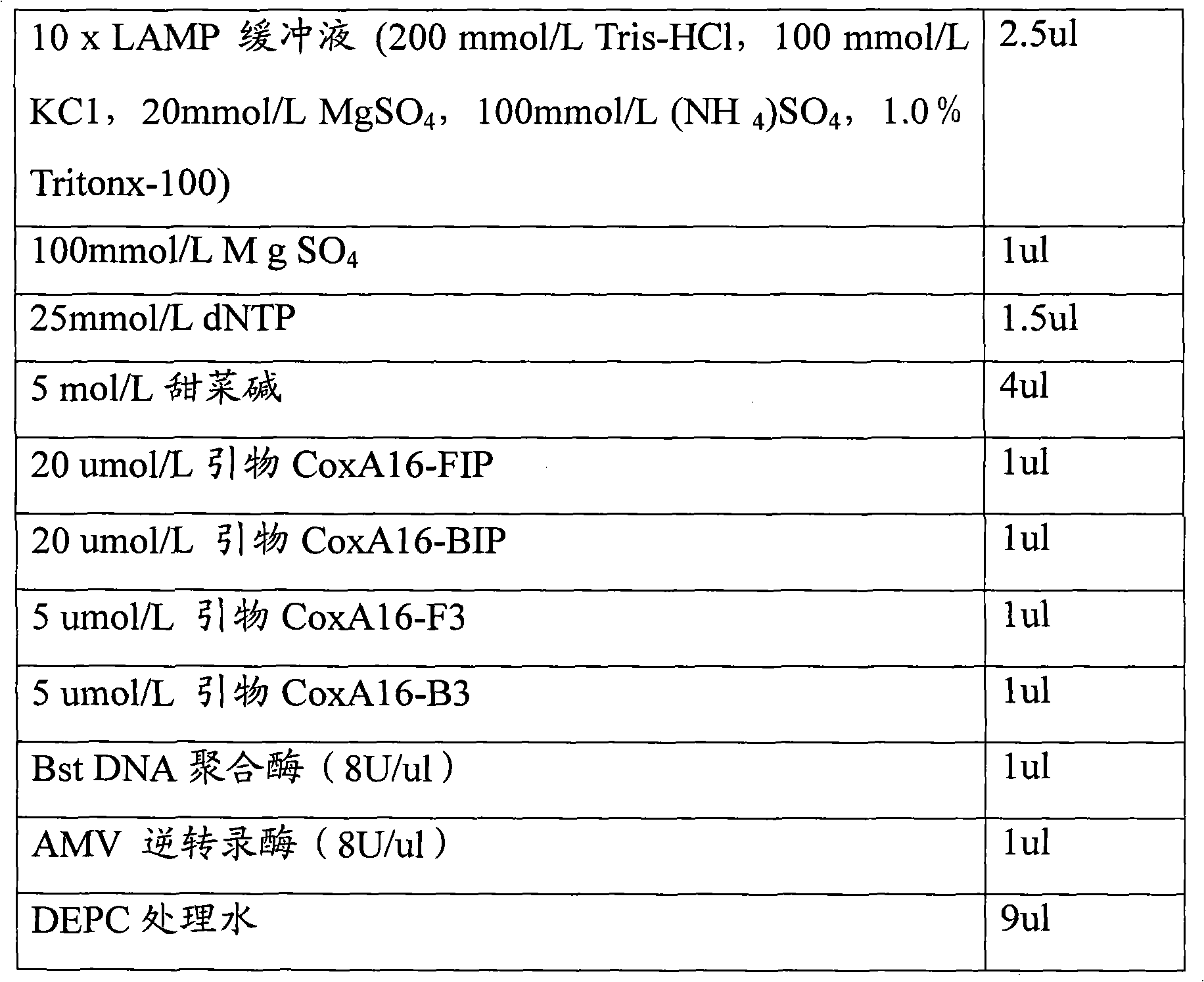
Loop-mediated isothermal amplification assay kit and detection method of hand, foot and mouth disease
InactiveCN102242223ASuitable for field applicationStrong specificityMicrobiological testing/measurementHand-foot-and-mouth diseaseCoxsackievirus a16
The invention belongs to the field of biotechnology, and relates to a loop-mediated isothermal amplification (LAMP) assay kit of Coxsackie A16 (Cox A16) and Enterovirus 71 (EV 71) which are main pathogens of hand, foot and mouth disease, and an establishment method and an application of the assay kit. The assay kit comprises four LAMP primers and LAMP reaction liquid for detecting Coxsackie A16, and four LAMP primers and LAMP reaction liquid for detecting Enterovirus 71. Tests prove that the assay kit has the advantages of good specificity and sensitivity, rapid amplification rapid, high efficiency and simple identification. A detection system provided by the invention can rapidly, conveniently, efficiently, high