Three-color fluorescent RT-PCR (Reverse Transcription-Polymerase Chain Reaction) combined detection method of enterovirus 71, Coxsackie virus A16 and other subtypes of enterovirus as well as kit thereof
A RT-PCR, Coxsackie virus technology, applied in biochemical equipment and methods, fluorescence/phosphorescence, microorganism-based methods, etc., can solve problems such as indistinguishable HFMD
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
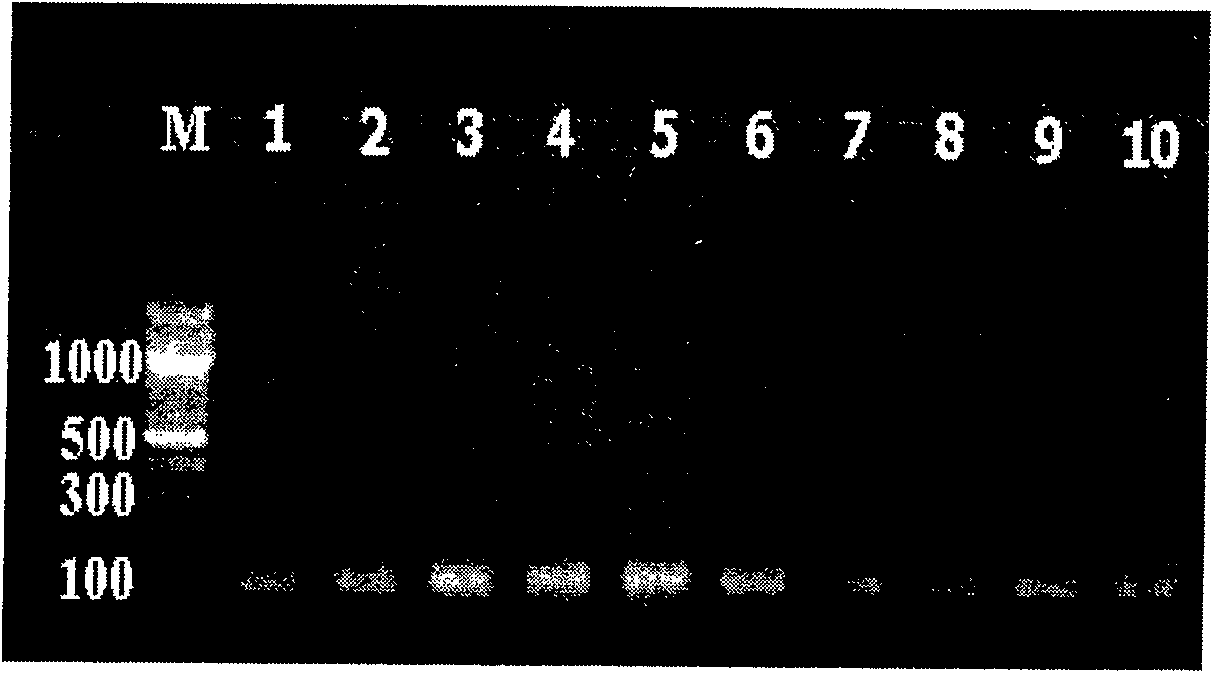
[0051] Example 1: Collection and delivery of specimens
[0052] Applicable specimen types include suspected patient feces, throat swab specimens, herpes fluid, cerebrospinal fluid or virus isolation and culture specimens, etc. Pharyngeal swab specimens: Collect throat swab specimens within 3 days of the patient's onset, use a special sampling cotton swab, and wipe the posterior pharyngeal wall and tonsils on both sides with moderate force, avoiding touching the tongue; quickly put the cotton swab into a container containing 1-2ml In the sampling tube of preservation solution (maintenance solution or saline), break off the cotton swab rod near the top, tighten the tube cap and seal it for inspection. Herpes solution: Disinfect the skin around the herpes with 75% alcohol, then use a sterile needle to pierce the herpes, dip a cotton swab into the herpes solution, and quickly put the cotton swab in it with 1-2ml of preservation solution (maintenance solution or normal saline) In ...
