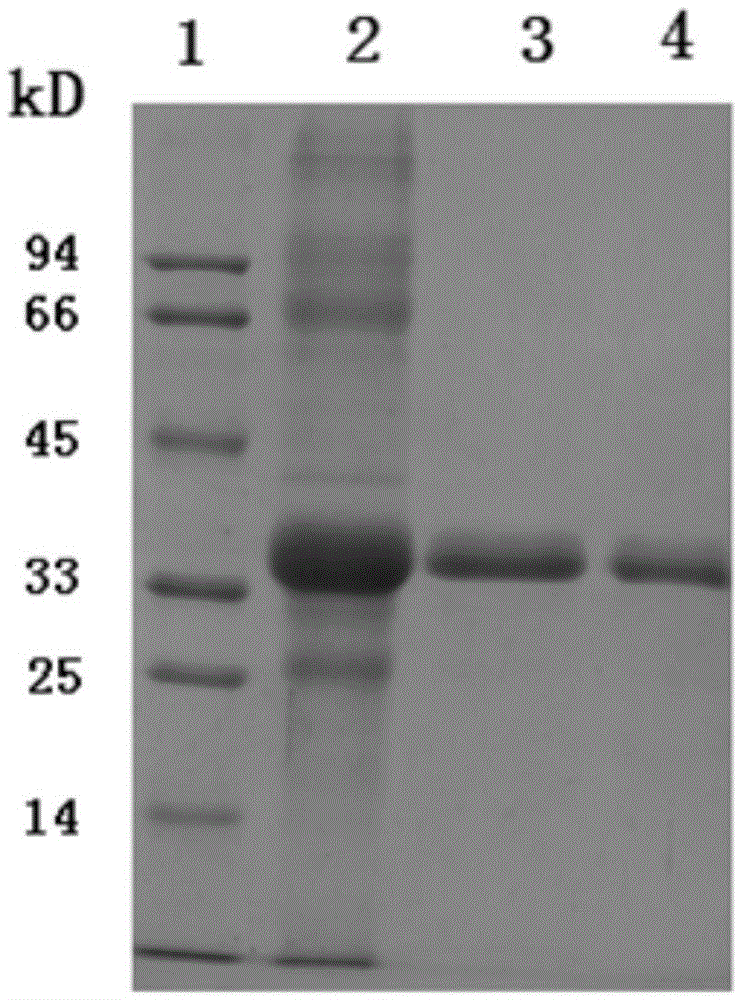
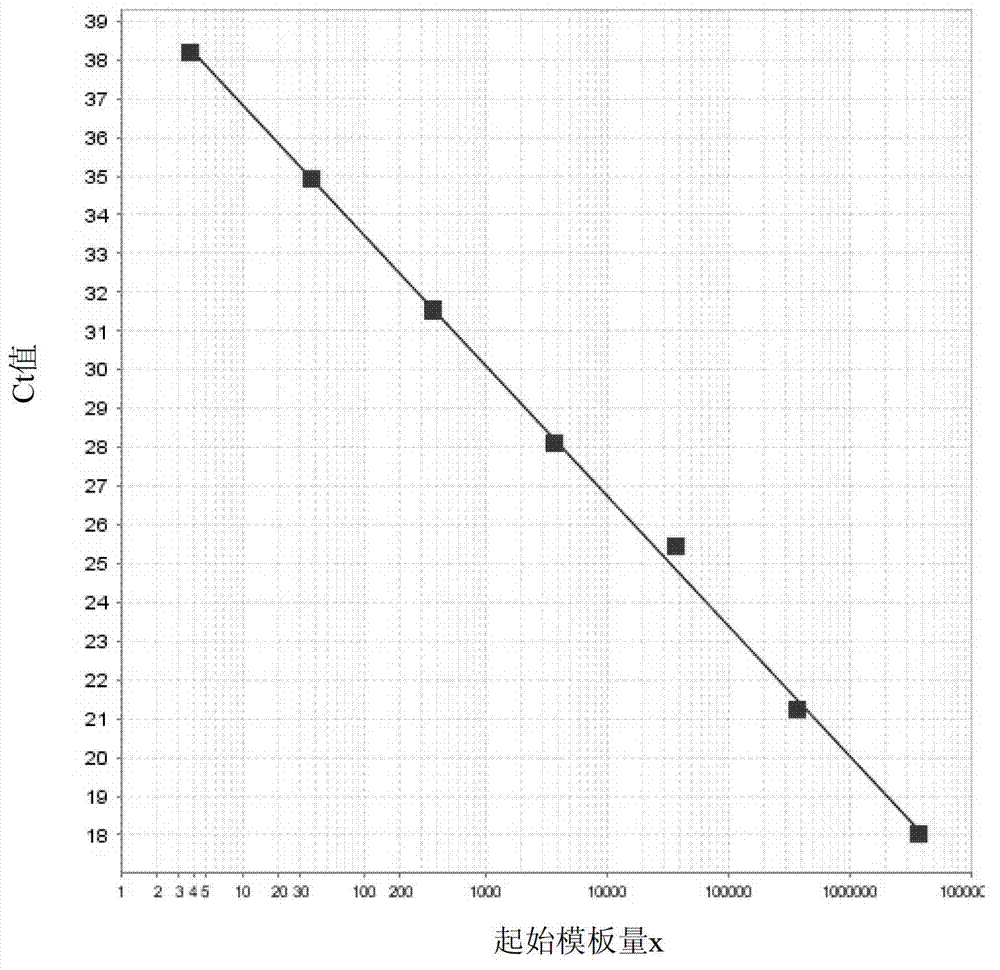
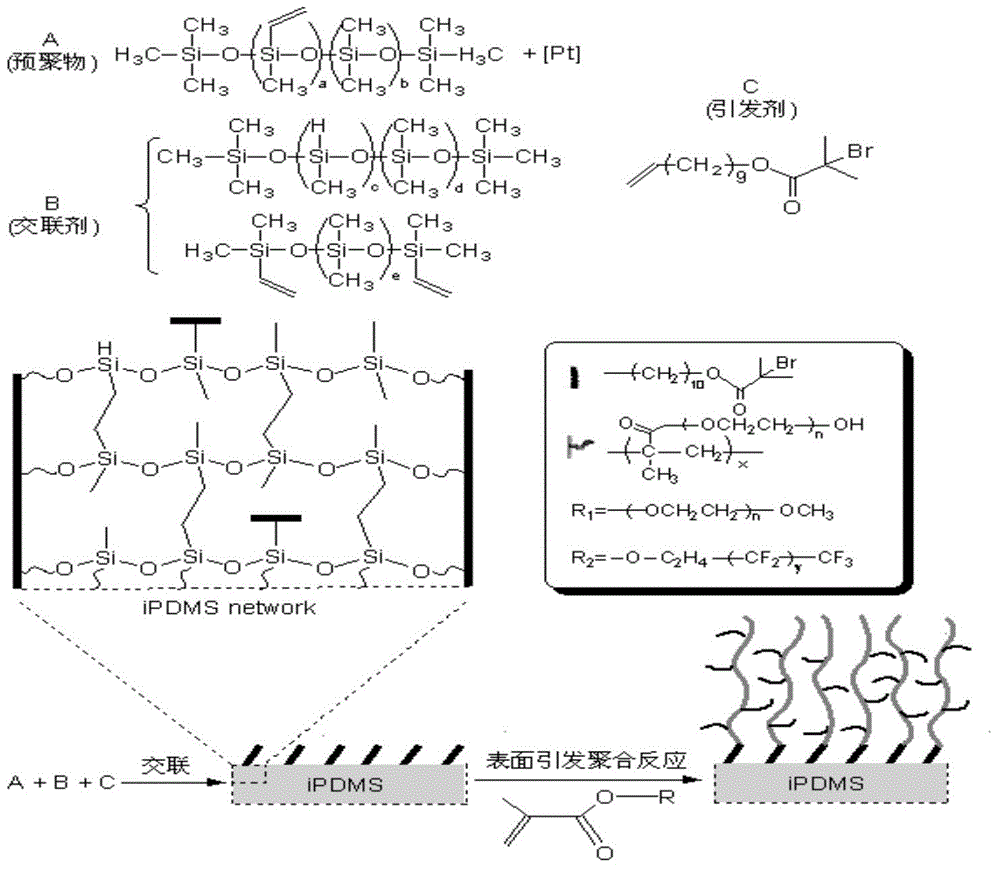
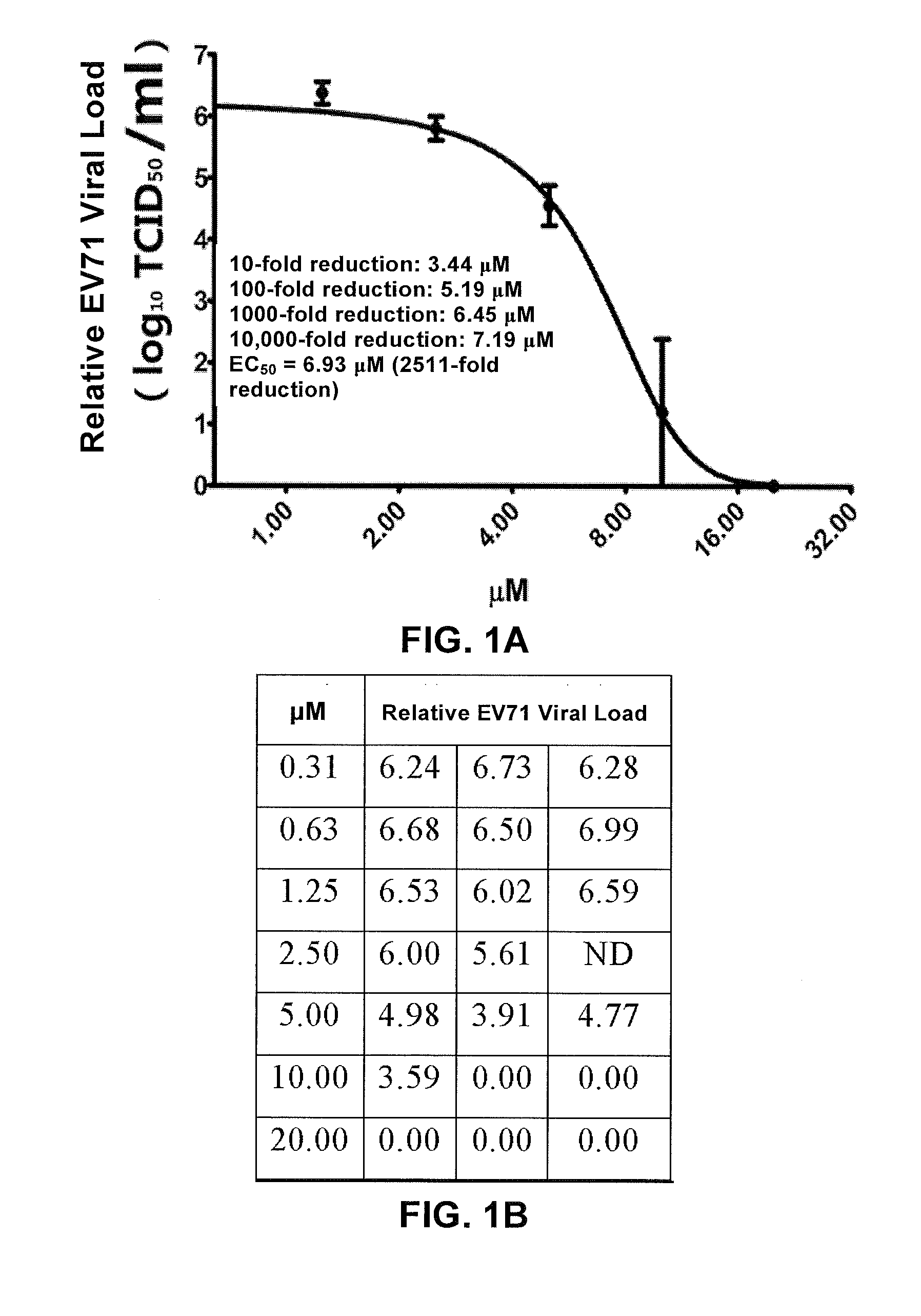
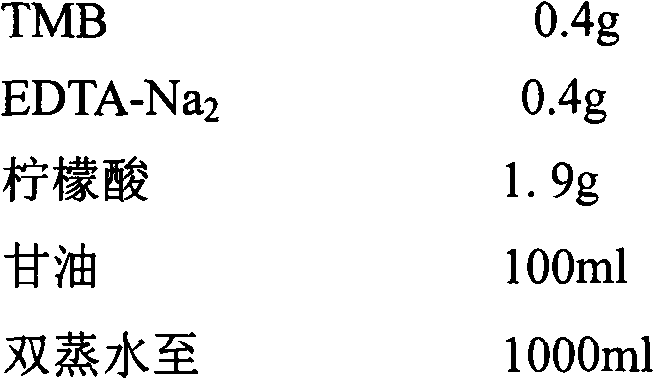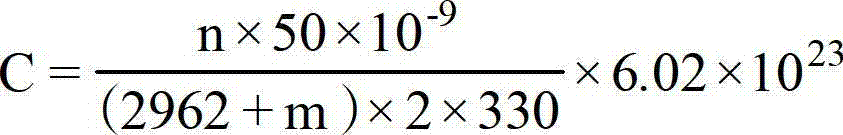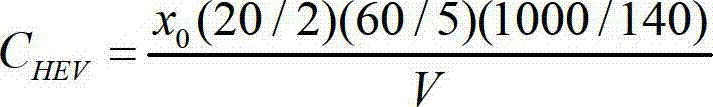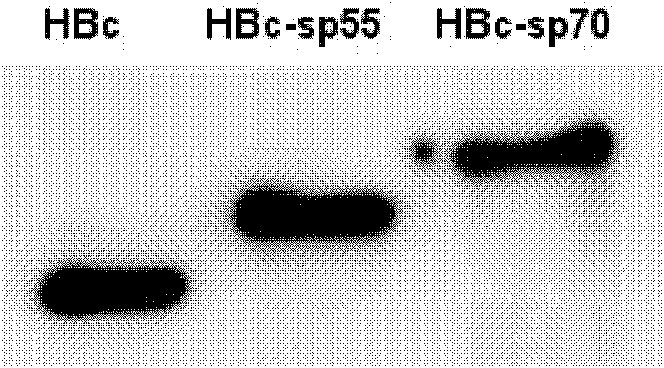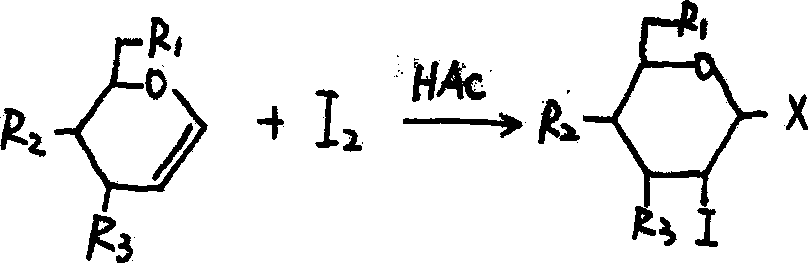Patents
Literature
83 results about "Human enterovirus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Nucleic acid sequences for the amplification and detection of respiratory viruses
InactiveUS20100279273A1Improve the situationSsRNA viruses negative-senseSsRNA viruses positive-senseSpecific detectionHuman respiratory virus
The present invention relates to methods of detection, as well as assays, reagents and kits for the specific detection of 15 clinically important respiratory viruses including influenza A and B viruses, human respiratory syncytial viruses, human metapneumoviruses, human enteroviruses, all serotypes of rhinoviruses, 7 serotypes of adenoviruses, parainfluenza viruses types 1, 2, 3, and 4, as well as coronaviruses NL, 229E, OC43, and SARS-CoV. The present invention allows for the detection of each of these respiratory viruses in a single assay.
Owner:UNIV LAVAL
Method for determining cross antigen region initiating human antigens in human enterovirus 71-type total protein
The invention belongs to the field of biomedicine, and provides a method for determining a cross antigen region initiating human antigens in human enterovirus 71-type (EV71) total protein. In the method, the human enterovirus 71-type total protein is identified through segmental expression; and cross reaction between immunogenic and inductive antibodies and the human antigens in each segment is performed, and different segments of the human enterovirus 71-type total protein are divided into three classes: (1) strongly-crossed immunogens initiating the human antigens; (2) weakly-crossed immunogens initiating the human antigens; and (3) no crossed immunogens existing between the antibodies and the human antigens. Thus, the method plays a guidance role in developing and preparing human enterovirus 71-type vaccines, and hand-foot-and-mouth disease vaccines having no cross reaction or weak cross reaction with human bodies can be designed according to the method.
Owner:INST OF LAB ANIMAL SCI CHINESE ACAD OF MEDICAL SCI
Antigens and Vaccines Directed Against Human Enteroviruses