specifically and high sensitively detect Coxsackie A16 and Enterovirus 71 without complex apparatuses thus can satisfy well clinical detection requirements of hand, foot and mouth disease, and is suitable for a large-scale promotion and an application.
Owner:上海吉美生物工程有限公司
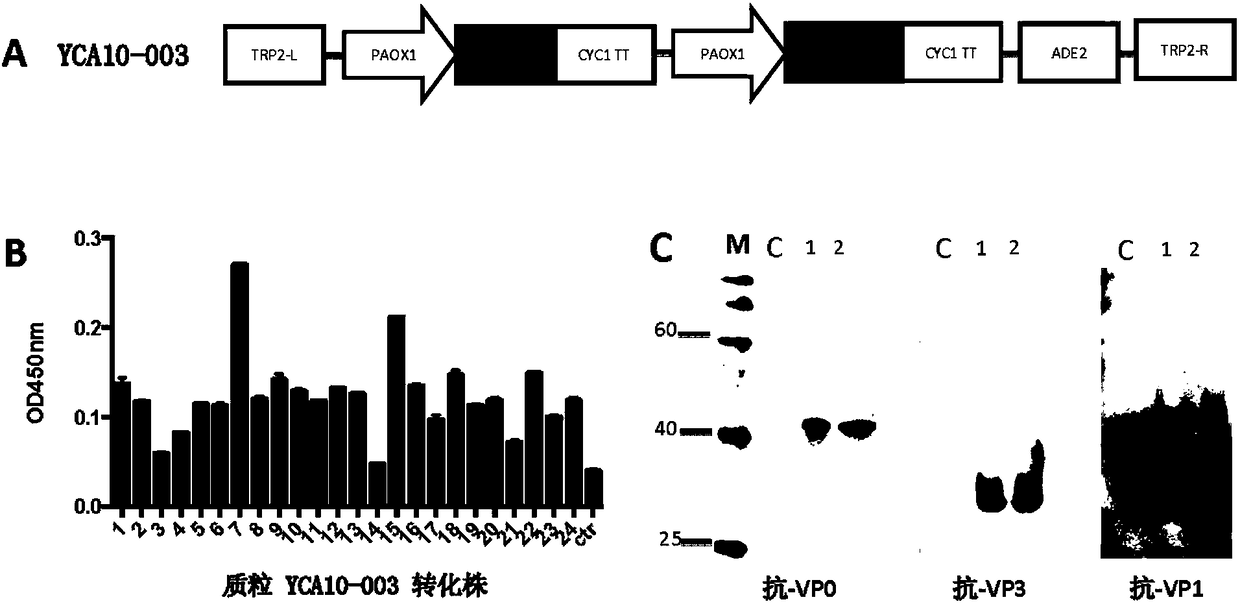
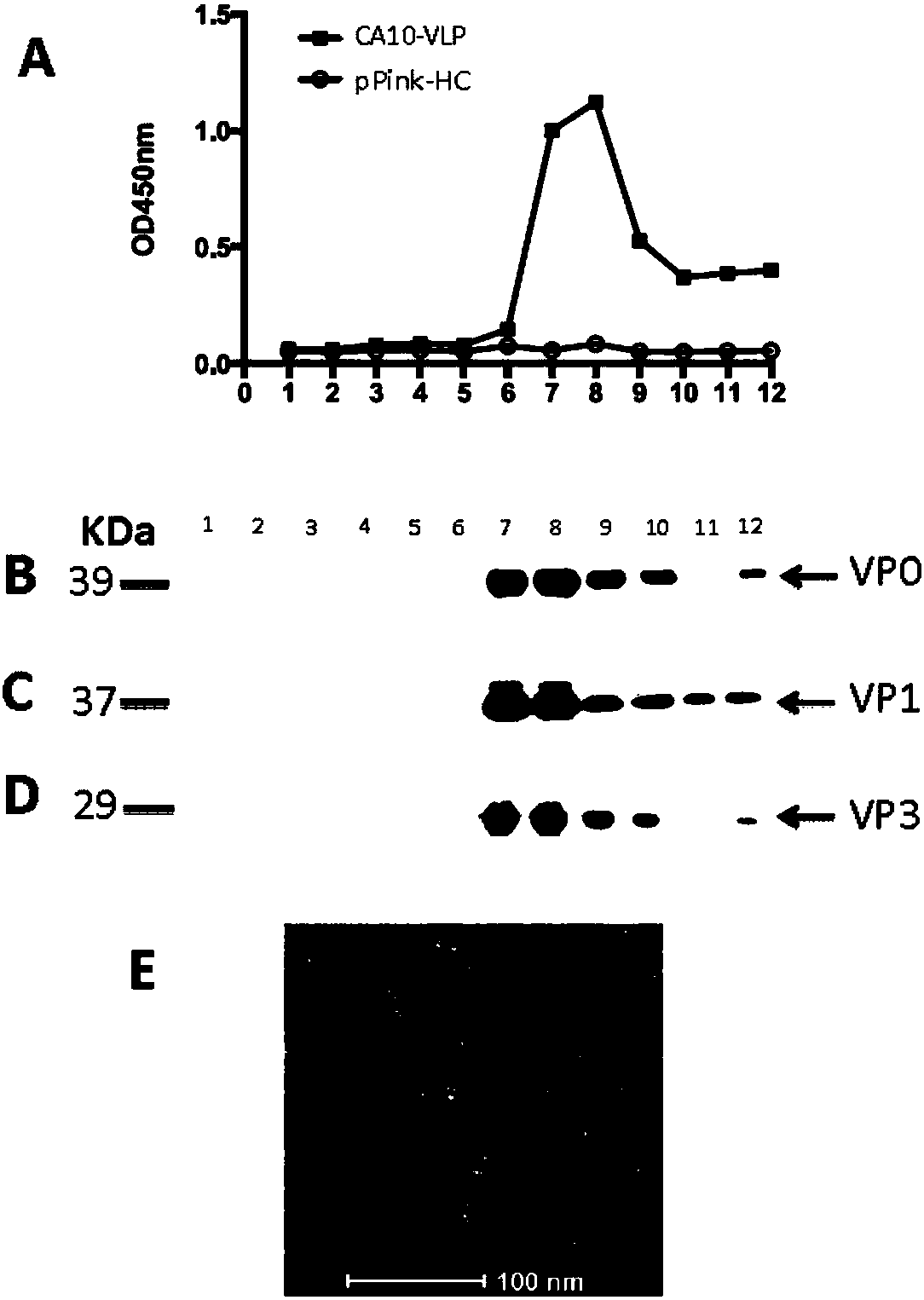
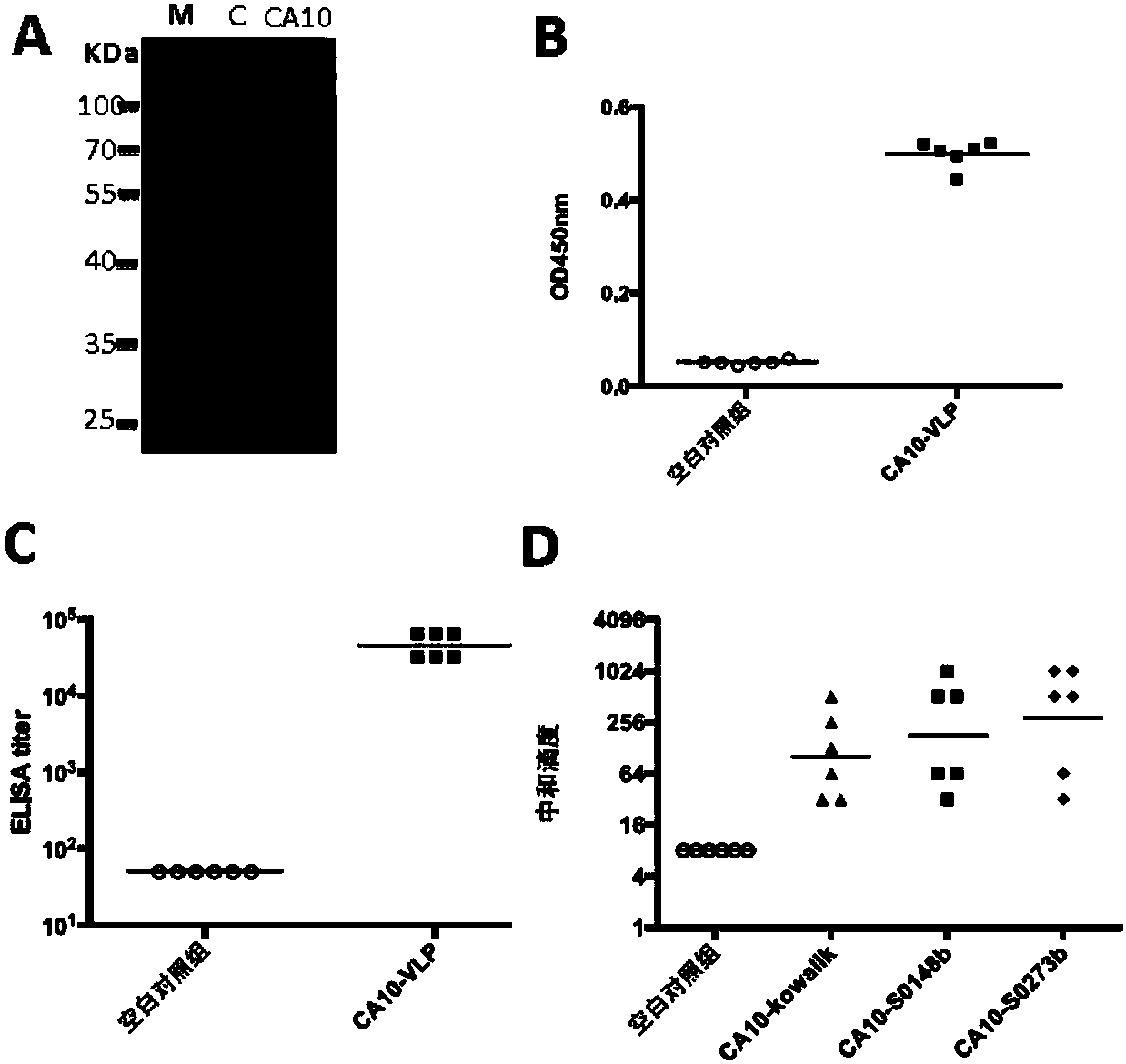
Yeast-expressed Coxsackievirus A10 virus-like particles and applications thereof
The present invention provides yeast-expressed Coxsackievirus A10 virus-like particles and applications thereof. According to the present invention, specifically the gene sequences of the P1 protein and the 3CD protein of Coxsackievirus A10 are transformed into yeast cells, and expression is performed to obtain the novel Coxsackievirus A10 virus-like particles; and the yeast-expressed Coxsackievirus A10 virus-like particles have characteristics of high expression, strong immunogenicity and good specificity.
