Enterovirus triple real-time fluorescent quantitative RT-PCR detection kit
A real-time fluorescence quantification and detection kit technology, applied in the biological field, can solve the problems of cumbersome operation, time-consuming, low sensitivity, etc., achieve the effect of unifying efficiency and technology, improving efficiency and time, and saving production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
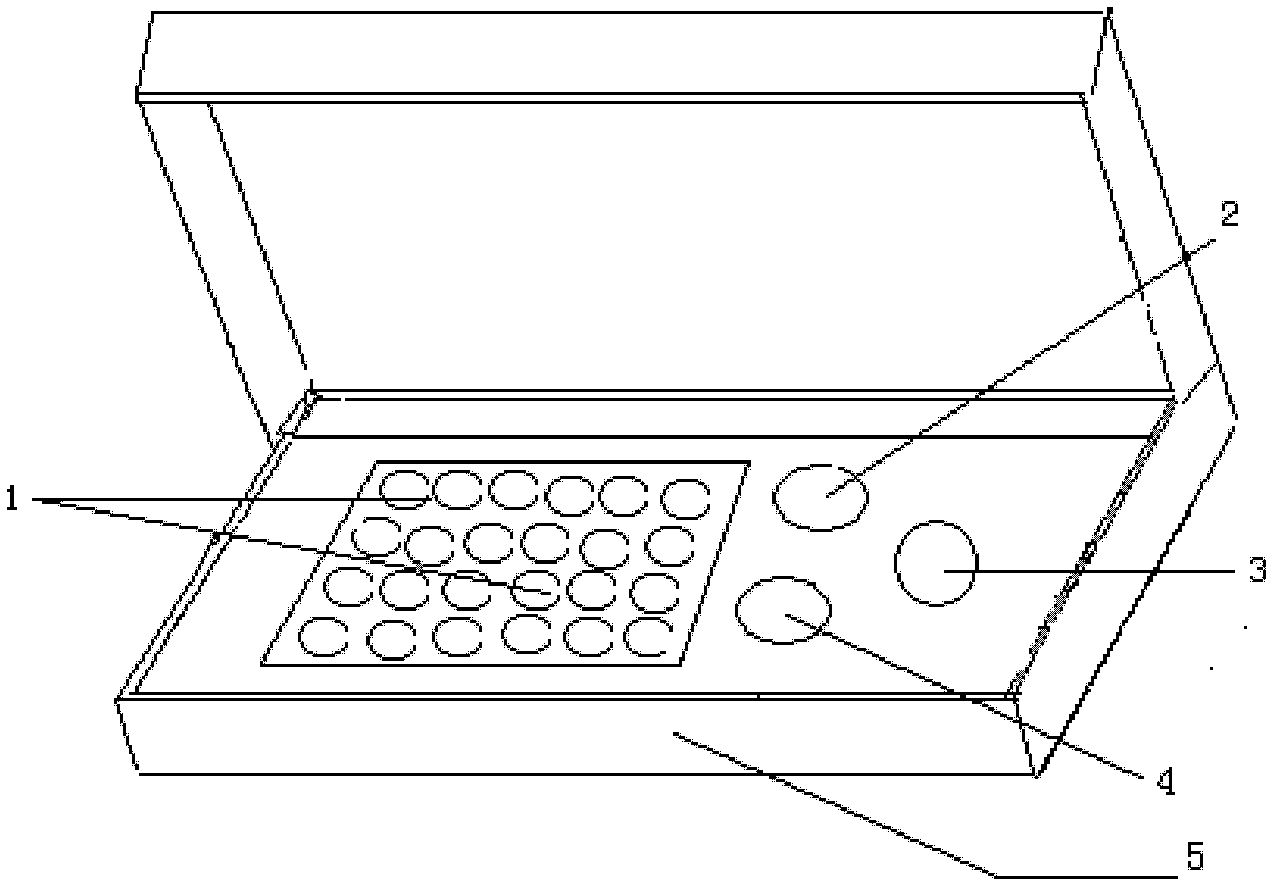
[0034] see figure 1 , the present invention provides a triplex real-time fluorescent quantitative RT-PCR detection kit for enterovirus, the reagent is composed of quantitative RT-PCR reaction solution 1, standard substance 2, positive control substance 3, negative control substance 4 and box body 5, Wherein quantitative RT-PCR reaction solution 1 contains RT-PCR buffer, MgCl 2 , dNTPs, EV universal primers, EV universal fluorescent probes, EV71 fluorescent probes, CA16 fluorescent probes, thermostable DNA polymerase (Taq DNA polymerase), reverse transcriptase (PrimeScript RTase enzyme).
[0035] Detection primers are divided into upstream primers and downstream primers:
[0036] The upstream primer sequence is: 5`- CCCTGAATGCGGCTAATCC -3`
[0037] The downstream primer sequence is: 5`- GATTGTCACCATAAGCAGCC -3`
[0038]Detection with each typing fluorescent probe sequence and respective fluorescein label:
[0039] EV universal fluorescent probe is: 5`FAM--ACACGGACACCCAAAGT...