The instant invention provides materials and methods for producing immunologically active antigens derived from members of the Picornaviridae virus family. The picornavirus antigens of the invention may be in a form for use as a vaccine administered to a subject in a therapeutic treatment or for the prevention of a picornavirus infection. The picornavirus antigens of the invention may be in the form of an immunogenic composition for use in vaccines which are administered for the prevention of an Enterovirus infection. The instant invention further encompasses immunogenic compositions comprising Human enterovirus A, Human enterovirus B, Human enterovirus C, Human enterovirus D antigens and their use in vaccines for the prevention of an Enterovirus infection.
Owner:SENTINEXT THERAPEUTICS
Human enterovirus(EV) fluorescence quantitative PCR detecting technology
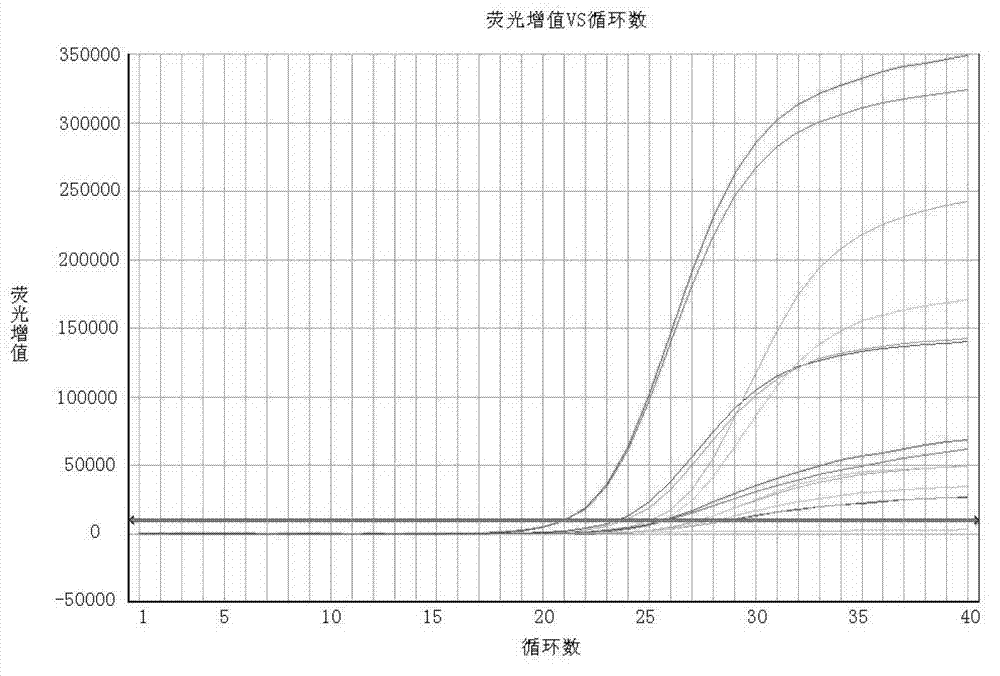
This invention relates to one human intestinal canal virus fluorescence meter test technique in the field of virus nuclear test technique, which adopts common upstream object, common downstream object and common fluorescence detector for EV each hypotype, wherein, the detector 5'end is labeled with fluorescence emission base group 6- carboxyl fluorandiol and 3'labeled fluorescence quencher base group and 6-carboxyl tetramethyl rhodamine. Due to the test agent case, it tests each EV hypotype standard blood serum for online test and to get each parameter as the following: abnormal property for 100 percent; sensitivity for 95 percent, repetitiveness CV less than 10 percent and minimum content of EV as 500 copy each ml.
Owner:河南省生物工程技术研究中心
Colloidal gold test strip for rapid detection of Human enterovirus 71 (EV71) IgM (immunoglobulin m)
The invention discloses a colloidal gold test strip for rapid detection of Human enterovirus 71 (EV71) IgM (immunoglobulin m), belonging to the field of medical detection consumables. The colloidal gold test strip is characterized in that a colloidal gold-labeled cellulose pad is coated with colloidal gold-labeled EV71 antibody; a control line C is coated with anti-mouse IgG polyclonal antibody; a test line T is coated with anti-Mu chain monoclonal antibody; and a sample cellulose pad is coated with EV71 recombinant antigen. The inventive test strip adopts antibody capture principle and colloidal gold technology to detect EV71 IgM in serum / plasma / whole blood samples simply, sensitively, precisely and rapidly; and can be used for the rapid detection of EV71 IgM and diagnosis of hand-foot-mouth disease.