Owner:INST PASTEUR OF SHANGHAI CHINESE ACADEMY OF SCI
Enterovirus triple real-time fluorescent quantitative RT-PCR detection kit
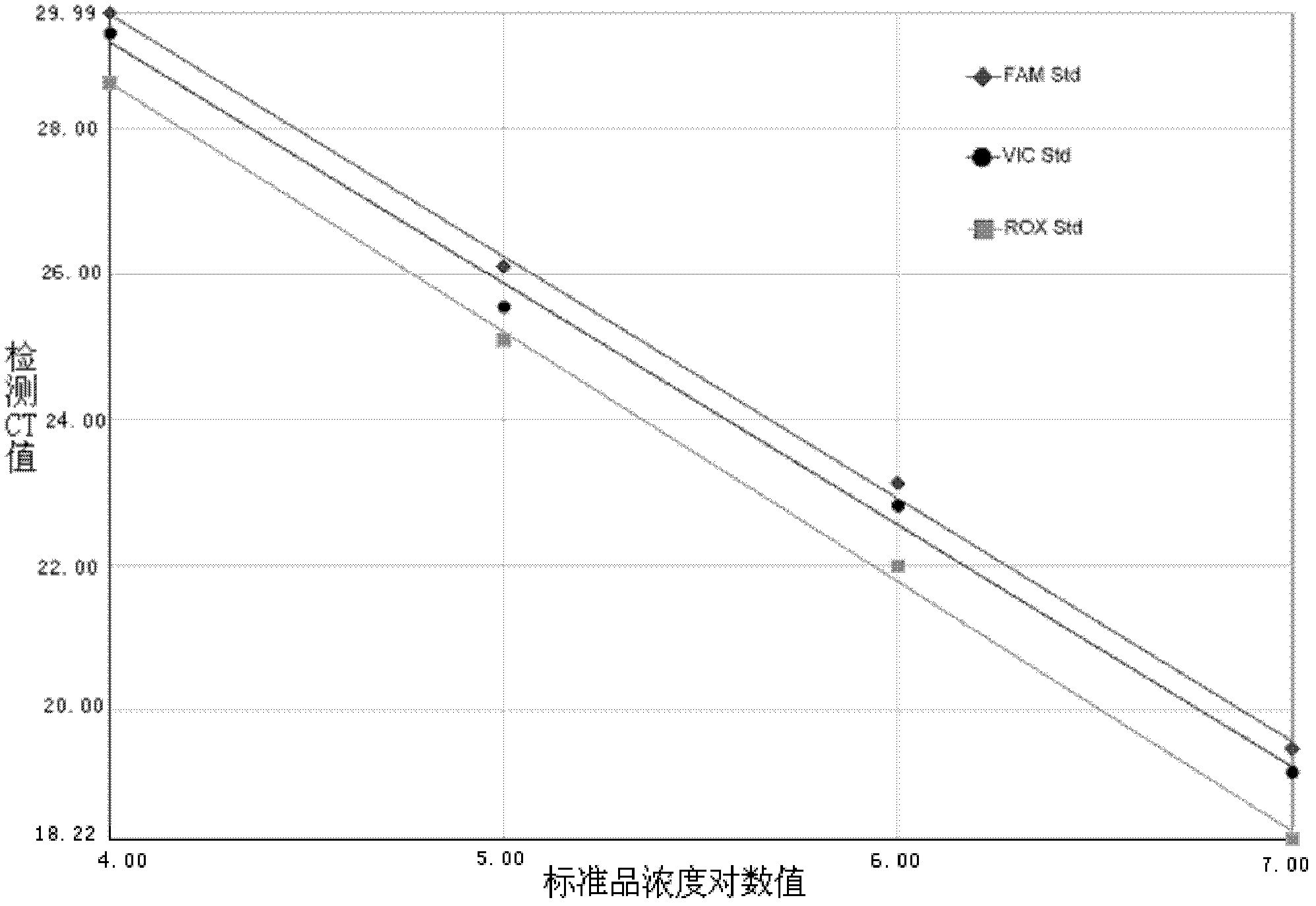
InactiveCN102367488AImprove detection efficiencyExtend detection timeMicrobiological testing/measurementMicroorganism based processesFluorescenceReverse transcriptase
The invention provides an enterovirus triple real-time fluorescent quantitative RT-PCR detection kit, which comprises a quantitative RT-PCR reaction solution, a standard substance and a reference substance, wherein, the quantitative RT-PCR reaction solution comprises a RT-PCR buffer solution, MgCl2, dNTPs, an EV universal primer, an EV universal fluorescent probe, an EV71 type fluorescent probe, a CA16 type fluorescent probe, heat resistant DNA polymerase and reverse transcriptase. The kit of the invention enables detection and genotyping to enterovirus universal type, enterovirus 71 type andcoxsackie virus group A 16 type by a one-step method. Compared with the current single detection kit, the kit of the invention is capable of saving the production cost and detection cost, raising theefficiency and time of the detection. Under the condition of ensuring no competition of multiple primers, the purpose of multi-channel detection can be achieved, so that the unification of the efficiency and technology is achieved.
Owner:ZHEJIANG UNIV
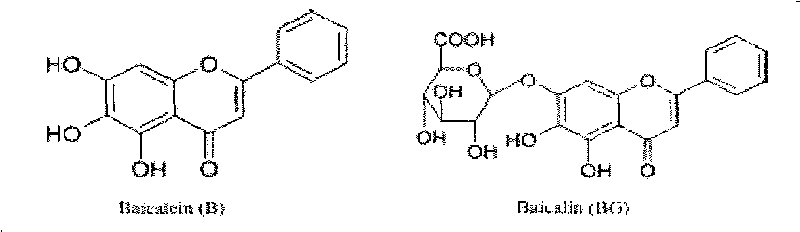
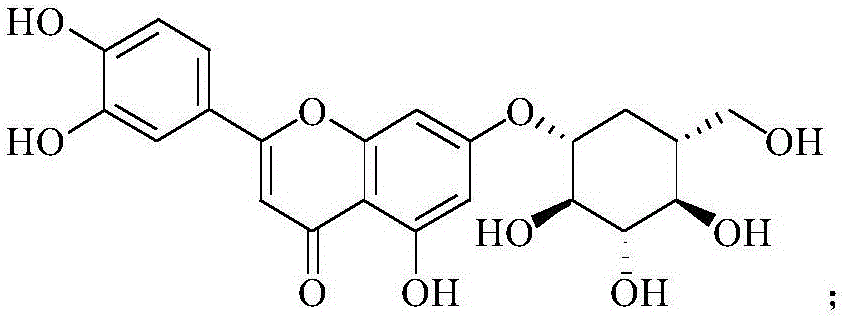
Application of scullcapflavone in preparing medicament for treating enterovirus infection
The invention discloses new application of scullcapflavone in preparing a medicament for treating enterovirus infection, in particular new application of scullcapflavone in preparing a medicament for treating or preventing a hand-foot-and-mouth disease caused by enterovirus infection. The scullcapflavone comprises an extract of baicalein, baicalin or scutellaria baicalensis general flavone; and the enterovirus indicates a coxsackie virus A group, a coxsackie virus B group, echovirus or enterovirus EV71 which causes the hand-foot-and-mouth disease. The scullcapflavone as an active substance is mixed with a pharmaceutically allowable excipient and made into a preparation which can be accepted by a patient and can be used for treating and preventing the hand-foot-and-mouth disease by injection administration, mucosa administration, oral administration or inhalation and has obvious treating effect and high safety.
Owner:BEIJING WEHAND BIO PHARMA CO LTD
Nucleic acid fluorescence PCR detection kit for universal enterovirus, coxsackievirus A16 and enterovirus 71
InactiveCN105420411AHigh sensitivityHigh amplification efficiencyMicrobiological testing/measurementMicroorganism based processesCoxsackievirus a16RNA extraction
The embodiment of the invention discloses a nucleic acid fluorescence PCR detection kit for a universal enterovirus, a coxsackievirus A16 and an enterovirus 71. The nucleic acid fluorescence PCR detection kit comprises an RNA extracting solution, an RNA eluant, internal standards, a PCR reaction solution, an EV / CA16 / EV71 enzyme mixed solution, EV / CA16 / EV71 positive reference substances and EV / CA16 / EV71 negative reference substances. According to the kit, the universal enterovirus, the coxsackievirus A16 and the enterovirus 71 can be simultaneously detected in a same sample, but other pathogen RNA cannot be detected, the detection sensitivity can reach 400 copie / ml, the detection range is 4.0 E+02-4.0E+08 copies / ml, and a reliable experimental evidence is supplied to early diagnosis on infection of the universal enterovirus, the coxsackievirus A16 and the enterovirus 71.