Embodiment 2
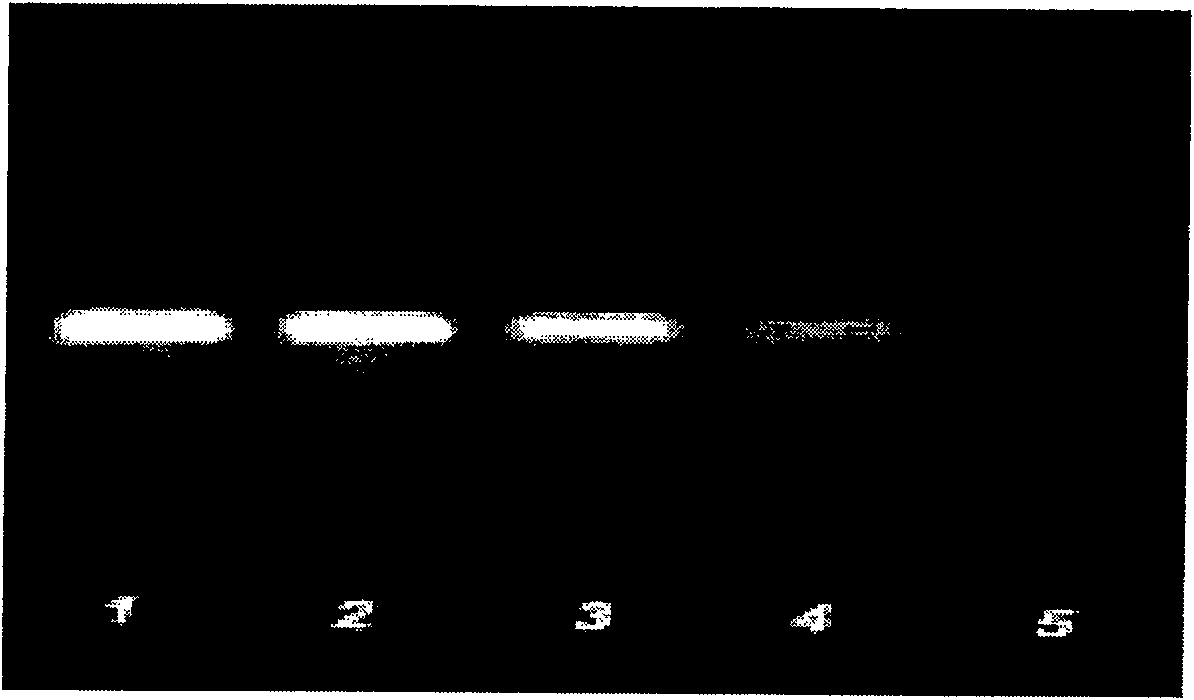
[0053] Embodiment 2: the extraction of enterovirus RNA
[0054] Sample treatment: Take 500 μl of sample solution and add 500 μl of virus concentrated solution to fully shake, centrifuge in a refrigerated centrifuge at 13,000 rpm for 10 minutes at 4°C, remove the supernatant, keep about 50 μl of sample solution, add 150ul Trizolreagents (RNA extraction solution) to fully shake, and place at room temperature for 5 minutes . Add 100ul of chloroform, shake vigorously for 15s, let stand at room temperature for 5min, and centrifuge at 13,000rpm at 4°C for 10min. Carefully transfer the upper aqueous phase to a clean centrifuge tube, add an equal volume of isopropanol, mix well, and centrifuge at 13,000rpm for 10min. Discard the supernatant, add 500 μl of 75% DEPC ethanol, mix well, centrifuge at 13,000 rpm for 10 min, and carefully absorb most of the ethanol. Open the extraction tube and dry it in the air at room temperature for 5 min until the ethanol evaporates, and dissolve the ...