Embodiment 2
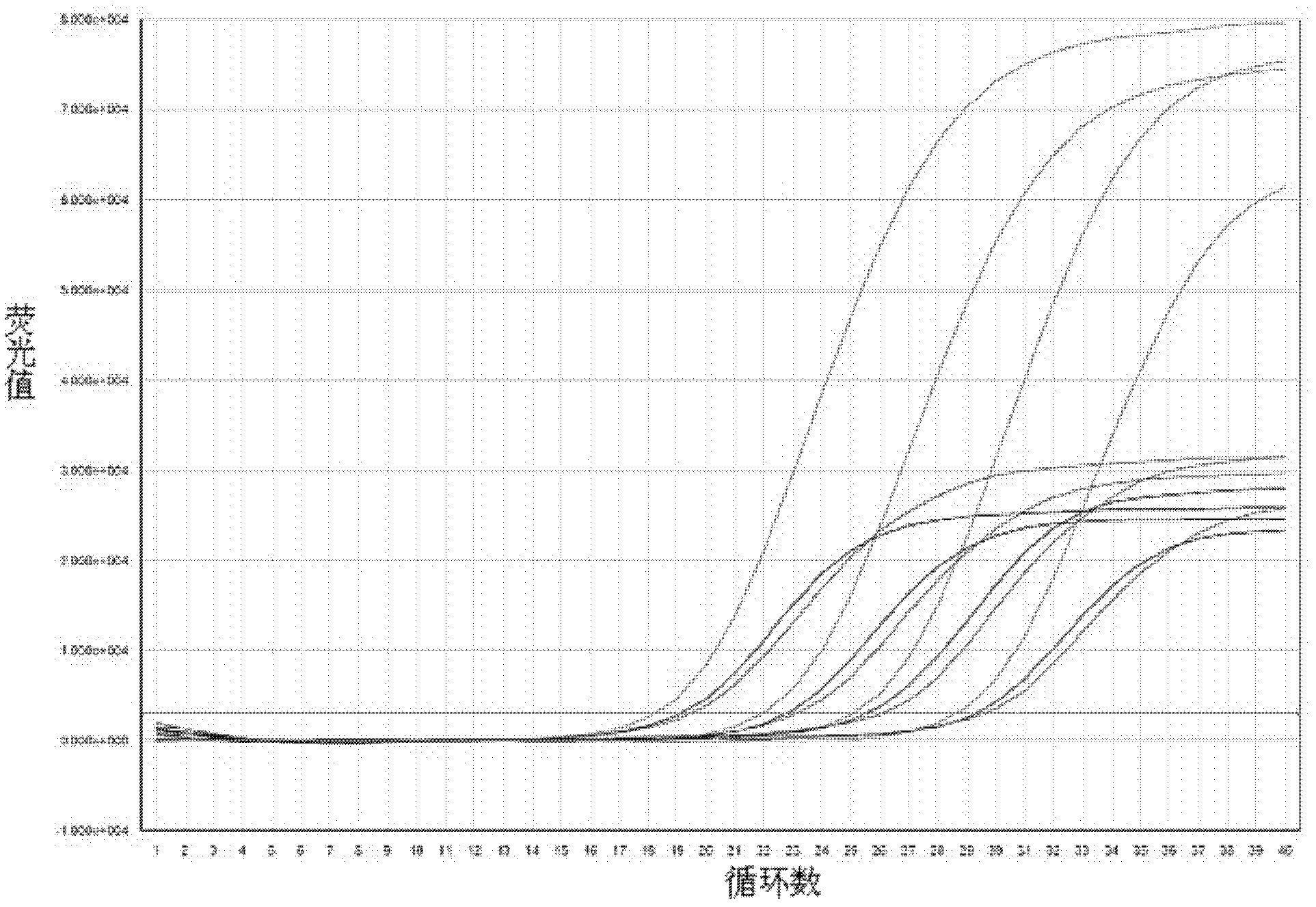
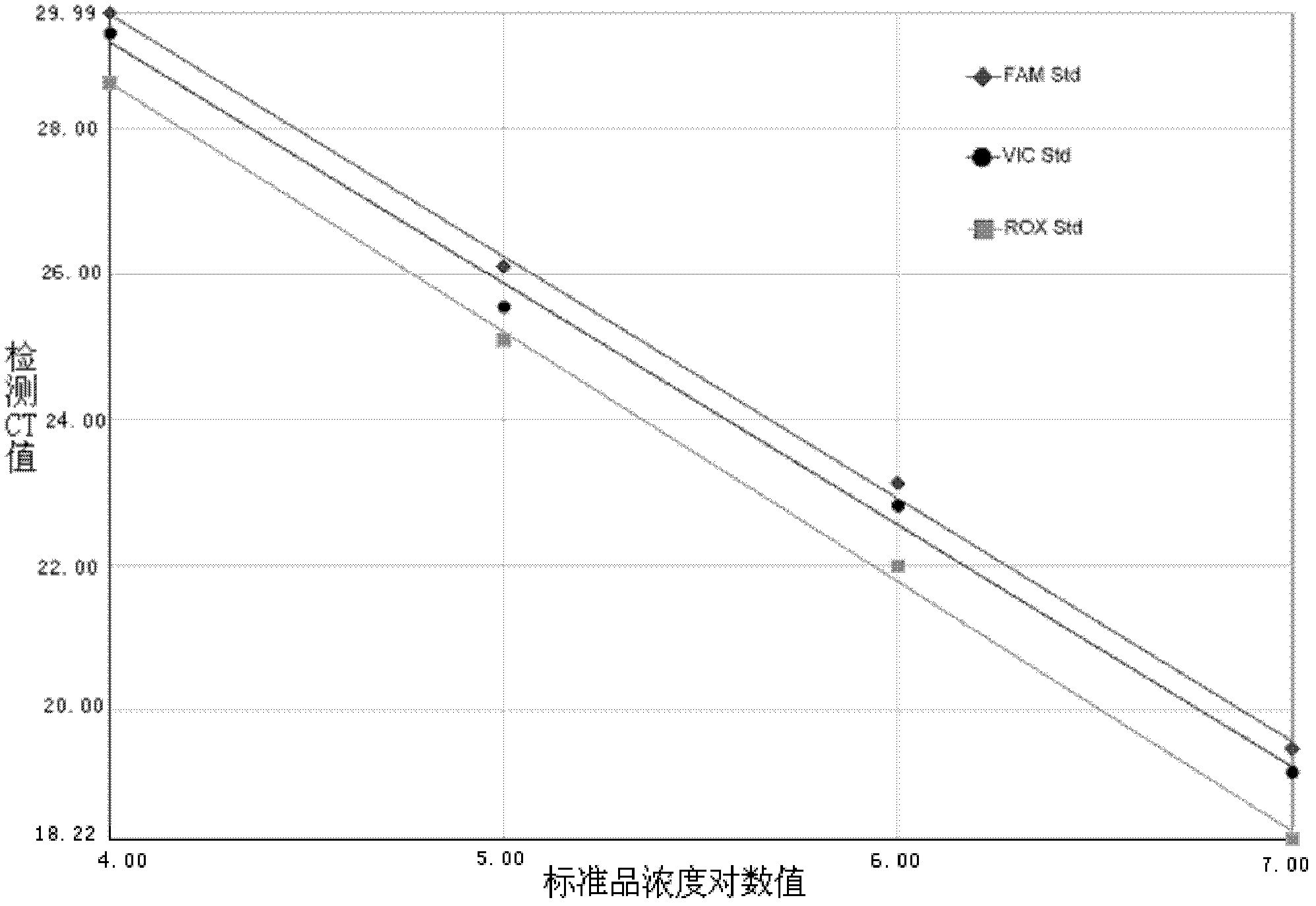
[0046] Example 2 Sensitivity and specificity experiment of enterovirus triple real-time fluorescent quantitative RT-PCR detection kit
[0047] 1. Materials:
[0048] The selected pathogenic microorganisms include the following: experimental group: coxsackievirus A group type 16, enterovirus EV71 type, coxsackievirus group B type 3 and echovirus type 30 and other enterovirus strains; control group: hepatitis A Viruses, rotavirus, beta virus, human cytomegalovirus and mycoplasma pneumoniae, etc., the above virus strains were purchased from Sun Yat-sen University Da'an Gene Company.
[0049] 2. Primer and probe design and synthesis:
[0050] Bioinformatic analysis of the nucleic acid sequence in the UTR region of enteroviruses, using software such as DNA STAR to analyze the sequence homology and differences of each type, designing and screening EV universal primers, EV universal fluorescent probes, EV71 fluorescent probes, etc. Needle, CA16 fluorescent probe.
[0051] The u...
Embodiment 3
[0061] Example 3 Application of Enterovirus Triple Real-Time Fluorescent Quantitative RT-PCR Detection Kit in Clinical Sample Detection
[0062] 1. Specimen testing:
[0063] During the epidemic period of HFMD from May 2010 to October 2010, 192 stool samples and 215 throat swab samples from outpatients and inpatients in our hospital with suspected HFMD were selected. The Enterovirus Universal Nucleic Acid Detection Kit, EV71 Nucleic Acid Detection Kit and CA16 Nucleic Acid Detection Kit provided by Sun Yat-Sen University Daan Gene Co., Ltd. and registered and approved by the State Food and Drug Administration were selected as controls. To evaluate the performance index of the application kit.
[0064] 2. Clinical sample control test results:
[0065] The results of 192 cases of stool samples and 215 cases of throat swab samples of enterovirus triple real-time fluorescent quantitative RT-PCR detection kit and single triple tube control reagent produced by Daan Company are sho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com