Owner:SANSURE BIOTECH INC
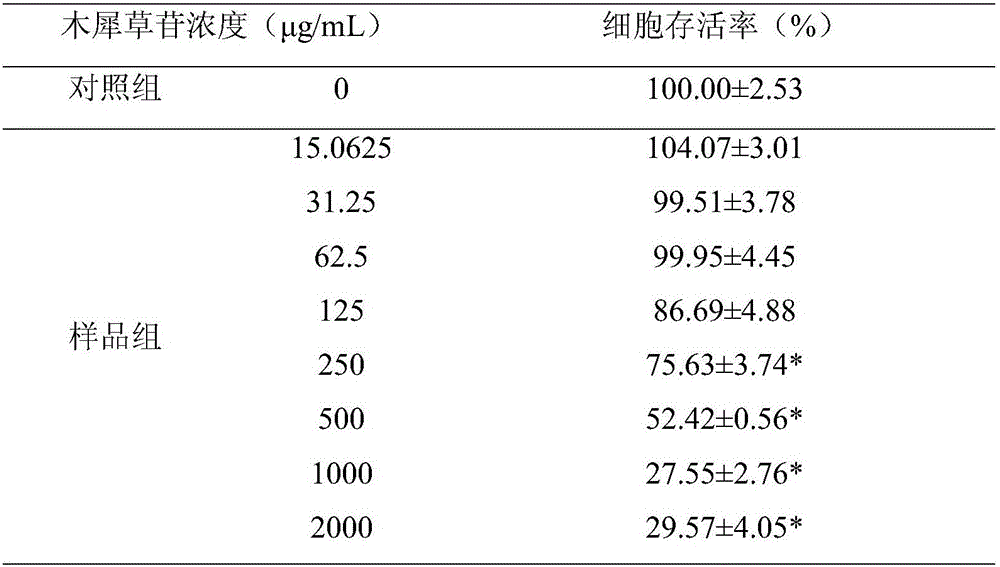
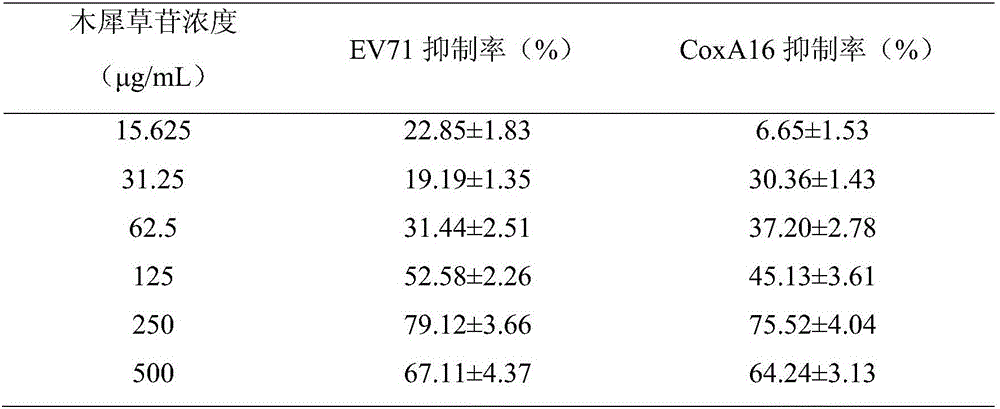
Application of cynaroside to preparation of medicine for treating or preventing hand-foot-and-mouth diseases
InactiveCN105920026ALow cytotoxicityAvoid infectionOrganic active ingredientsAntiviralsInfected cellSide effect
The invention discloses application of cynaroside to preparation of a medicine for treating or preventing hand-foot-and-mouth diseases. The hand-foot-and-mouth diseases are caused by an enterovirus type 71, a type 16, type 4, type 5, type 7, type 9 and type 10 of a coxsackie virus group A, a type 2 and type 5 of a coxsackie virus group B or an echovirus. The medicine takes the cynaroside as an active component and is mixed with a pharmaceutically acceptable auxiliary material to prepare any one clinically acceptable dosage form. The application disclosed by the invention provides a new way for treating the hand-foot-and-mouth diseases; the cynaroside can be developed as a new medicine for preventing the hand-foot-and-mouth diseases; the toxic and side effects are small; the lesion of infected cells is inhibited, a dose-dependent effect is realized and the medicine is a broad-spectrum antiviral medicine; virus replication is inhibited, the virus load in host cells is reduced and viruses are promoted to become negative; the case fatality rate is reduced or eliminated and the survival time of illness cases is prolonged; the content of cell factors is reduced and inflammations caused by virus infection is alleviated; a plurality of administration manners are provided and the medicine can be administrated by injection and can also be orally taken.
Owner:JIANGSU KANION PHARMA CO LTD
Vaccine for preventing and treating diseases caused by Coxsackie virus and preparation method and application thereof
The invention discloses a vaccine for preventing and treating diseases caused by Coxsackie virus and a preparation method and application thereof. The preparation method of the vaccine for preventing and treating the diseases caused by Coxsackie virus comprises the following steps: taking an inactivated Coxsackie virus A6 strain as an active ingredient to prepare the vaccine; preservation number of the Coxsackie virus A6 strain in a general microorganism center of China Committee for Culture Collection of Microorganisms is No. CGMCC No. 13393. The vaccine and CVA6 antiserum prepared by CVA6 strain WF057R have the functions of treating and preventing the diseases caused by CVA6, and a parent antibody of CVA6 strain WF057R has protective effect on neonatal suckling mice.
Owner:TAISHAN MEDICAL UNIV
CV-A10 virus species and inactivated vaccine thereof for human
PendingCN109536460AIncrease productivityImprove cultivation efficiencySsRNA viruses positive-senseViral antigen ingredientsAntigenAluminium hydroxide
The invention discloses a CV-A10 virus species and an inactivated vaccine thereof for human. The classification name of the species is coxsackie virus A group 10-type with the preservation number being CGMCC No.16218; and the prepared CV-A10 inactivated vaccine for human consists of 100 [mu]g / ml of CV-A10 inactivated purified antigens and 1mg / ml of aluminium hydroxide. Results indicate that the vaccine products have favorable immunogen and safety.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
Kit for detecting coxsackie virus A16-type nucleic acid and detection method thereof
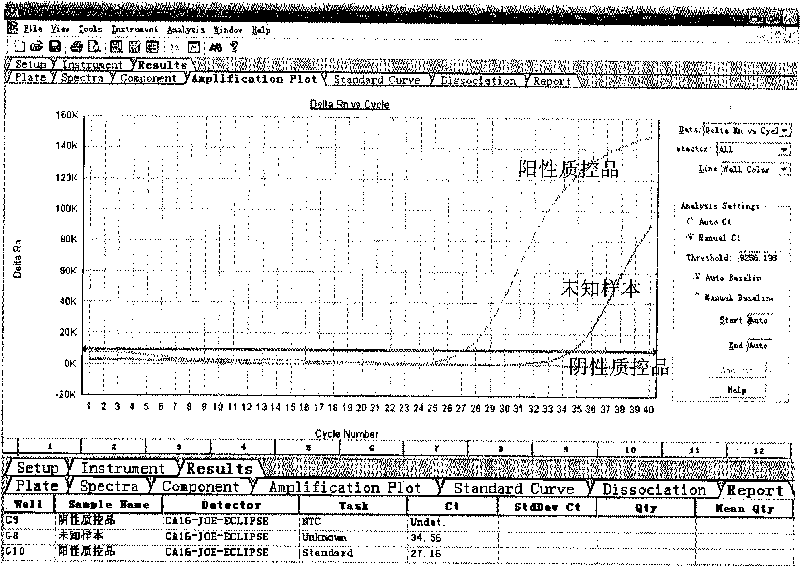
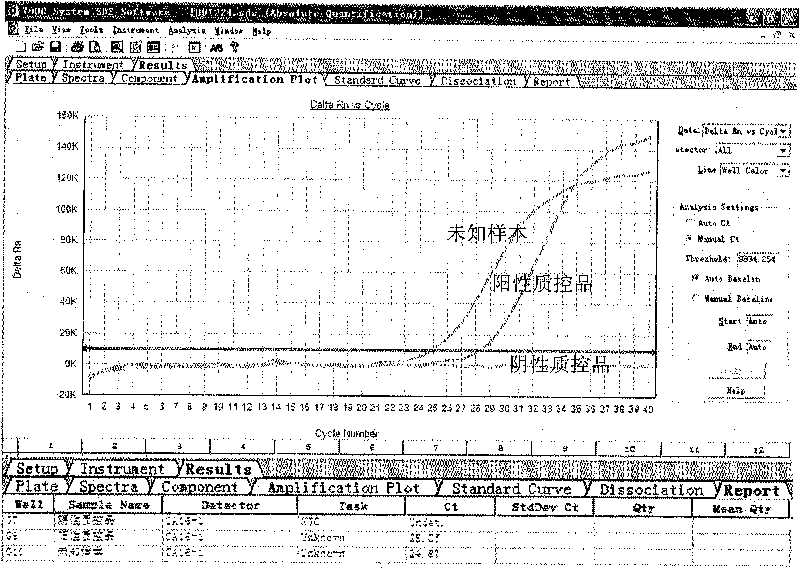
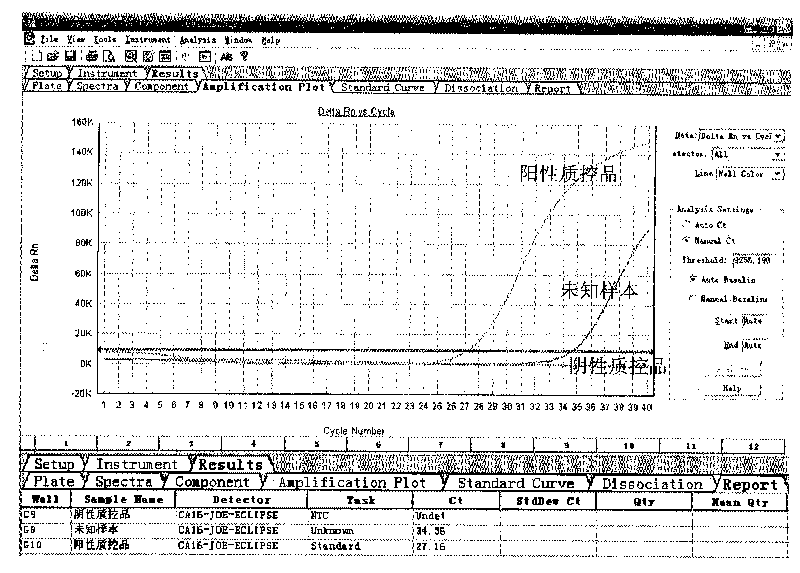
ActiveCN101713003ASimple and fast operationMicrobiological testing/measurementFluorescence/phosphorescenceForward primerFluorescence
The invention discloses a kit for detecting coxsackie virus A16-type nucleic acid and a detection method thereof. The kit comprises PCR reaction enzyme, PCR reaction liquid, a negative quality control product and a positive quality control product, wherein the PCR reaction enzyme contains a hot start Taq enzyme, a MLV reverse transcriptase and a RNA enzyme inhibitor; the PCR reaction liquid contains DEPC treating water, dNTPs, 10*PCR Buffer, a coxsackie virus A16-type forward primer, a coxsackie virus A16-type reverse primer and a coxsackie virus A16-type probe, the sequence of the coxsackie virus A16-type forward primer is 5'-CGCTGCCGATACTGAAGCACCG-3', the sequence of the coxsackie virus A16-type reverse primer is 5'-CTGTCTCCGCGGCTTGTAG-3', the sequence of the coxsackie virus A16-type probe is 5'-ACAGATTAGGCACTGGTGTTGTACCGTA-3'; the negative quality control product is the DEPC treating water; and the positive quality control product is the prepared transcription in vitro RNA standard product. The method for fast detecting the sequence of the coxsackie virus A16-type nucleic acid by using a real-time fluorescence quantitative PCR technology has the advantages of specificity, sensitiveness, high speed as well as simple and convenient operation.