Owner:蓝十字生物药业(北京)有限公司 +1
Human enterovirus 71 type specific polypeptide and application thereof
InactiveCN101891805AStrong immune responseImmunoglobulins against virusesAntiviralsSerum igePoliovirus
The invention belongs to the field of medical immunology, and discloses human enterovirus 71 type specific polypeptide and application thereof. The amino acid sequence of the human enterovirus 71 type specific polypeptide, which is capable of triggering an immunological response to EV71, is derived from the polypeptide 1 derived and has an amino acid sequence SEQ ID NO.1 of an amino acid sequence which is formed by substituting, losing or adding 1 to 10 amino acid residues in amino acid sequence SEQ ID NO.1, or a polypeptide fragment which has the function of triggering the immunological response to EV71 and of which the length of the amino acid sequence is at least 15 continuous amino acid residues in the SEQ ID NO.1. The polypeptide provided by the invention can be specifically reacted with the blood serum of human enterovirus 71 type patients, and simultaneously not cross reacted with poliomyelitis viruses. Therefore, the human enterovirus 71 type specific polypeptide can be applied to clinical test and diagnosis of human enterovirus 71 type infection.
Owner:BEIJING KYNING BIOSCI
Typing of human enteroviruses
The present invention discloses a method for detecting the presence of an enterovirus in a clinical sample. The invention additionally discloses a method for typing an enterovirus in a clinical sample. Both methods employ a set of primer oligonucleotides for reverse transcription and amplification that hybridize to conserved regions of the enterovirus genome, and that provide amplicons that include significant portions of the VP1 region that are characteristic of the various serotypes. In the typing method, the invention further provides a database consisting of nucleotide sequences from prototypical enteroviral serotypes, which is used to type the clinical sample by comparing the sequence of its amplicon with each prototypical sequence in the database. The invention additionally provides mixtures of primer oligonucleotides, and a kit for use in conducting the typing method that includes a mixture of the primer oligonucleotides,
Owner:US DEPT OF HEALTH & HUMAN SERVICES
EV71 (human enterovirus 71) antigen enzyme-linked reaction detection kit and its preparation method
The invention relates to the biotechnical field, concretely relates to a virus antigen content detection kit and its preparation method, and more concretely relates to an enzyme-linked reaction kit for qualitatively and quantitatively detecting the EV71 virus antigen content, and its preparation method. The double-antibody enzyme-linked reaction detection kit adopted in the invention and its preparation method have the characteristics of good specificity, high sensitivity, convenience, fastness, economy and the like.
Owner:BEIJING HUAWEI BRAVOBIO
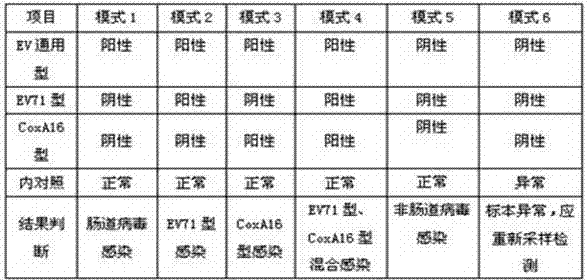
Human enterovirus four-color fluorescence RT-PCR detection kit and detection method
InactiveCN102851391AEffective monitoringStrong specificityMicrobiological testing/measurementMicroorganism based processesForward primerReverse transcriptase
The invention discloses a human enterovirus four-color fluorescence RT-PCR detection kit and a detection method; the detection kit comprises multiple fluorescence RT-PCR reaction mother liquor, AMV reverse transcriptase liquid, Taq DNA polymerase liquid, a positive control, and a negative control; the detection kit is characterized by further comprising an internal control, a multiple 10*PCR reaction buffer, bovine serum albumin with a concentration of 20 mg / ml, various forward primers and reverse primers with a concentration of 100 muM, and various specific probes with a concentration of 50 muM, wherein sequences of the forward primers, reverse primers, specific probes and the internal control are as shown in SEQIDNO. 1, NO. 2, NO.3, NO.4, NO.5, NO.6, NO.7. NO.8, NO.9, NO.10., NO.11., NO.12., and NO.13. The detection kit of the invention has the advantages of high detection sensitivity, good specificity, accuracy, high speed, and no false positivity.
Owner:NINGBO ACAD OF SCI & TECH FOR INSPECTION & QUARANTINE
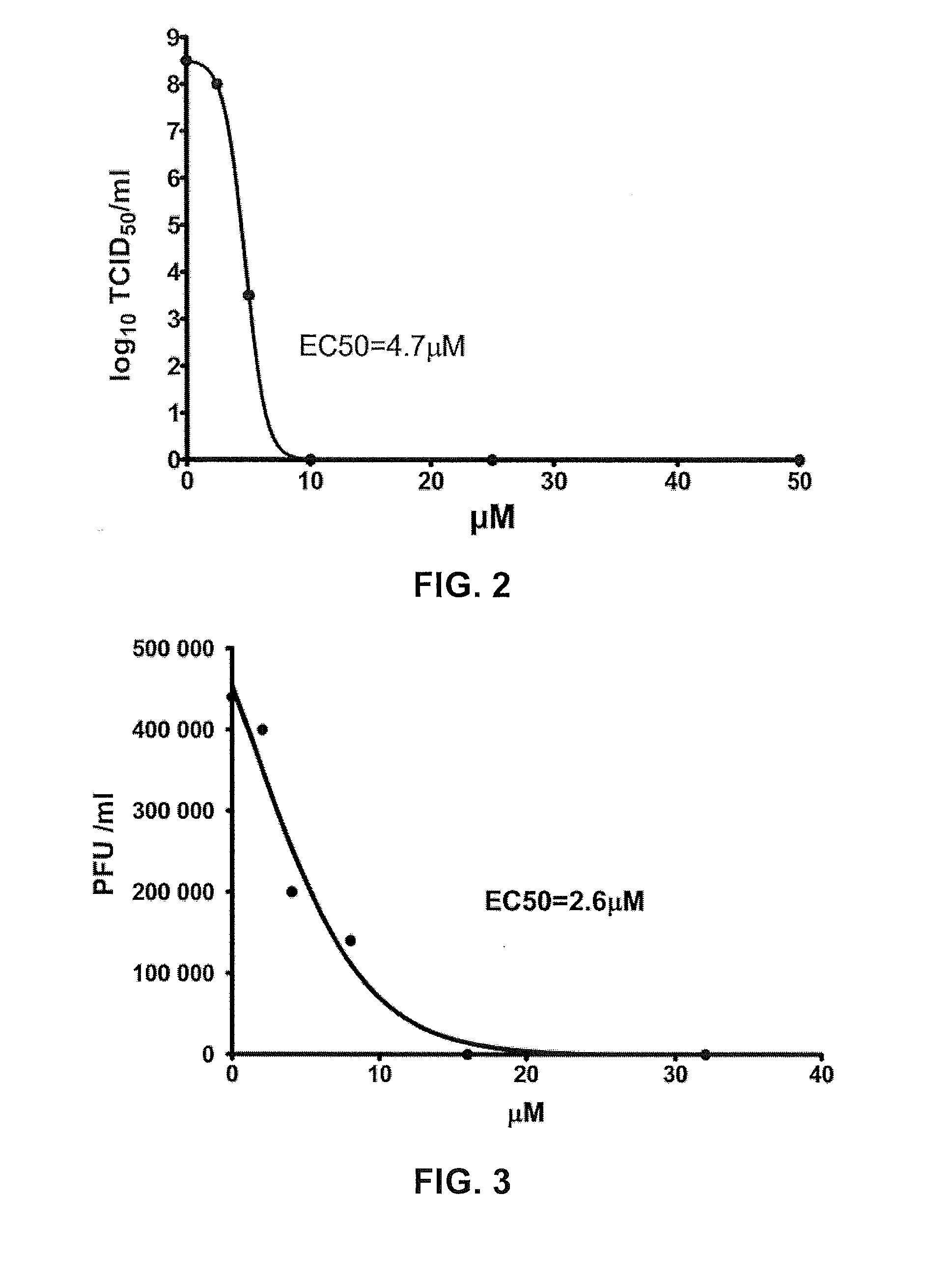
Application of lycorine in preparing medicament for treating diseases caused by human enterovirus 71 type infection
ActiveCN102178678AReduce mortalityRelieve symptomsOrganic active ingredientsNervous disorderViral MyocarditisPulmonary edema
The invention belongs to the field of medicaments, discloses application of lycorine, lycorine salt, lycorine hydrate, lycorine optical isomer or lycorine prodrug in preparing medicaments for treating diseases caused by human enterovirus 71 type infection, preferably treating hand-foot-and-mouth disease, herpangina, viral meningitis, viral encephalitis, flaccid paralysis, pulmonary edema and vital myocarditis. In vitro and in vivo tests prove that the lycorine can inhibit replication and lesion of enterovirus (EV) 71 in cells, has excellent function of inhibiting the EV 71 virus, and has clinical application prospect.
Owner:INST OF LAB ANIMAL SCI CHINESE ACAD OF MEDICAL SCI
EV71 subunit vaccine of mixed adjuvant and preparation method thereof
InactiveCN105535963AHigh School and ValenceGood immunogenic responseSsRNA viruses positive-senseViral antigen ingredientsEscherichia coliImmunogenicity
The invention discloses an EV71 subunit vaccine of a mixed adjuvant and a preparation method thereof. According to the vaccine, C4 sub-type EV71 virus VP1 protein is used as an antigen, and aluminum hydroxide and monophosphoryl lipid A are used as a mixed adjuvant. VP1 protein which is expressed and purified by escherichia coli and does not have any label is used as an antigen; the prepared protein is combined with an aluminum adjuvant under a denaturing condition, is subjected to detergence determination, and is mixed with an MPLA adjuvant to immune mice; and the prepared polyvalent antibody can be used for specifically neutralizing the EV71 virus to generate a neutral protective antibody having a titer nearly 1:128. Compared with an EV71 inactivated vaccine in current clinical tests, the human enterovirus 71 type subunit vaccine has the characteristics of low cost, simple operation, high safety, easy large-scale production and the like in the production process. The vaccine can generate relatively good immunogenicity in a human body, has a relatively high titer of the neutralizing antibody, and is an alternative vaccine with potential clinical application value.
Owner:BEIJING KYNING BIOSCI
Method for detecting content of human enteroviruses and non-human enteroviruses in environmental water
InactiveCN102888469AHigh sensitivityHigh precisionMicrobiological testing/measurementFluorescence/phosphorescenceReaction systemBiology
The invention discloses a method for detecting content of human enteroviruses and non-human enteroviruses in environmental water. On the basis of the established method for quantitatively detecting the enteroviruses in the environmental water, primers and probes for the human enteroviruses are selected, recombinant plasmids are prepared as standard substances, a real-time fluorescent quantitative PCR reaction system for quantitatively detecting the human enteroviruses is established, the content of the human enteroviruses and the enteroviruses in environmental water is quantitatively detected to further determine a distribution condition of the non-human enteroviruses. The method has good specificity and high sensitivity, accurately quantifies the human enteroviruses and the enteroviruses and determines relationship between the human enteroviruses and the non-human enteroviruses in the environmental water.
