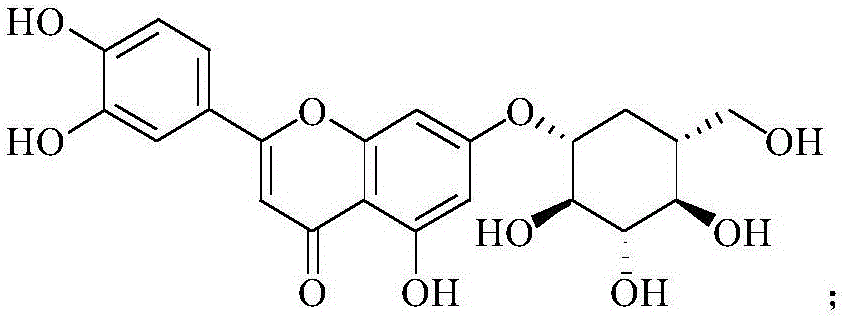
Application of cynaroside to preparation of medicine for treating or preventing hand-foot-and-mouth diseases
A technology for hand, foot and mouth disease and luteolin, applied in the field of medicine, can solve the problem that no literature reports luteolin against hand, foot and mouth disease, etc., and achieve the effect of various administration methods.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
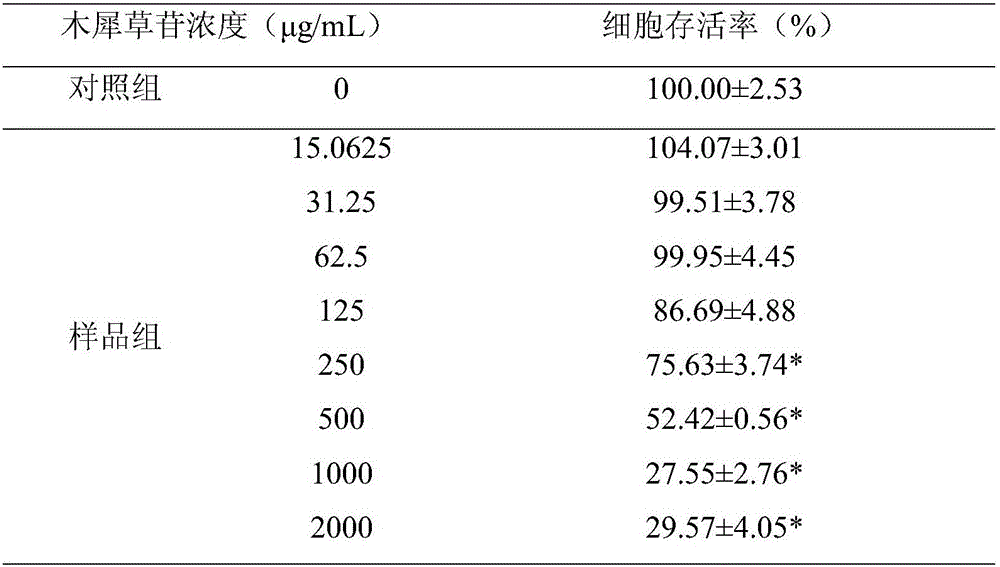
[0024] Example 1 Vero cytotoxicity of luteolin
[0025] 1. Materials and methods
[0026] 1.1 Cells and culture methods
[0027] African green monkey kidney cells (Vero cells) were used as the cell model (provided by the Institute of Microbial Epidemiology, Academy of Military Medical Sciences, the same below), and cultured in M199 containing 10% fetal bovine serum (Gibco company, batch number: 1376154, the same below). Base (Hyclone company, batch number: NZC1116, the same below), placed at 37 ° C, 5% CO 2 Cultivate in an incubator, and subculture when the cells grow to 90% density, and the ratio of cell subculture is 1 / 3-1 / 4.