Owner:IPE BIOTECHNOLOGY CO LTD
Hand-foot-mouth disease detection reagent kit and its detection method
InactiveCN102643927ASuitable for field applicationStrong specificityMicrobiological testing/measurementHand-foot-and-mouth diseaseCoxsackievirus a16
The invention belongs to biological field, and relates to a reagent kit for detecting main pathogen Coxsackievirus A16 (Cox A16) and enterovirus 71 of hand-foot-mouth disease, construction method and uses thereof. The reagent kit comprises reaction solutions for detecting four primers of Coxsackievirus A16 and four primers of enterovirus 71.As detected, the reagent kit has a good performance of specificity, sensitivity, rapid amplification, high efficiency and simple identification. The detection system of the invention can detect Coxsackievirus A16 and enterovirus 71 quickly, conveniently, high-effectively, high-specifically and highly-sensitive under temperature of 64 DEG C without need of complicated instruments, which can preferably satisfy clinical detection for hand-foot-mouth disease and be easily popularized in large scope.
Owner:刘志学 +1
Test kit for coxsackie virus A6 nucleic acid and test method
InactiveCN104878126AShort amplification timeReduced Chances of ContaminationMicrobiological testing/measurementMicroorganism based processesForward primerFluorescence
The invention belongs to the technical field of biology, and particularly relates to a real-time fluorescence quantification reverse transcription PRC test kit for a rapid coxsackie virus A6 and a test method. The test kit for coxsackie virus A6 nucleic acid comprises a coxsackie virus A6 forward primer shown by a nucleotide sequence such as SEQ ID NO.1, a reverse primer shown by a nucleotide sequence such as SEQ ID NO.2 and a special fluorescence probe shown by a nucleotide sequence such as SEQ ID No.3. The test kit for the coxsackie virus A6 nucleic acid is high in sensitivity, strong in specificity, rapid, easy and convenient to operate, precise and stable in result and wide in application range, and suitable for screening of a coxsackie virus A6 crowd.
Owner:安阳市疾病预防控制中心
New application of luteolin
The invention relates to new application of luteolin, in particular to application of luteolin in preparing medicine for preventing and treating hand-foot-mouth diseases, particularly, medicine used for resisting enterovirus 71 and / or coxsackievirus A16.
Owner:JILIN UNIV +1