Owner:XI'AN UNIVERSITY OF ARCHITECTURE AND TECHNOLOGY
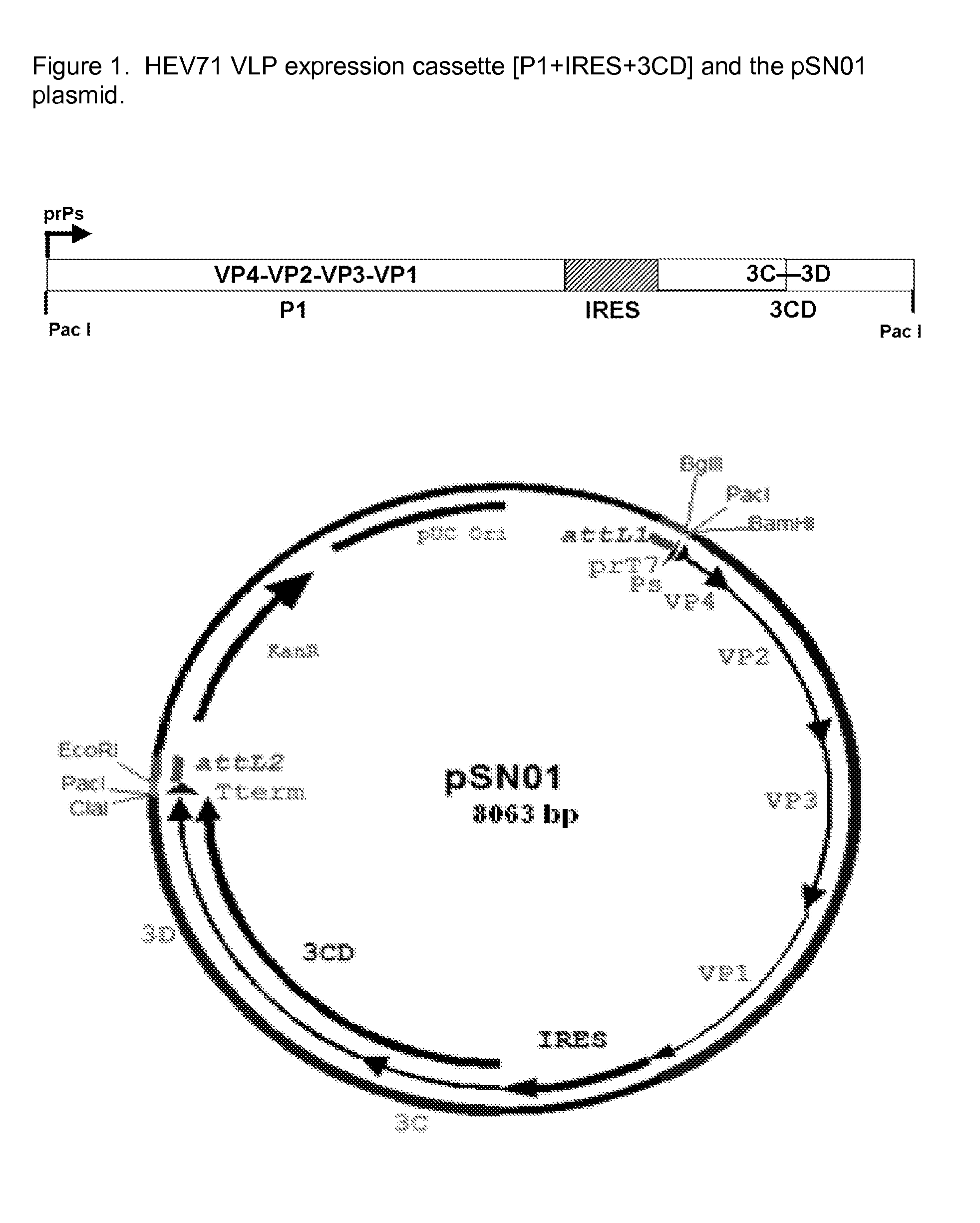
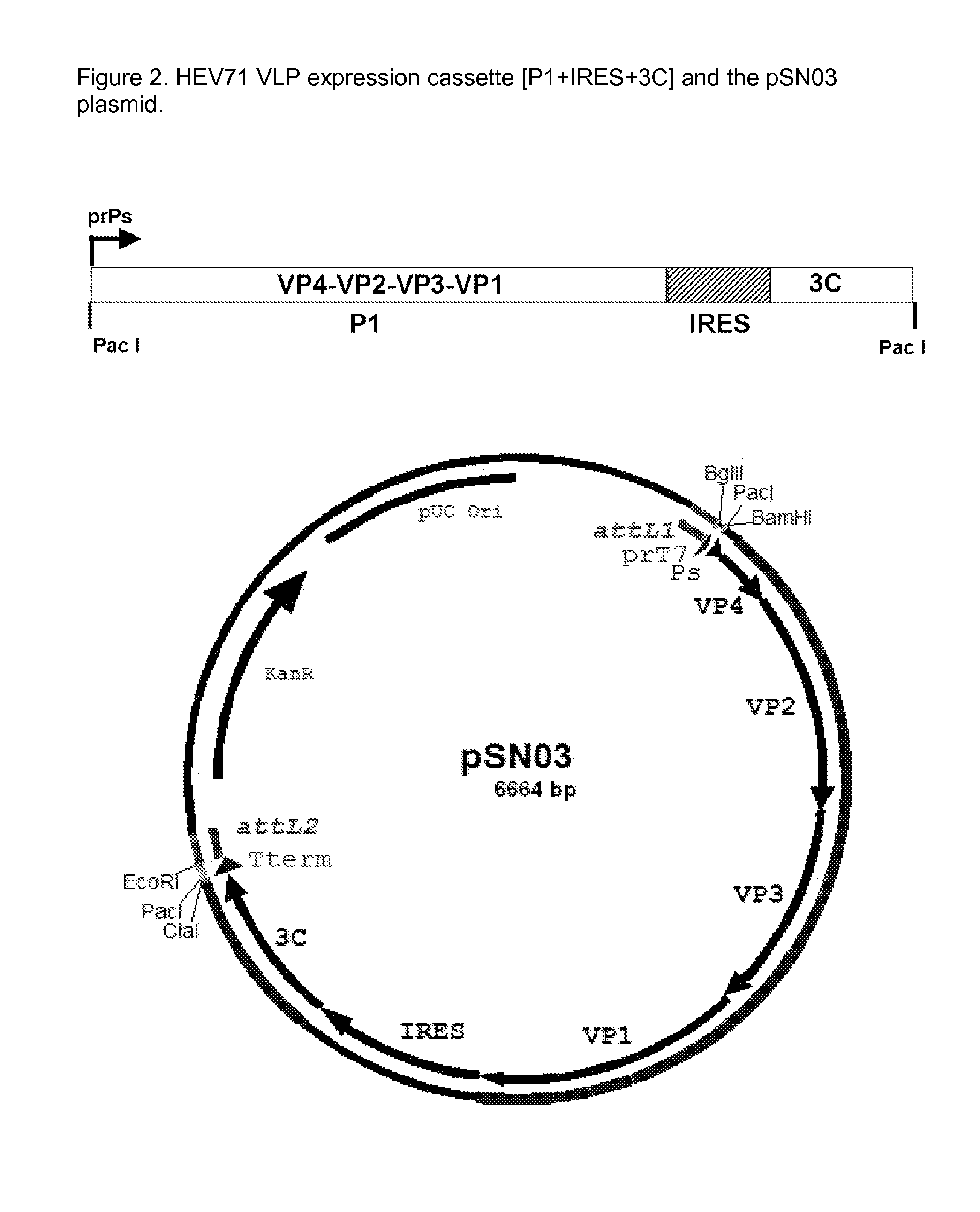
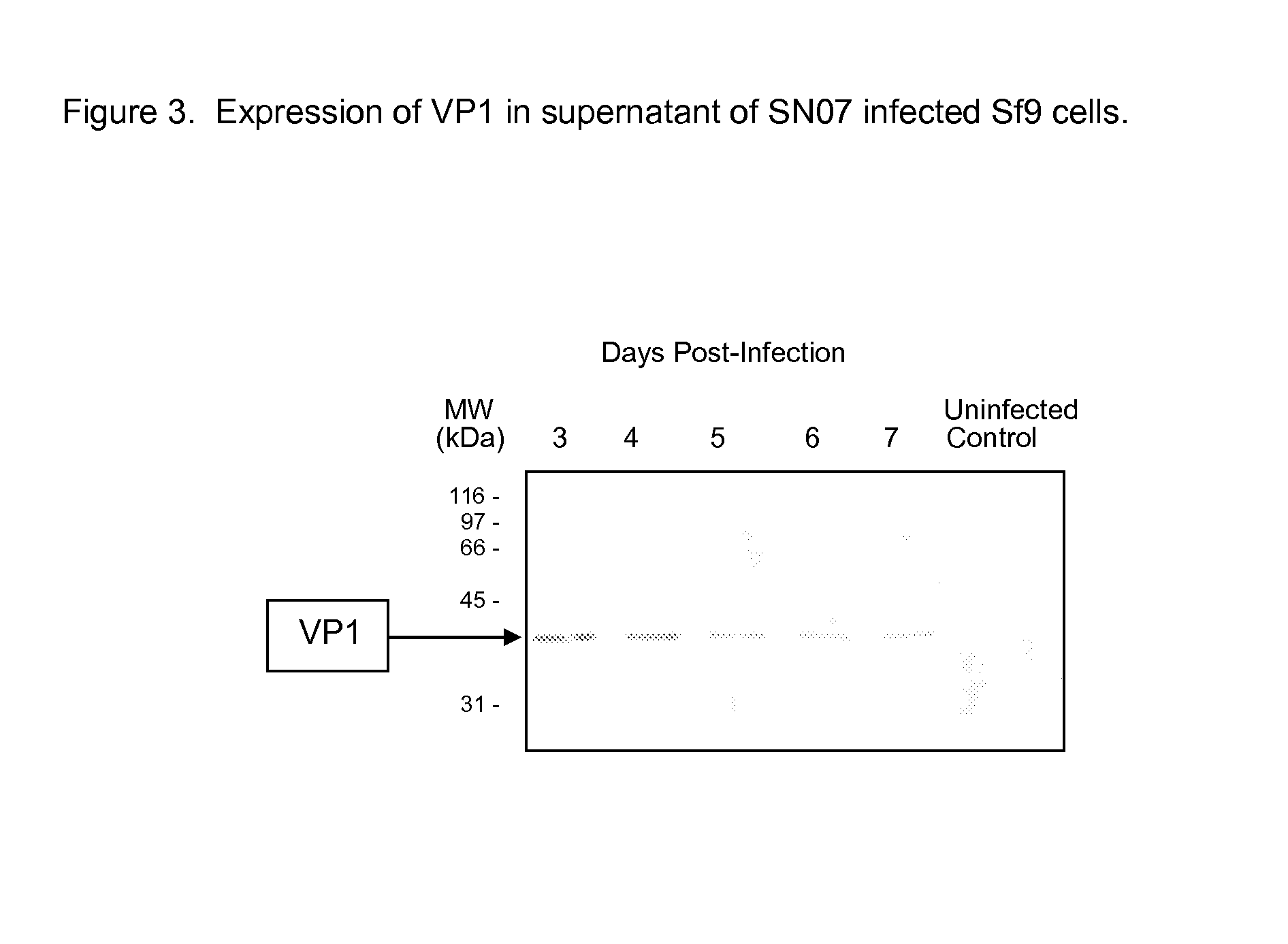
Recombinant adenovirus expressing human enterovirus 71 capsid protein and vaccine prepared from same and application thereof
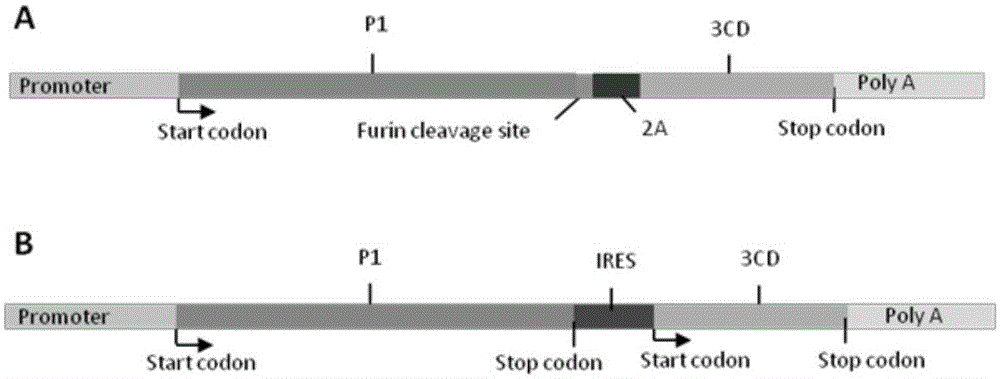
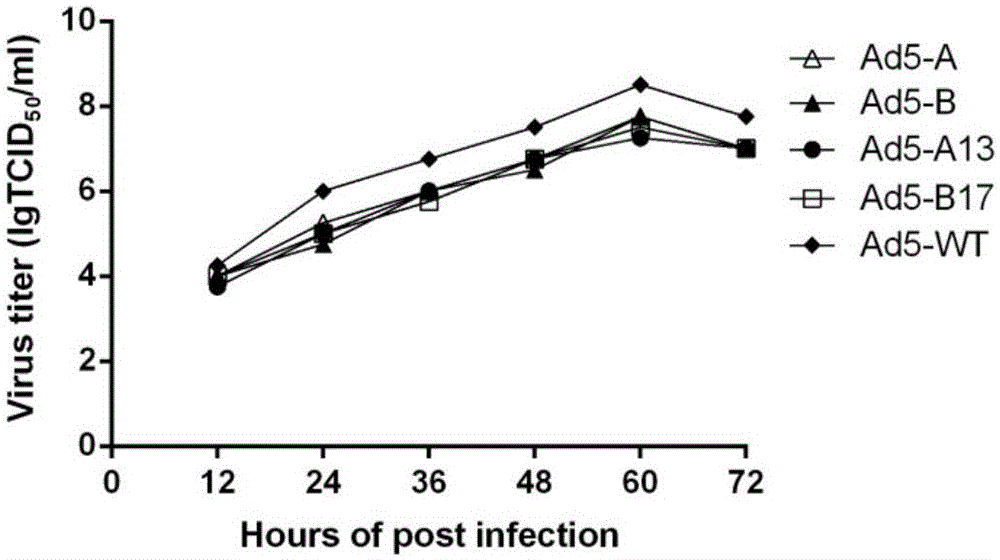
ActiveCN106318955AImprove immune activityGood antigenicityMicroorganism based processesAntiviralsInfected cellNeutralizing antibody
The invention discloses a recombinant adenovirus expressing human enterovirus 71 (EV71) capsid protein and a vaccine prepared from same and an application of the recombinant adenovirus. In the invention, two groups of EV71 sub-genome containing the P1 gene and 3CD gene of EV71 capsid protein are constructed from foot and mouth disease virus 2A gene fused with an alkaline protease cleavage site and a foot and mouth disease virus IRES element as Linker; in the invention, the sub-genome is recombined into an adenovirus expression vector, and a recombinant adenovirus with good replication capacity, genetic stability and high protein expression is obtained by screening and can efficiently express P1 and 3CD proteins in the infected cells while the 3CD can correctly cut the P1; and the recombinant adenovirus can induce a high-level EV71 specific broad-spectrum neutralizing antibody in a mouse, has remarkably better ability of inducing anti-EV71 specific cellullar immunologic response than an inactivated whole-virus antigen, and can be used for preventing and treating human hand-foot-and-mouth disease caused by EV71.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI +1