[0028] 1.2 Reagents
[0029] MTS Cell Proliferation Quantitative Detection Kit (purchased from Promega, batch number: 00000657694, the same below); Luteolin (purchased from Shanghai Eternal Biotechnology Co., Ltd., batch number: 20100423, the same below).
[0030] 1.3 Instruments
[0031] Microplate reader (purchased from Molecular Devices, model...
Embodiment 2
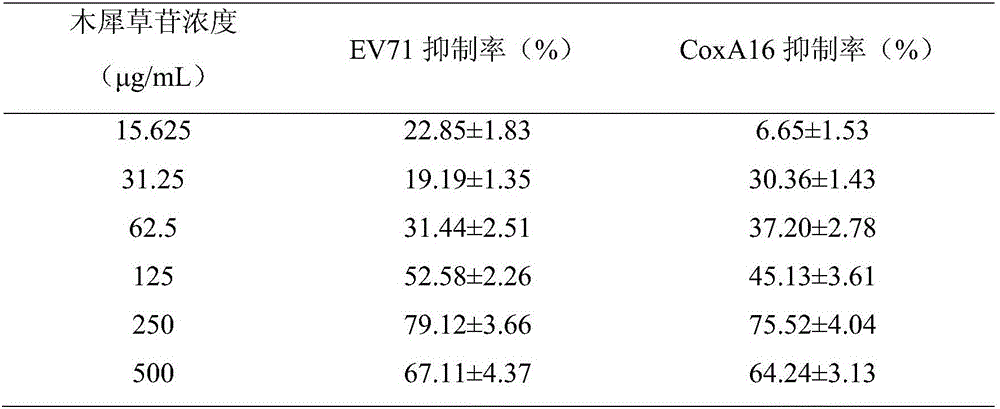
[0041] Example 2 Protection of luteolin on Vero cells infected by EV71 and CoxA16 viruses and inhibition of virus replication
[0042] 1. Materials and methods
[0043] 1.1 Cells and culture methods
[0044] African green monkey kidney cells (Vero cells) were used as the cell model, and M199 medium containing 10% fetal bovine serum was used at 37°C and 5% CO 2 Cultivate in an incubator, and subculture when the cells grow to 90% density, and the ratio of cell subculture is 1 / 3-1 / 4.
[0045] 1.2 Virus strains
[0046] EV71 virus BJ09 / 07 strain, GenBank accession number JQ319054.1; CoxA16 virus TS10 / 08 strain, GenBank accession number JX068829, all provided by the Institute of Microbial Epidemiology, Academy of Military Medical Sciences, the same below. Determine the half cytopathic dose (TCID) of EV71 virus before use 50 ) is 10 7 / mL, CoxA16TCID 50 for 10 7.5 / mL.
[0047] 1.3 Reagents
[0048] MTS cell proliferation quantitative detection kit (purchased from Promega, ...
Embodiment 3
[0066] Example 3 Broad-spectrum study of luteolin against hand-foot-mouth virus
[0067] 1. Materials and methods
[0068] 1.1 Cells and culture methods
[0069] African green monkey kidney cells (Vero cells) were used as the cell model, and the cells with the number of passages between 130 and 145 were used in the experiment. Cells were cultured in M199 medium containing 10% fetal bovine serum at 37°C, 5% CO 2 Cultivate in an incubator, and subculture when the cells grow to 90% density, and the subculture ratio is 1 / 3-1 / 4.
[0070] 1.2 Virus strains
[0071] Coxsackieviruses A groups 4, 5, 7, 9 and 10 (CoxA4, CoxA5, CoxA7, CoxA9 and CoxA10), Coxsackieviruses B groups 2 and 5 (CoxB2 and CoxB5) and echoviruses (ECHO) . Measure the TCID of each virus before use 50 10 respectively 6 、10 7 、10 7.5 、10 5 、10 8 、10 6.5 、10 7.5 and 10 6 / mL, the above viruses were provided by the Wuhan Institute of Virology, Chinese Academy of Sciences.
[0072] 1.3 Reagents
[0073] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com