Method for detecting neutralizing antibody in coxsackievirus A6 (CV-A6), and recombinant virus applied in method
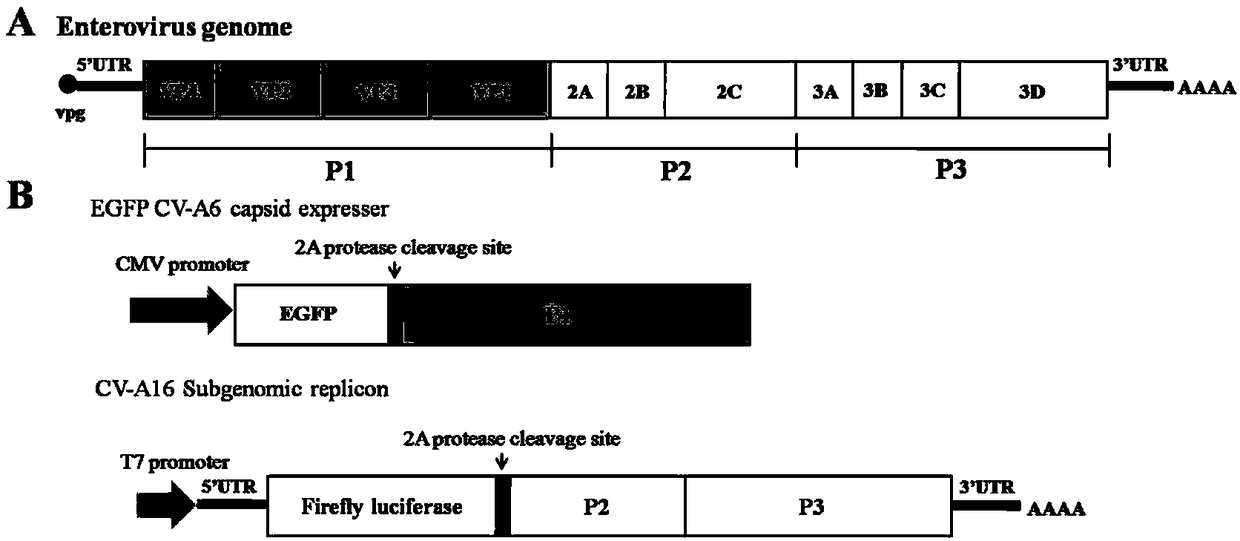
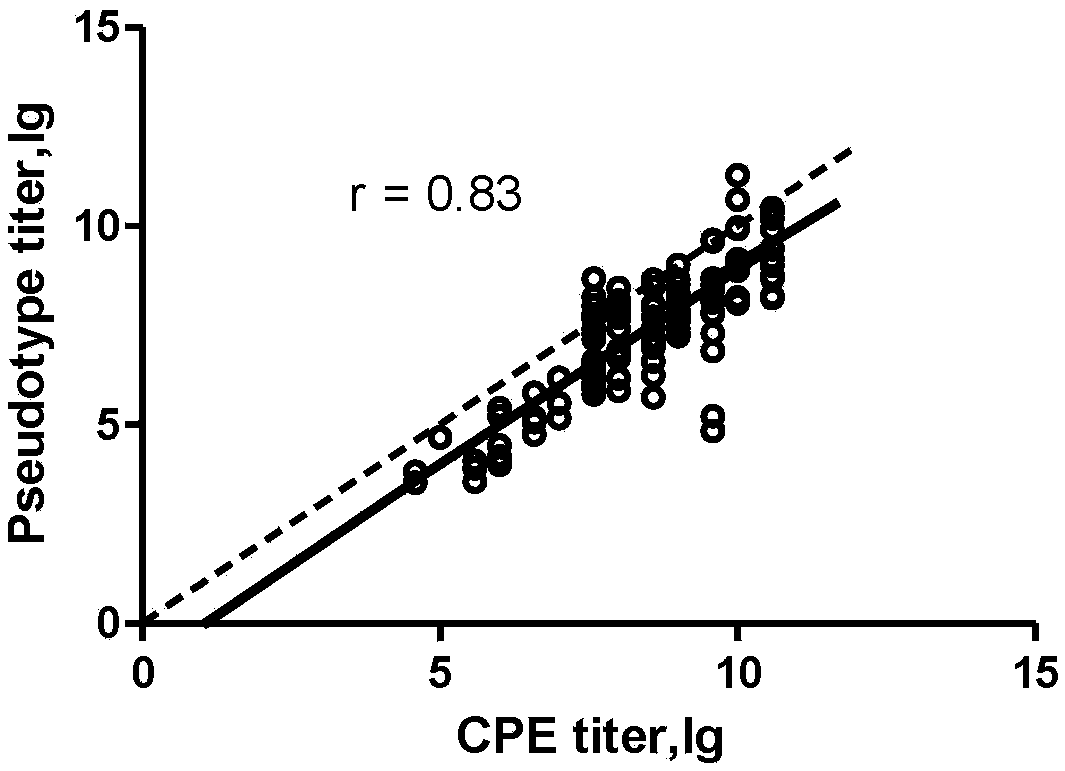
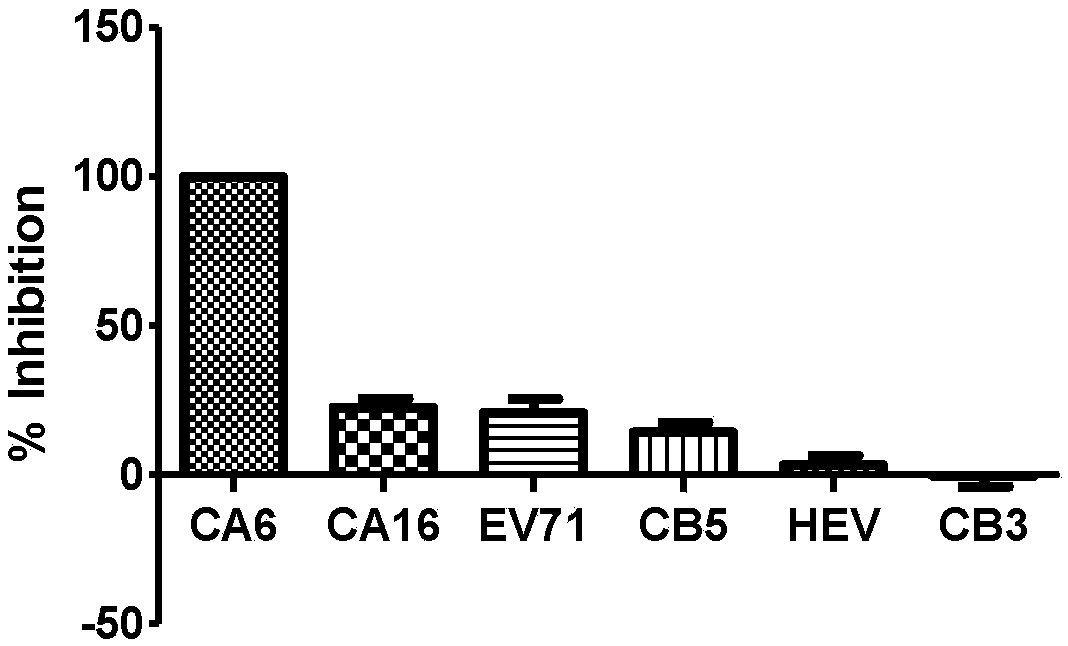
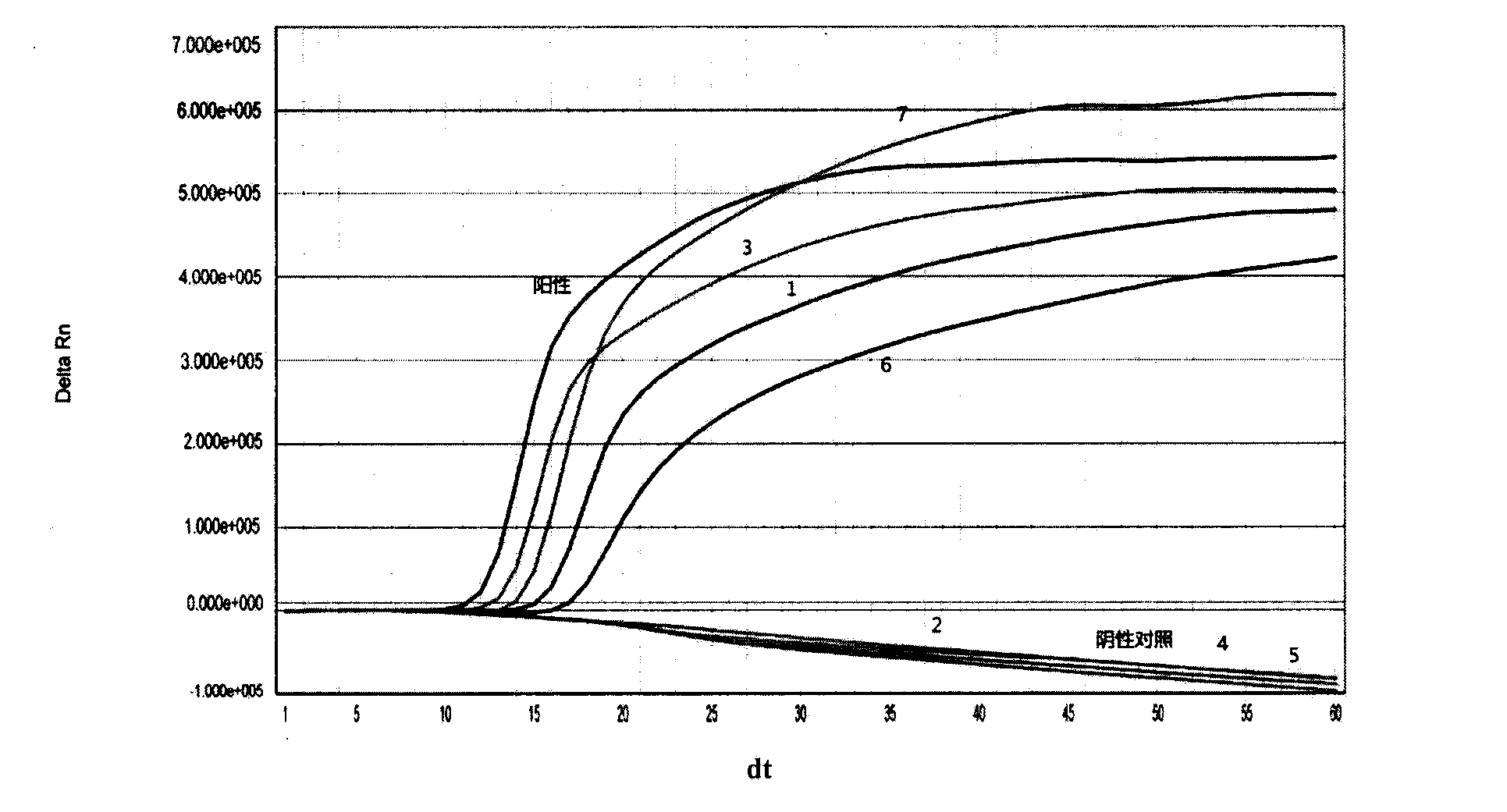
ActiveCN108060172ALower requirementGood linear relationshipSsRNA viruses positive-senseVector-based foreign material introductionViral VaccineNeutralizing antibody
The invention discloses a method for detecting a neutralizing antibody in coxsackievirus A6 (CV-A6), and a recombinant virus applied in the method. According to the method, the neutralizing antibody is detected by using a pseudovirus system packaged by two types of recombinant plasmids; by adopting a single-cycle infected pseudovirus, the safety problem caused by use of live viruses is avoided. The results of a plurality of tests prove that the pseudovirus detection system is a CV-A6 neutralizing antibody detection method which is safe, sensitive, rapid, specific, simple and convenient, and low in cost. Based on the characteristics, the pseudovirus detection system is very suitable for the tests for rapid and large-scale detection of the neutralizing antibody, and has an import applicationvalue for development of a CV-A6 viral vaccine and detection of CV-A6 specific neutralizing antibody level of patients and populations.
Owner:NAT INST FOR FOOD & DRUG CONTROL
Coxsackie virus type A16 (CA16) real-time fluorescent nucleic acid isothermal amplification detection kit
ActiveCN103388032AReduce pollutionEfficient captureMicrobiological testing/measurementMicroorganism based processesCoxsackievirus a16Throat swab
The invention discloses a Coxsackie virus type A16 (CA16) real-time fluorescent nucleic acid isothermal amplification detection kit comprising reagents such as a capturing probe, CA16 amplification primers T7 primer and nT7 primer, a CA16 detection probe, M-MLV reverse transcriptase, T7 RNA polymerase, and the like. The kit can be used for detecting CA16 RNA in throat swab or stool, and has the characteristics of high specificity, high sensitivity (reaching 10copies / reaction), low pollution (amplification product RNA can be easily degraded under natural environment), and fast detection (conventionally detection can be finished within 60min). The kit can perform important effect in clinical diagnosis of CA16 early-stage infection, and can be widely applied.