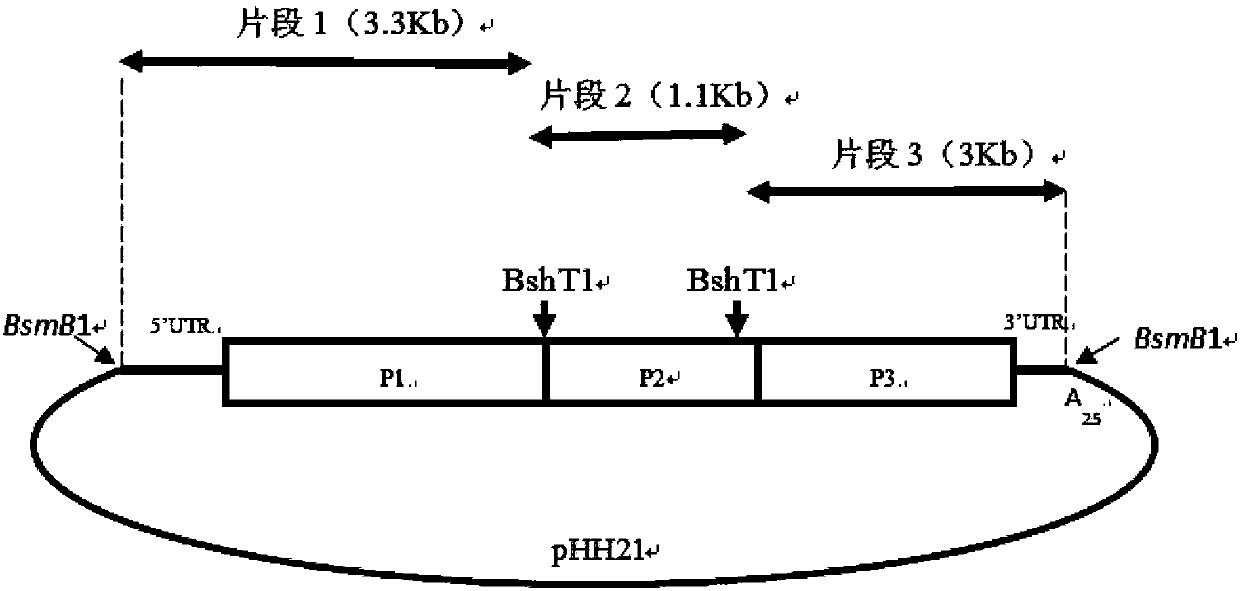
Human enterovirus D68 type infectious clone and construction method and application thereof
InactiveCN107893083AAvoid pollutionSave experimental stepsSsRNA viruses positive-senseGenetically modified cellsVirulent characteristicsRestriction site
The invention belongs to the field of biotechnology, and particularly relates to a human enterovirus D68 type infectious clone and a construction method and application thereof. The infectious clone is obtained by using a RNA polymerase I system and inserting the full-length cDNA of EVD68Fermon strain; the 1882 and 2593 BsmB1 restriction sites of the full-length cDNA are subjected to the mutationwithout changing amino acids, and the BsmB1 restriction sites are inserted at both ends. The recombinant plasmid containing the complete genomic cDNA clone of EV-D68 strain Fermon fills in the gap ofthe Chinese human enterovirus D68 type infectious clone, and the construction method is simple and highly efficient. At the same time, the application of the human enterovirus D68 type infectious clone provides a powerful tool for the search for the virulence decision sites of enterovirus 68 type and the development of drugs and vaccines resistant to enterovirus D68 type viruses.
