CV-A6 virus species and human inactivated vaccine thereof
A CV-A6, inactivated vaccine technology, applied in the direction of positive-sense single-stranded RNA viruses, viruses, antiviral agents, etc., can solve problems such as difficulty in control, lack of CV-A6 vaccine research and development, and achieve the effect of increasing production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment one: the isolation, adaptation and purification method of the virus strain involved in the present invention
[0044] (1) Obtain virus samples
[0045] Collect excrement samples from hand, foot and mouth disease patients identified as CV-A6 infection by the Pediatric Department of the hospital, take 1g of feces and resuspend in 5mL of normal saline, centrifuge at 3000g for 30 minutes to get the supernatant, and filter it with a 0.45μm filter (purchased from Millipore Company) , stored at -20°C for later use.
[0046] (2) Adaptation of virus on RD cells
[0047] Select the RD cells that have grown into a dense monolayer, discard the old culture medium, wash the cell surface with an appropriate amount of PBS solution, and add an appropriate amount of 0.125% trypsin to digest the cells. Inoculate 2ml of the culture medium into each well of a 24-well plate, inoculate 200μl of the filtered sample on the cell surface, incubate at 37°C for 3 to 4 days, observe the...
Embodiment 2
[0054] Example 2: Cultivation and vaccine preparation method of CV-A6 strain YNKA1205
[0055] (1) The virus was cultured in KMB17 cells
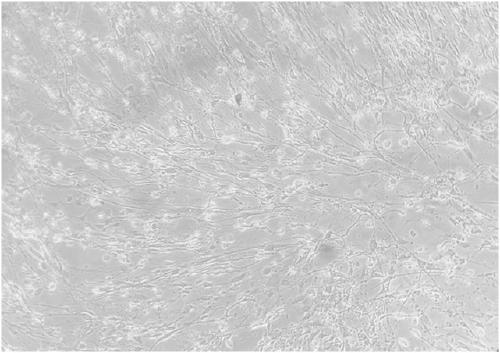
[0056] Select the P28 generation KMB17 cells that have grown into a dense monolayer, discard the old culture medium, wash the cell surface with an appropriate amount of PBS solution, add an appropriate amount of 0.125% trypsin to digest the cells, and gently pat the cell bottle, the cells slide down from the bottle wall like quicksand, Add cell culture medium, press 2×10 5 Inoculate small square flasks with cells / bottle, inoculate 3-4 days at 37°C, inoculate 200 μl of CV-A6 virus on the cell surface, adsorb at 37°C for 1 hour, then add MEM cell maintenance solution without calf serum, incubate at 37°C and Observe the cell lesions and the virus CPE reaches 90%. Harvest the virus and freeze it for later use. attached Figures 1a-1d For the CV-A6 strain to produce pathological changes in KMB17 cells (cultured for 4 days), cytopathic effects...
Embodiment 3
[0063] Embodiment three: the identification method of virus seed
[0064] (1) Sterility inspection and mycoplasma detection experiment
[0065] Take 4 tubes of thioglycolate liquid medium and 2 tubes of tryptone soy liquid medium, and inoculate each tube with 0.5 ml of virus. After inoculation, 2 tubes of thioglycolate fluid medium were cultivated at 30-35°C; 2 tubes of thioglycolate fluid medium and 2 tubes of tryptone soytone liquid medium were cultured at 20-25°C; method as a negative control. The results were judged after 14 days of culture. Take 4 tubes of semi-solid medium and broth medium for mycoplasma inspection, boil and melt the semi-solid medium, and cool to 56°C. Add 2.5ml of incapacitated calf serum and double-antibody mixture mixed at a ratio of 4:1 to each of the two media, shake quickly, and then add 0.5ml of the sample to be tested to each medium for primary culture. Incubate and observe at 35°C. On the 7th day, take out 2 tubes of each of the two primar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com