Owner:SHANGHAI RENDU BIOTECH
Immune globulin and its prepn and application
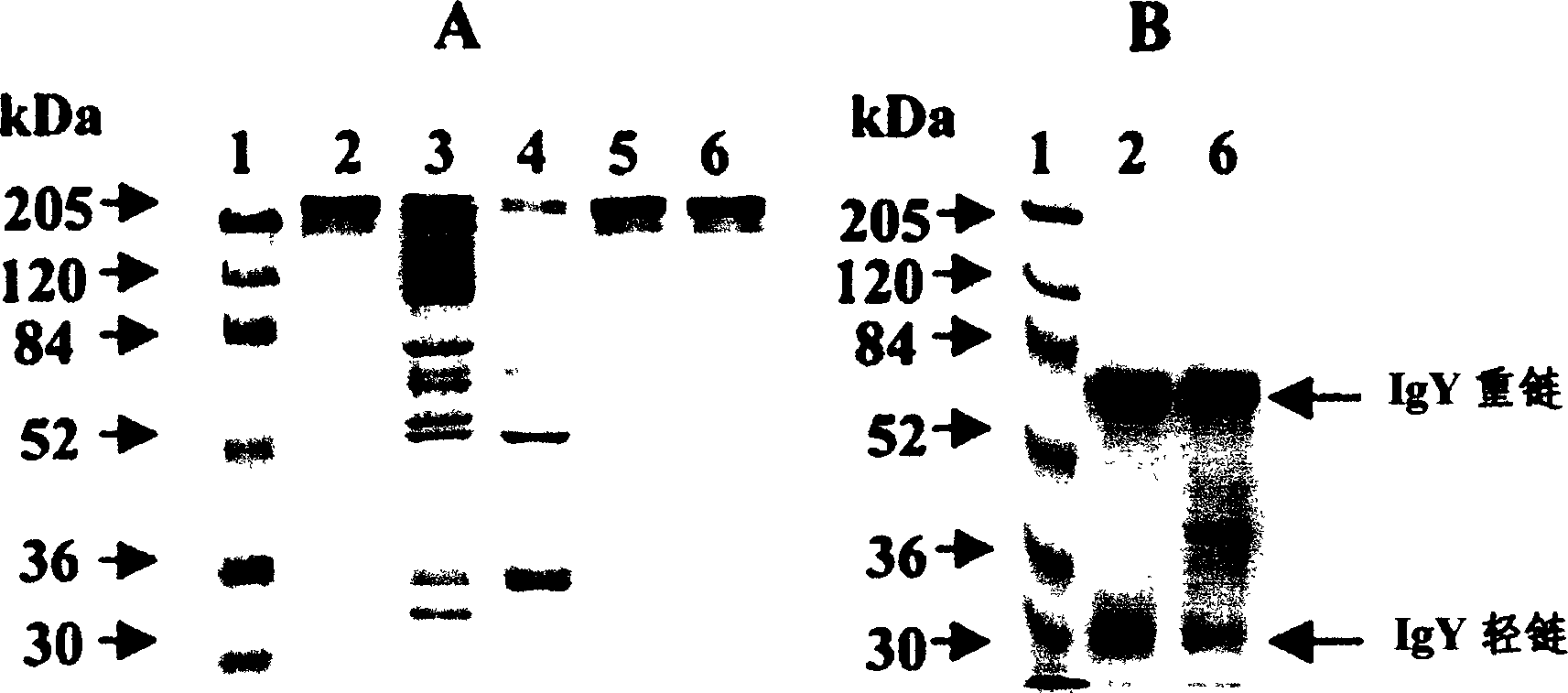
InactiveCN1526735AStrong specificityHigh cure rateSenses disorderEgg immunoglobulinsYolkConventional medicine
The present invention discloses one kind of immune globulin and its preparation and application. The immune globulin is type-70 enterovirus resisting and type-A24 Coxsachie virus resisting chicken yolk immune globulin and is prepared into product with antibody activity. The refined effective component has high purity and high activity, and is ideal product for eye drop and eye ointment. The immune globulin is used in preparing medicine for preventing and treating acute haemorrhagic conjunctivitis caused by type-70 enterovirus and type-A24 Coxsachie virus infection, and the medicine has high specificity, high curative rate and effect superior to conventional medicine.
Owner:KUNMING BEIOUSIDA BIOLOGICAL SCI TECH
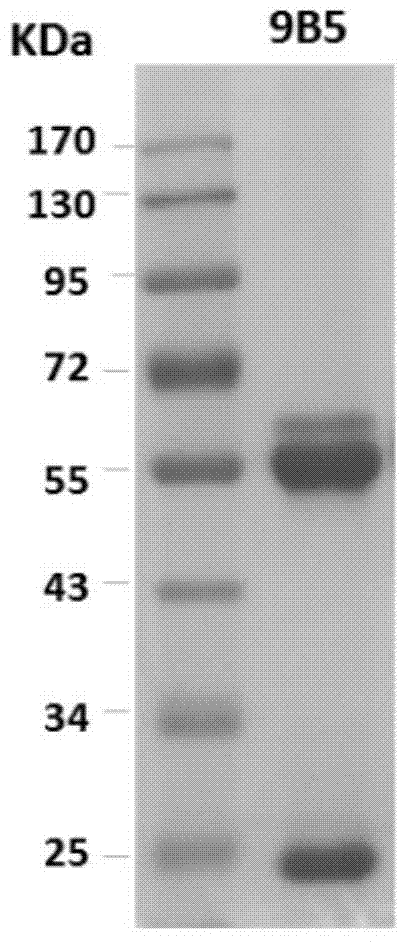
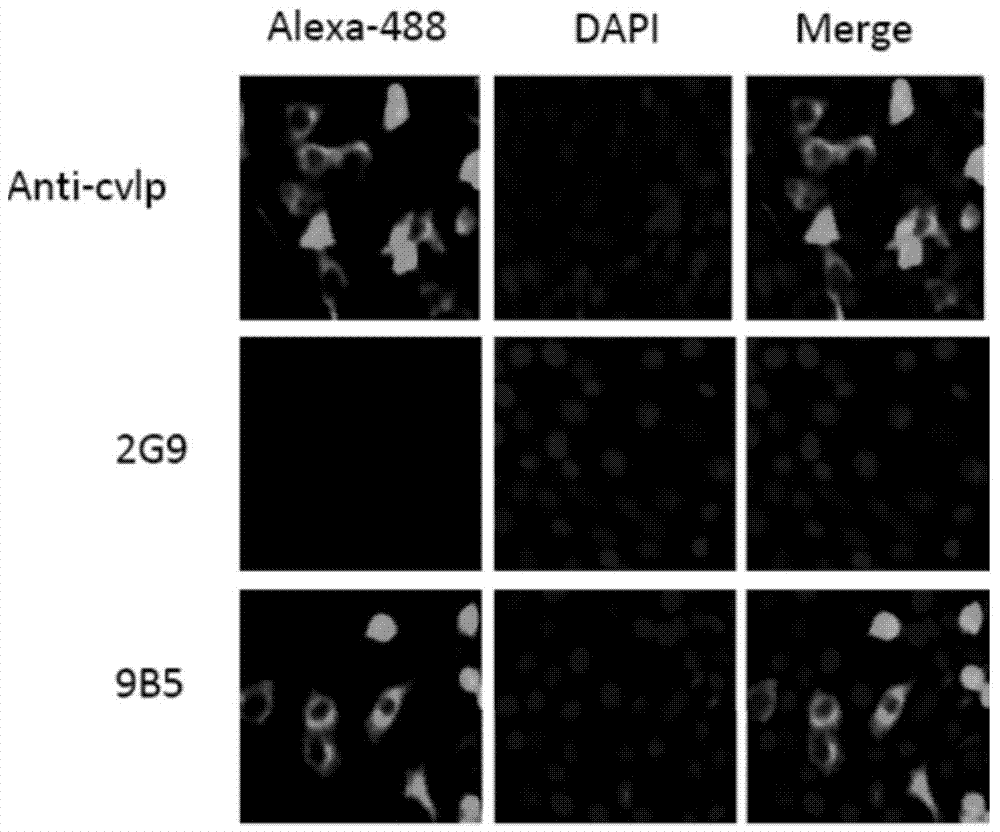
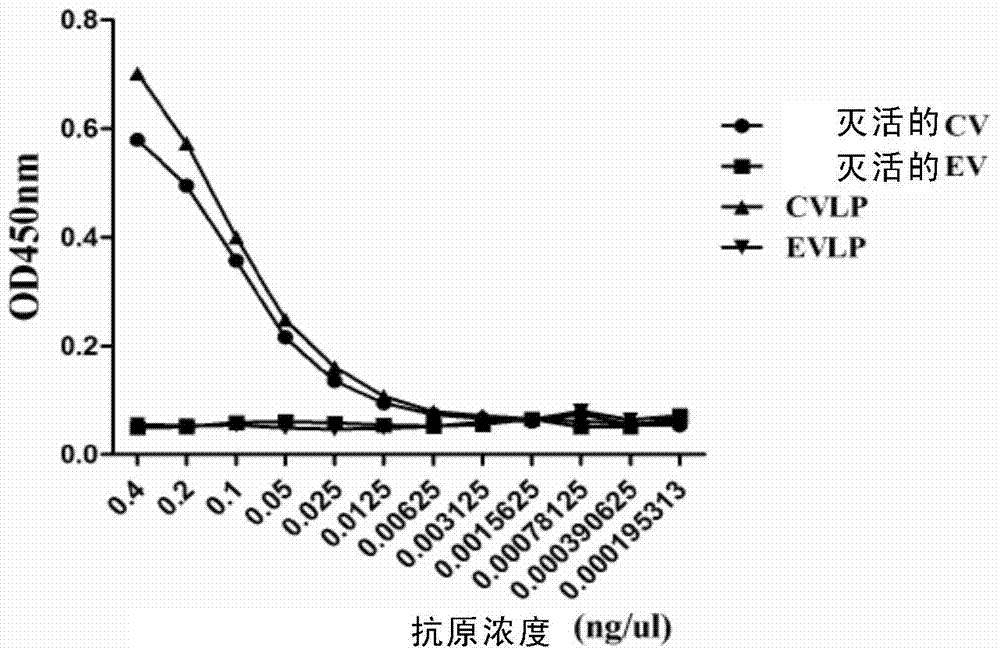
Preparation and application of anti-Coxsackie virus A16 monoclonal antibody
The invention relates to preparation and application of an anti-Coxsackie virus A16 (CA16) monoclonal antibody. The invention reveals a mouse source anti-CA16 monoclonal antibody, which has good binding specificity, can be used for sensitive detection of Coxsackie A16 virus, and has strong anti-virus infection capacity.