Owner:TIANJIN UNIV
Subunit mixed vaccine of human enterovirus 71
ActiveCN101947316APlay a protective effectPotential clinical application valueAntiviralsAntibody medical ingredientsAnti virusSide effect
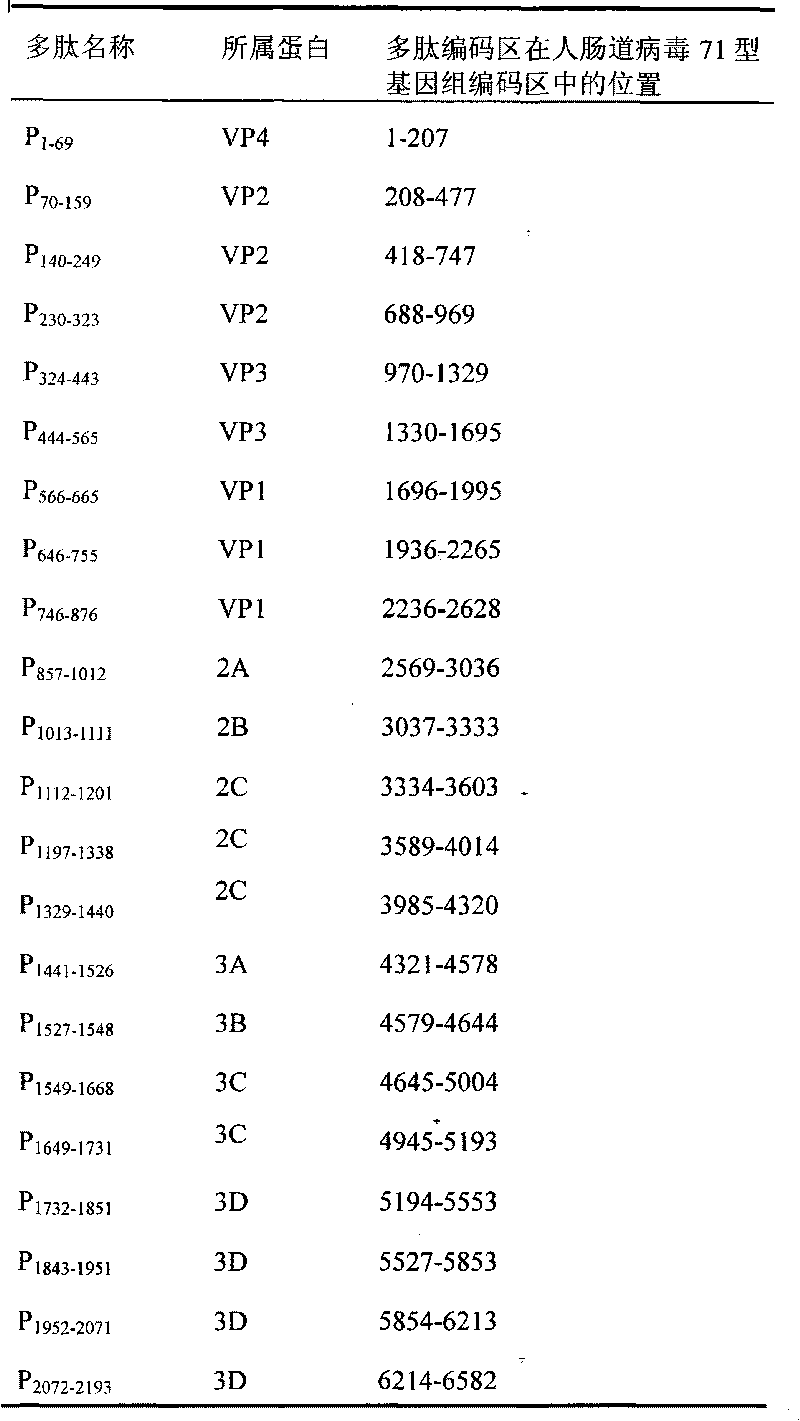
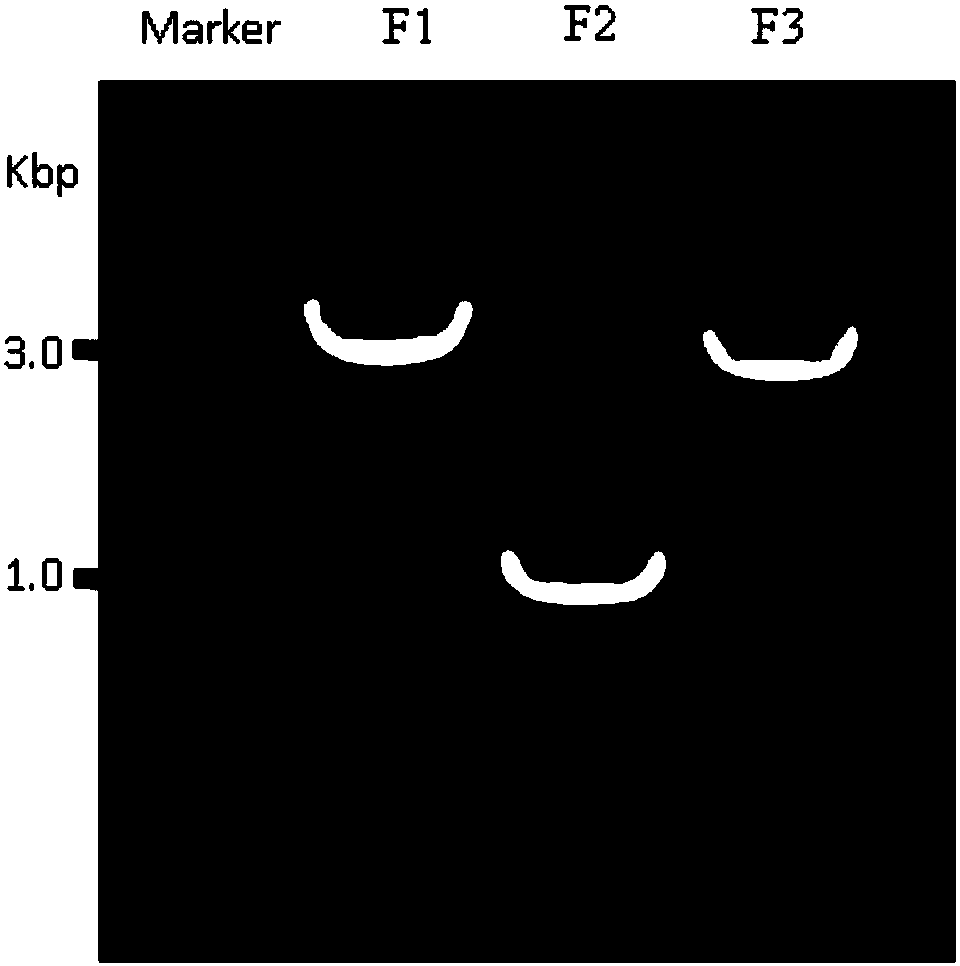
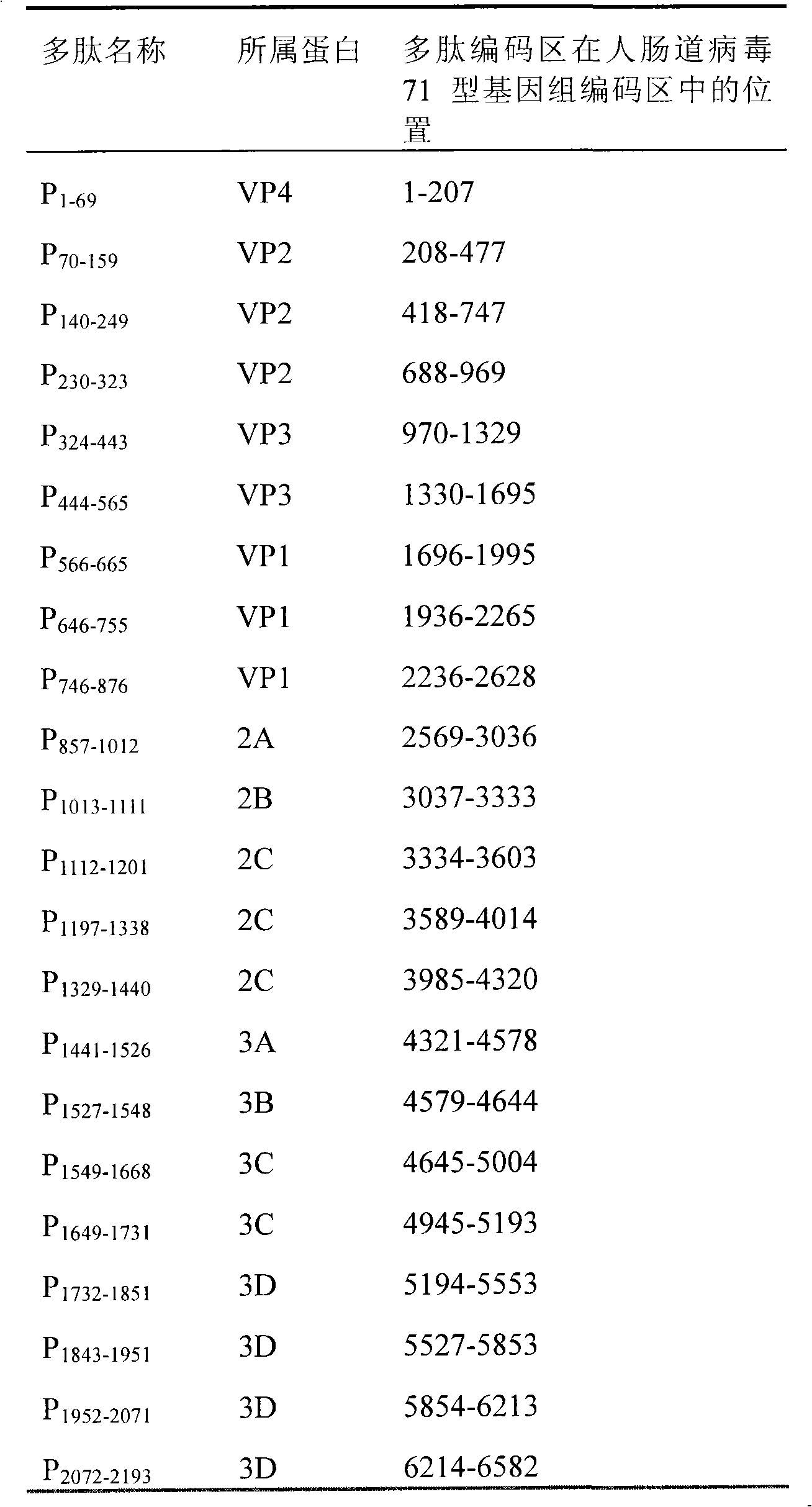
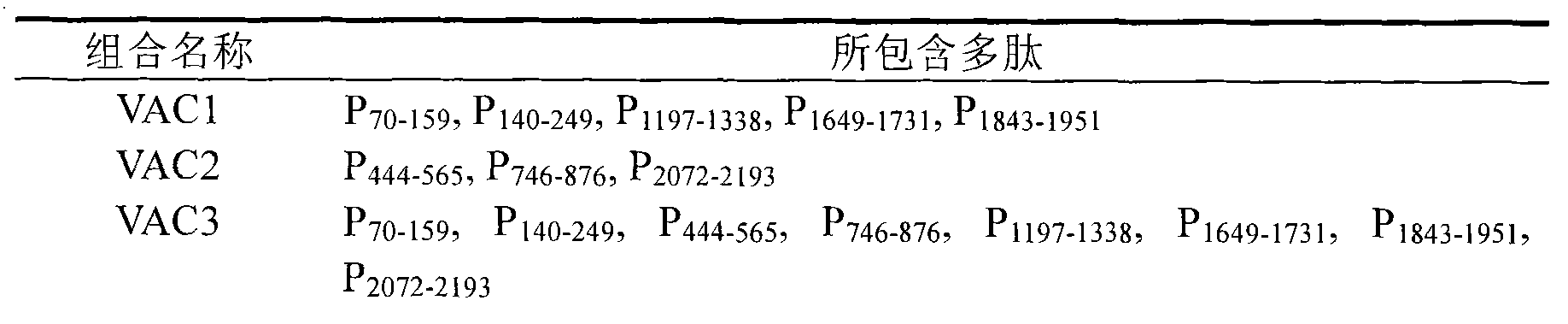
The invention belongs to the biomedical field and provides a subunit mixed vaccine of the human enterovirus 71. The subunit mixed vaccine contains one or more of the polypeptides of the human enterovirus 71 polypeptide chain and necessary adjuvants, wherein the polypeptides are P1-69, P70-159, P140-249, P324-433, P444-565, P566-665, P746-876, P1197-1338, P1441-1526, P1549-1668, P1649-1731, P1732-1851, P1952-2071, P2072-2193 and P1843-1951. The vaccine has no potential toxic or side effect, has the protective function of anti-virus infection in vitro and in vivo and is a vaccine candidate with the potential clinical application value.