Embodiment 3
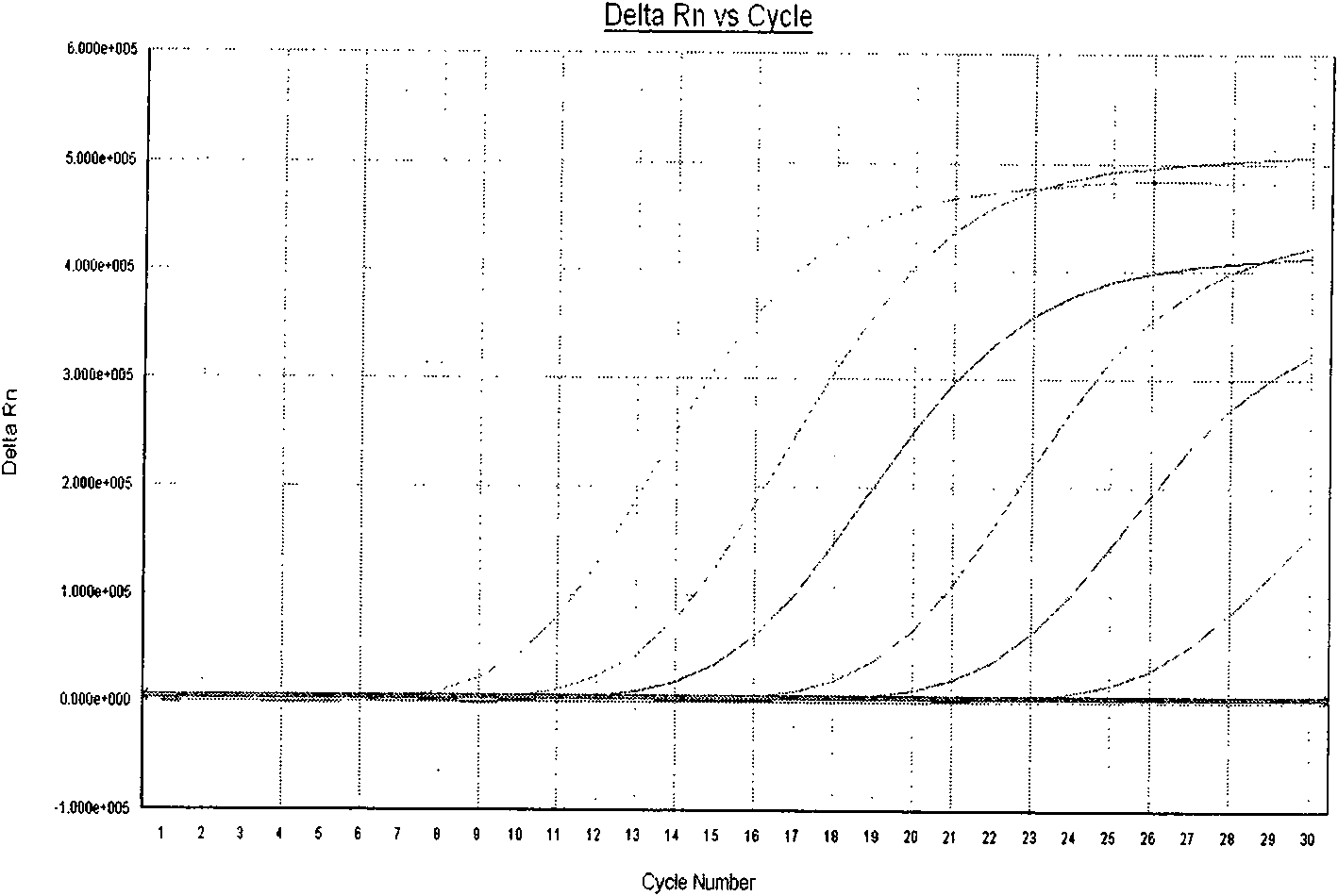
[0055] Embodiment 3: Applicable instruments for this detection method and kit
[0056] Applicable instruments mainly include ABI GeneAmp PCR System7700, ABI GeneAmp PCRSystem7500, ABI PRISM 7300, LightCycler, etc., capillary instruments such as LightCycler, MJOpticon series or qualified quantitative PCR instruments.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com