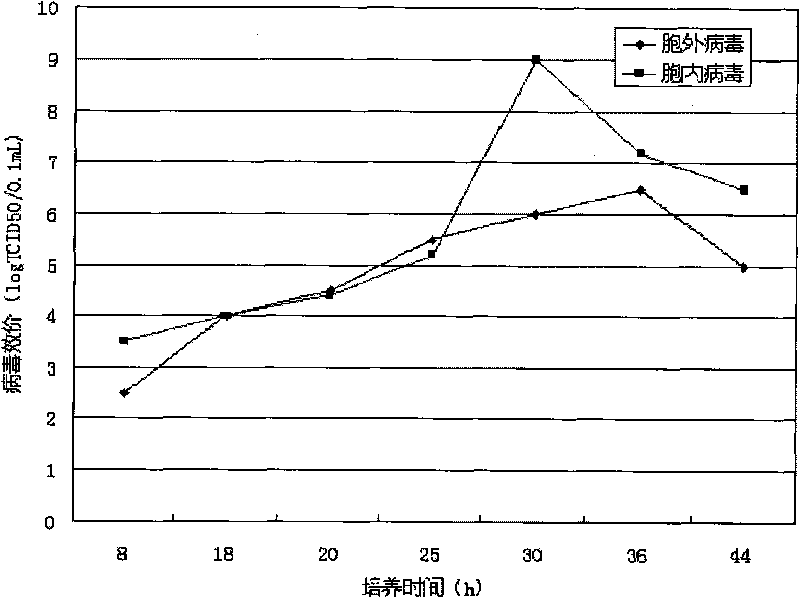
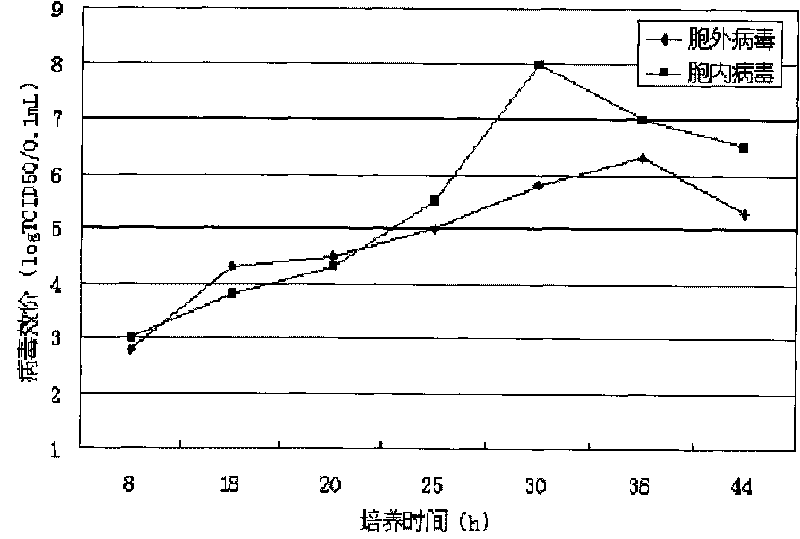
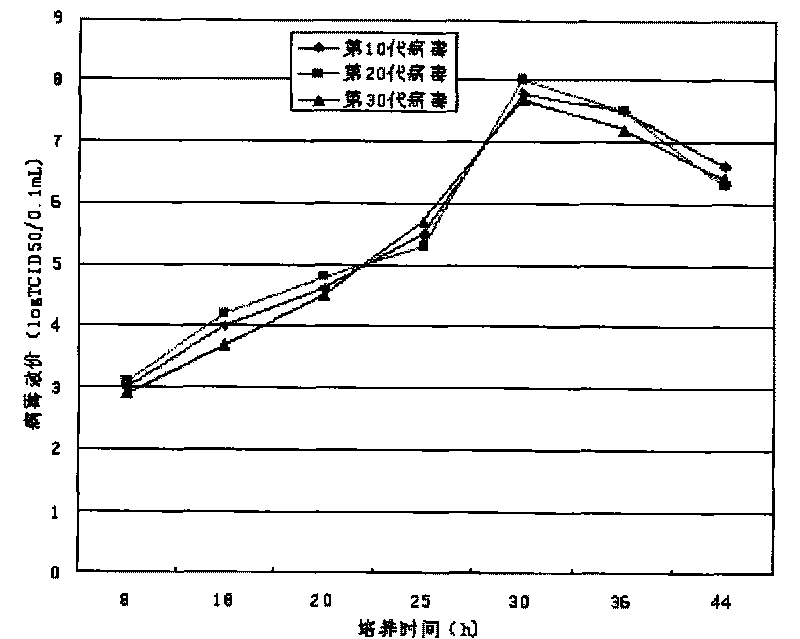
Univalent and bivalent inactivated vaccine for hand-foot-and-mouth disease and preparation method thereof
A technology for inactivated vaccines and hand, foot and mouth disease, applied in botany equipment and methods, biochemical equipment and methods, microorganism-based methods, etc., can solve the problem of no prevention
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Embodiment 1: Preparation of hand, foot and mouth disease inactivated vaccine of the present invention
[0050] 1. Detection of enterovirus EV71 and Cox.A16 strains
[0051] (1) Viral titration determination
[0052] The Reed&Muench method is adopted to measure, and the highest virus dilution factor that can cause more than 50% of Vero cell lines to present the typical enterovirus cytopathic phenomenon is taken as the virus potency value (TCID 50 ):
[0053] Using the 199 medium without fetal bovine serum as the diluent, make ten-fold serial dilutions of the tested virus strains, and obtain different dilution ratios (1-10 10 ) virus strains were inoculated with monolayer Vero cell strains (2×10 4 cells / well) 8 replicate wells, and inoculate the same volume of 199 medium as negative control. In 199 medium containing 2% fetal bovine serum, 5% CO 2 and cultured at 37°C. After culturing, observe the results of the typical enterovirus cytopathic phenomenon of the Vero ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com