Owner:INST OF LAB ANIMAL SCI CHINESE ACAD OF MEDICAL SCI
Recombinant human type 3 adenovirus as well as preparation method and application thereof
InactiveCN105219740AAvoid infectionPrevention of Hand, Foot and Mouth DiseaseMicroorganism based processesAntiviralsGenetic engineeringTGE VACCINE
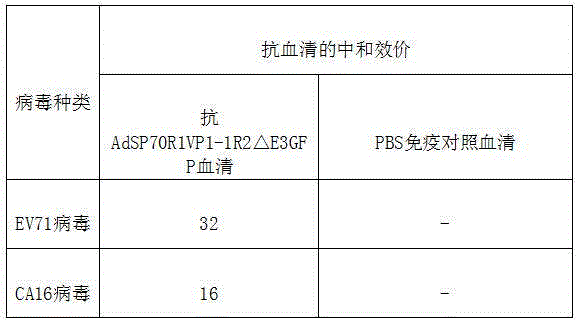
The invention belongs to the technical field of recombinant virus genetic engineering, and in particular relates to a novel human enterovirus 71 (EV71)-coxackievirus A16 (CA16)-recombinant type 3 adenovirus (Had 3) candidate vaccine strain using recombinant human type 3 adenovirus as a vector, and a preparation method of the strain; hexon of the human type 3 adenovirus is simultaneously interpolated with a neutralizing epitope SP70 of the EV71 and a neutralizing epitope VP1-1 of the CA16, wherein the amino acid sequence of the neutralizing epitope SP70 of the EV71 is shown as YPTFGEHKQEKDLEYC and the amino acid sequence of the neutralizing epitope VP1-1 of the CA16 is shown as PKPTSRDSFAWQTAT. The recombinant human type 3 adenovirus is capable of simultaneously preventing EV71 and CA16, and the adenovirus has a significant effect on preventing hand-foot-and-mouth disease.
Owner:东莞市第八人民医院
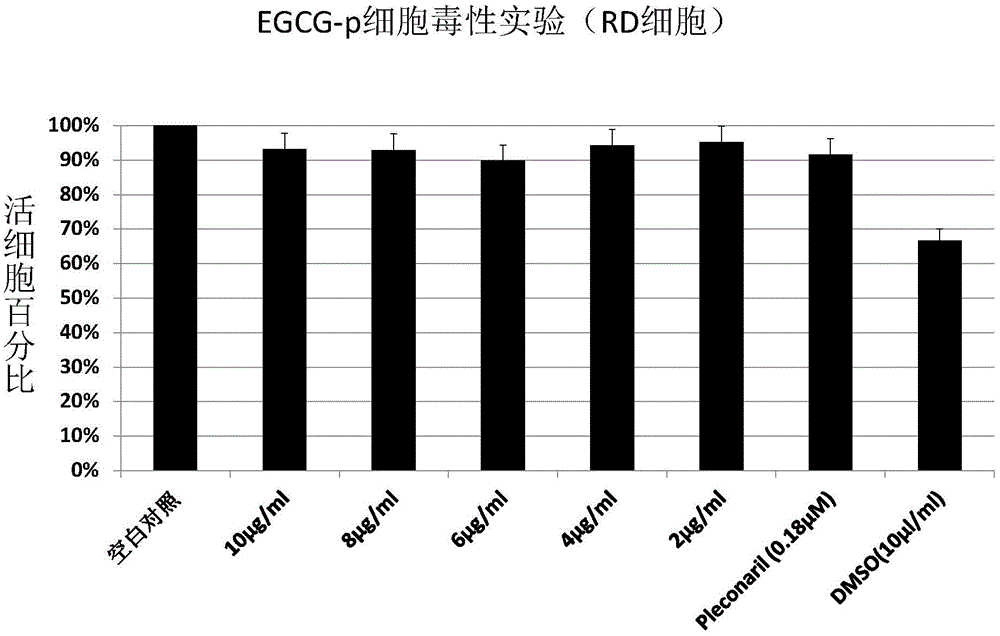
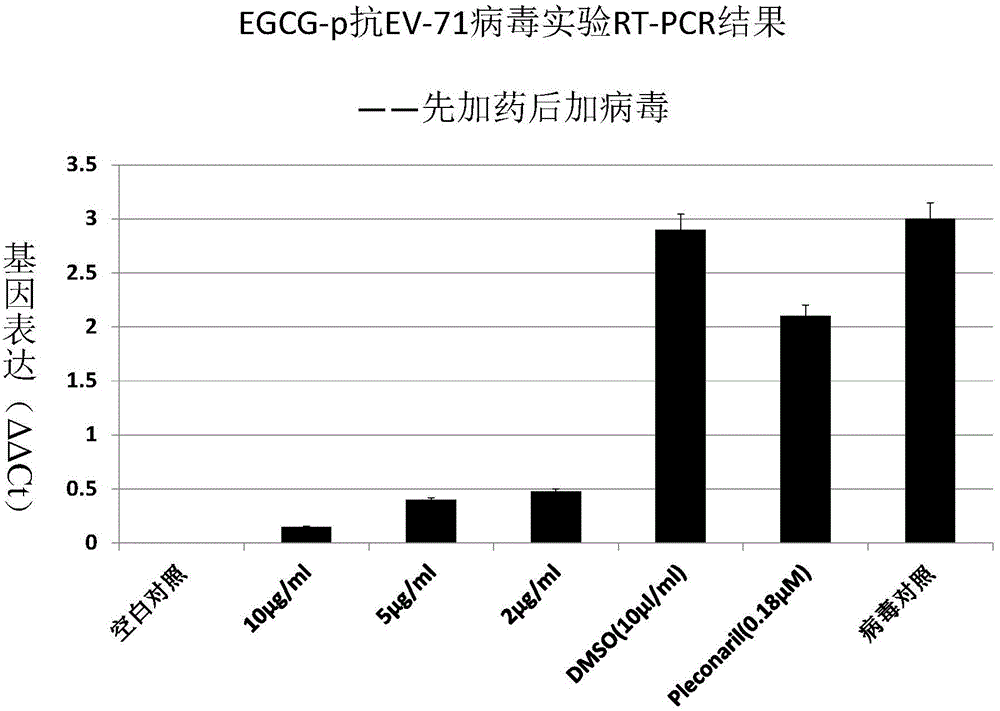
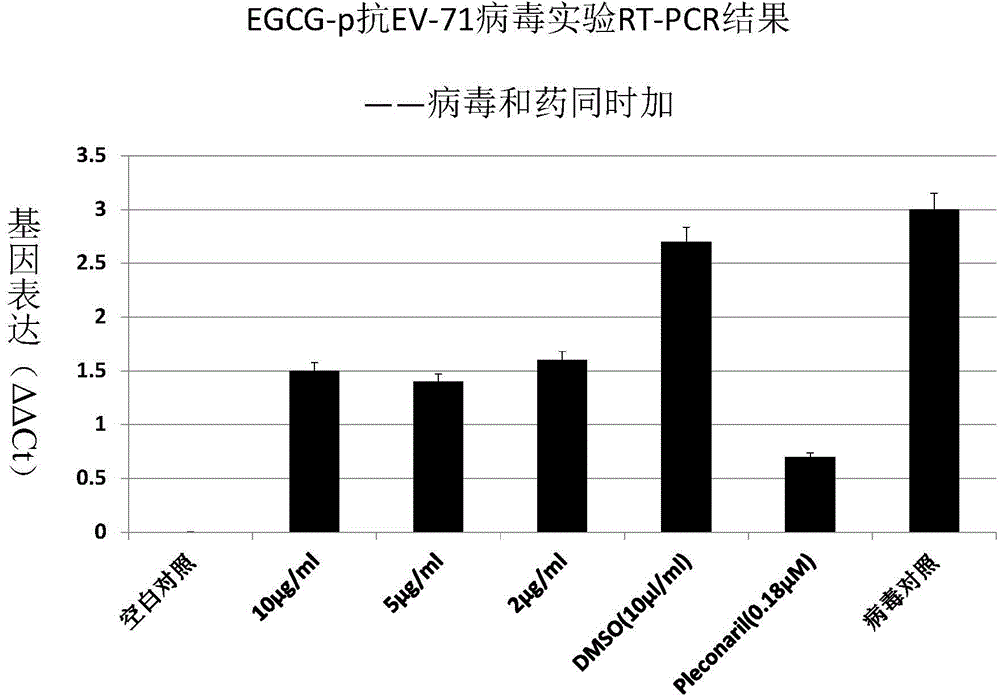
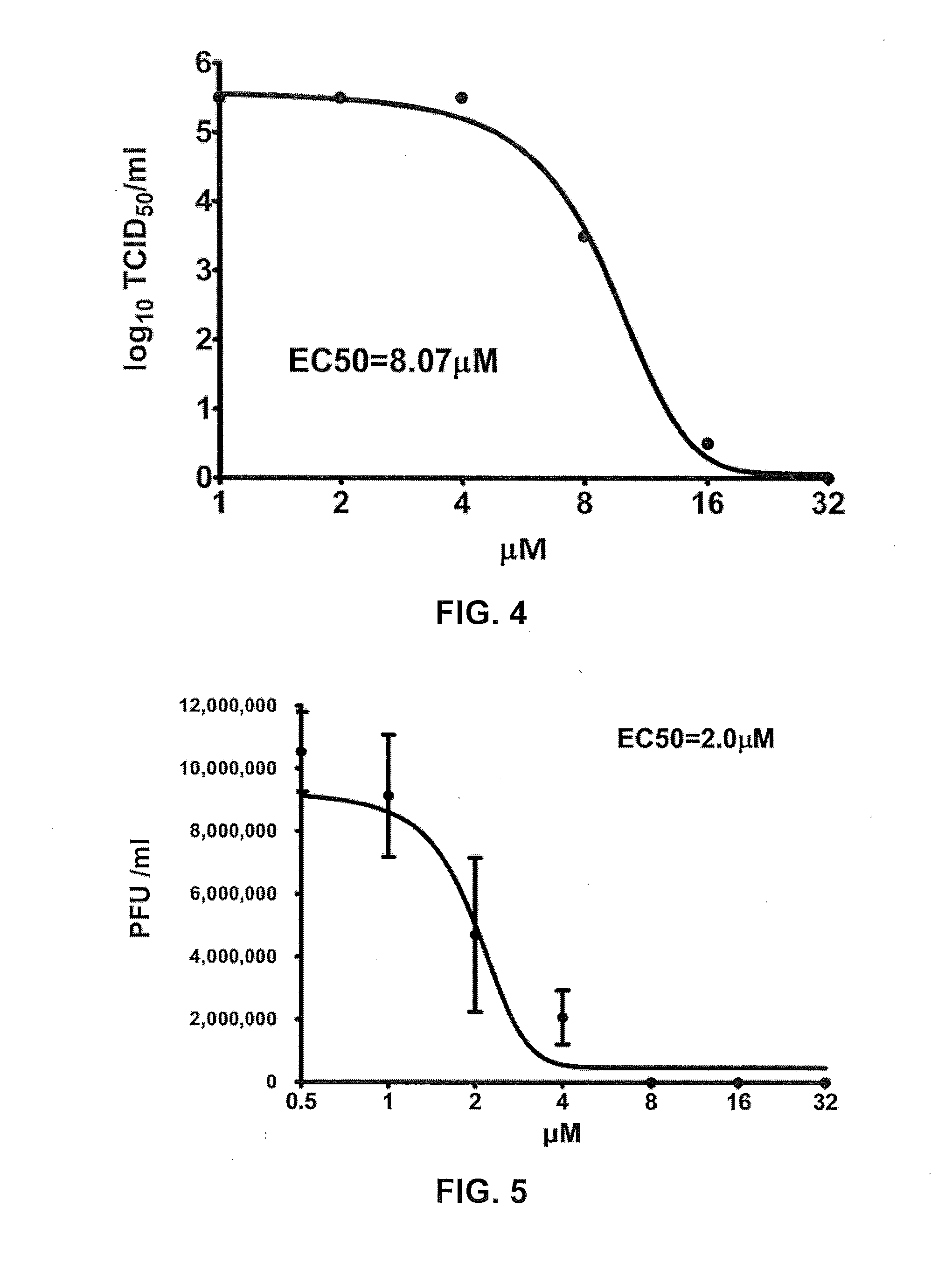
Application of EGCG palmitate in preparing medicines for treating or preventing human enterovirus 71 infection
InactiveCN104095842AIncrease productionEasy to get materialsOrganic active ingredientsDigestive systemPositive controlPalmitates
The invention relates to the technical field of novel applications of medicines and particularly discloses an application of EGCG palmitate in preparing the medicines for treating or preventing human enterovirus 71 (EV 71) infections. The invention testifies that the EGCG palmitate has favorable antivirus action in EV 71 infection cell test in vitro and the EV 71 infection can be prevented; compared with the positive control medicine Pleconaril, the efficacy of the EGCG palmitate is more remarkable, which shows that the medicine has the prospect of being developed into the medicines to resist EV 71.