Owner:INST PASTEUR OF SHANGHAI CHINESE ACADEMY OF SCI
CA-193 virus strain and application thereof to preparation of inactivated vaccine
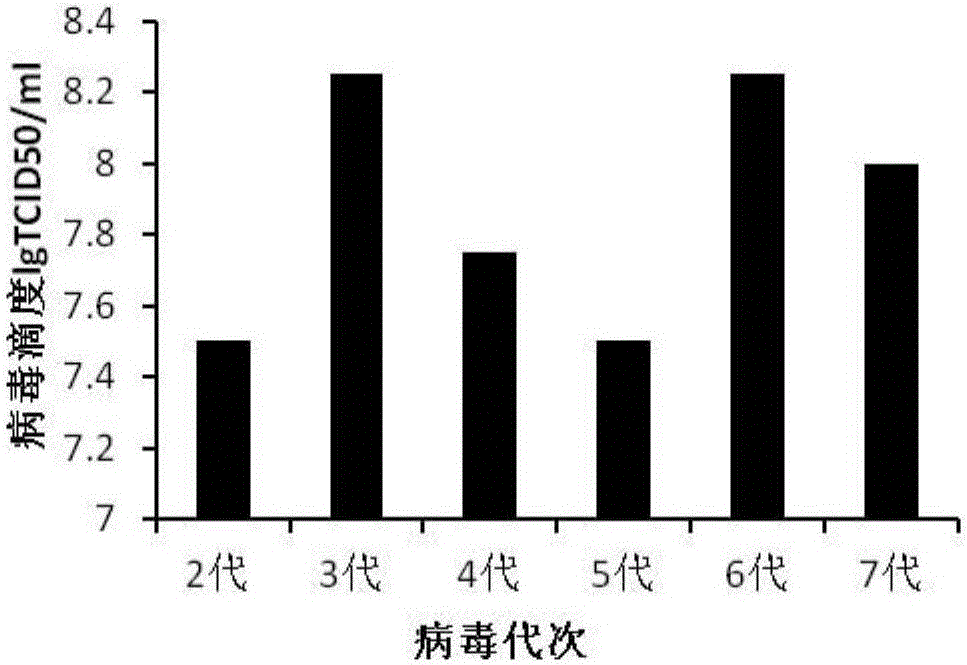
ActiveCN106367398ARich preparation methodImproving immunogenicitySsRNA viruses positive-senseViral antigen ingredientsFiltrationVaccine virus
The invention discloses a CA-193 virus strain (Coxackievirus A16) and application thereof to preparation of an inactivated vaccine. For the application, MRC-5 cells are used for proliferating the CA-193 virus strain; virus proliferation liquid is subjected to centrifugation, concentration and column chromatography filtration and purification after inactivation by formaldehyde or beta-propiolactone; and a CA16 inactivated vaccine is obtained. The invention provides the vaccine virus strain capable of being used for preparing a human CA16 inactivated vaccine; the subtype of the vaccine virus strain belongs to the main epidemiological subtype B1 in Chinese Mainland; the vaccine virus strain has good growth characteristics on the MCR-5 cells; and the virus titer (7.5 to 8.251gTCID50 / ml) with high stability can be obtained. A preparation method of the vaccine is complete; the impurity content is low; the immunogenicity and the immunizing protection performance are good; meanwhile, cell culture substrates are MRC-5 cells; the safety is high; and the international and CFDA specifications and requirements on the vaccine research and development can be well met.
Owner:ZHEJIANG CENT FOR DISEASE CONTROL & PREVENTION
Coxsackie virus type a16 (ca16) igm antibody detection test strip
ActiveCN102262156ASave manpower and material resourcesThe result is clear and easy to distinguishMaterial analysisCoxsackievirus a16Nitrocellulose
The invention provides a detection test strip for a coxsackie virus A16 type (CA16) IgM (Immune Globulin M) antibody. According to the detection test strip, a nitrocellulose membrane (NC membrane) is coated by a CA16 gene antigen (VP1) and an anti-mouse IgG; and by combining a tihuman IgM monoclonal antibody labeled by colloidal gold, a CA16 specificity IgM antibody in an infected human speciment is detected by applying a membrane chromatography capture method. The test strip for detection, which is disclosed by the invention, has the advantages of simpleness, convenience, quickness and fastness in operation, no need of special instrument or special training, distinct and easily-distinguished result and easiness for popularization; and the detection test strip is suitable for field detection and early-stage diagnosis and assisting function for CA16 infected diagnosis.
Owner:辽宁迪浩生物科技有限公司
Coxsackie virus A6 type and A10 type combined detection kit and application of same
PendingCN107881257AImprove detection efficiencyLess specimenMicrobiological testing/measurementMicroorganism based processesFluorescenceCoxsackievirus A
Owner:杨兴林
Nucleic acid fluorescent PCR detection kit for coxsacki evirus A16 and human enter ovirus 71
InactiveCN105368986AHigh purityHigh yieldMicrobiological testing/measurementMicroorganism based processesEnterovirusRNA extraction
The embodiment of the invention discloses a nucleic acid fluorescent PCR detection kit for coxsacki evirus A16 and human enter ovirus 71. The nucleic acid fluorescent PCR detection kit comprises the following components: RNA extract solution, RNA eluant, internal standard substance, PCR reaction liquid, CA16 / EV 71 enzyme mixed liquid, CA16 / EV 71 positive control substance and CA16 / EV 71 negative control substance. According to the nucleic acid fluorescent PCR detection kit, two kinds of enteroviruses including the coxsacki evirus A16 and human enter ovirus 71 are simultaneously detected in one sample, other pathogen RNAs can not be detected, and RNAs are extracted by a paramagnetic particle method, so that the detection sensitivity, accuracy and stability are improved, the detection sensitivity achieves 400 copie / ml, the detection range is (4.0E+02)-(4.0E+08) copies / ml, and reliabile experimental evidences are provided for early diagnosis of the infection of the coxsacki evirus A16 and the human enter ovirus 71.
Owner:SANSURE BIOTECH INC
CV-A6 virus species and human inactivated vaccine thereof
InactiveCN109609467AImprove cultivation efficiencyVaccines on the marketSsRNA viruses positive-senseViral antigen ingredientsCoxsackievirus AImmunogenicity
The present invention discloses a CV-A6 virus species and a human inactivated vaccine thereof. A classification naming of the virus species is Coxsackie virus group A type 6, a preservation number isCGMCC No. 16217, and the prepared human CV-A6 inactivated vaccine consists of 100 [mu]g / ml of CV-A6inactivated purified antigen and 1 mg / ml of aluminum hydroxide. The vaccine product has good immunogenicity and safety through animal experiments.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com