Owner:武汉胜达康生物科技有限公司
Human enterovirus specific antibodies and their uses in diagnostics
Owner:TEMASEK LIFE SCIENCES LABORATORY
Recombination broad-spectrum vaccine specific to Human enterovirus 71
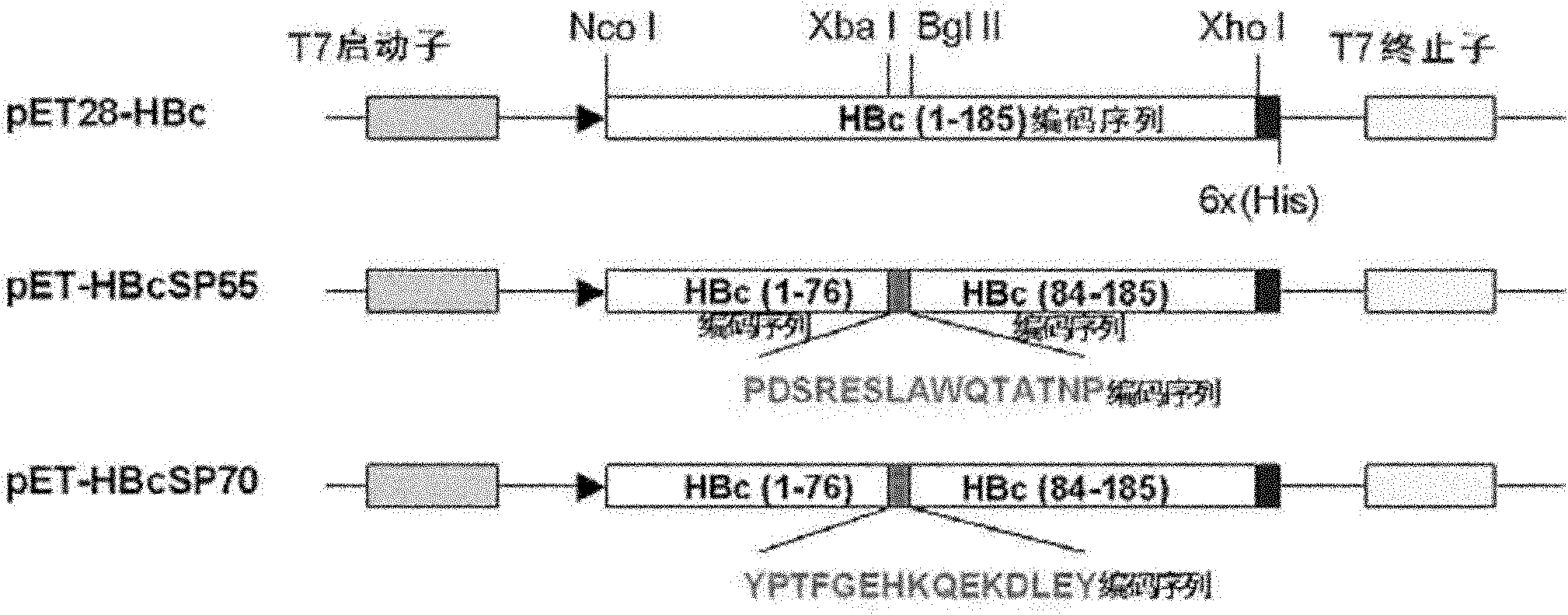
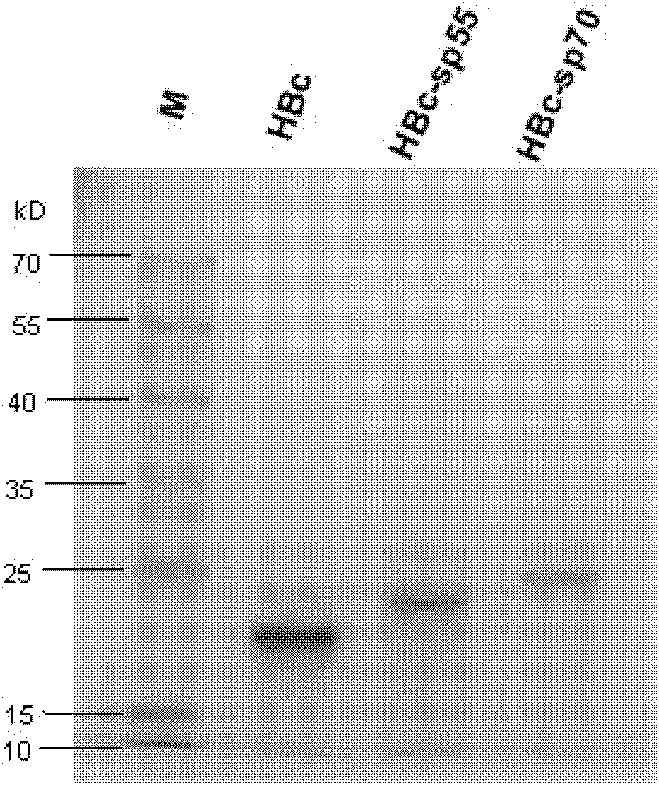
The invention relates to a recombination broad-spectrum vaccine specific to Human enterovirus 71. The invention constructs a fusion protein which comprises at least one copied peptide SP55 and / or SP70 of Human enterovirus 71 VP1 protein and a hepatitis B virus core antigen. The fusion protein can renature and assemble by self in a solubility manner after being expressed, denatured and purified, thereby forming virus-like particles with strong immunogenicity; and the virus-like particles can induce the generation of a broad-spectrum neutralizing antibody with high valence in vivo, thereby being capable of serving as the vaccine for preventing diseases related to Human enterovirus 71 infection. According to the invention, suitable carriers for forming the virus-like particles are found for antigens deriving from the enterovirus 71 VP1 protein, thereby the formed virus-like particles properly expose the antigens and increase the immunogenicity of the antigens.
Owner:INST PASTEUR OF SHANGHAI CHINESE ACADEMY OF SCI
Antigens and Vaccines Directed Against Human Enteroviruses
InactiveUS20150140027A1SsRNA viruses positive-senseViral antigen ingredientsEnteroviral infectionsProviding material
The instant invention provides materials and methods for producing immunologically active antigens derived from members of the Picornaviridae virus family. The picornavirus antigens of the invention may be in a form for use as a vaccine administered to a subject in a therapeutic treatment or for the prevention of a picornavirus infection. The picornavirus antigens of the invention may be in the form of an immunogenic composition for use in vaccines which are administered for the prevention of an Enterovirus infection. The instant invention further encompasses immunogenic compositions comprising Human enterovirus A, Human enterovirus B, Human enterovirus C, Human enterovirus D antigens and their use in vaccines for the prevention of an Enterovirus infection.
Owner:SENTINEXT THERAPEUTICS
Fluorescent quantitative PCR kit for quickly detecting human enterovirus 71
InactiveCN102108422AQuantitatively accurateThe detection process is fastMicrobiological testing/measurementRNA extractionQuality control
The invention discloses a fluorescent quantitative polymerase chain reaction (PCR) kit for quickly detecting human enterovirus 71, relates to a technology for detecting gene of pathogen causing various diseases, and is suitable for qualitative and quantitative detection of the human enterovirus 71. The kit comprises RNA extracting solution, fluorescent quantitative PCR reaction solution, an EV71 standard positive template PMD18-T-EV71 and a negative quality control standard, wherein the fluorescent quantitative PCR reaction solution contains a human enterovirus 71 specific primer pairs and a fluorescent probe. The kit is accurate in quantity, high in detection speed, and high in specificity and sensitivity, can identify and detect the human enterovirus 71, and is simple in using steps and high in repeatability. The kit can quickly perform qualitative and quantitative detection on the human enterovirus 71, and can replace the traditional culture method.
Owner:WUHAN BIOTECH GENE ENG
Real-time fluorescence quantitative PCR detection kit for human enterovirus 71
ActiveCN101580882AHigh sensitivityEffective monitoringMicrobiological testing/measurementThroat swabFluorescence
The invention relates to a real-time fluorescence quantitative PCR detection kit for human enterovirus 71, in particular to a kit for rapidly diagnosing the human enterovirus 71 infection at the early stage by using the real-time fluorescence quantitative polymerase chain reaction technology. The detection and the quantitative analysis to the human enterovirus 71 in various samples of feces, blood serum, throat swab, cerebrospinal fluid, herpes fluid, and the like can be realized through the kit.
Owner:DAAN GENE CO LTD
Polypeptide, detection device containing polypeptide and detection kit containing device
The invention relates to a polypeptide, a detection device containing the polypeptide and a diagnostic kit containing the detection device. By applying the kit to the early screening and clinical auxiliary diagnosis of human enterovirus 71, a satisfactory effect can be achieved.
Owner:SUZHOU EPITOPE BIOTECH INC
Component and method for treating viral disease
ActiveUS20150045318A1Preventing and alleviatingBiocideAntiviralsSingle-Stranded RNATherapeutic effect
A method for treating viral infection includes administering to a subject in need thereof a composition containing P2X receptor antagonists. The methods may achieve preventive or therapeutic effect on hand foot and mouth disease by inhibiting viruses. The P2X receptor antagonists can inhibit infection by a positive-sense single-stranded RNA picornavirus. The virus may be an enterovirus or a Coxsackie virus, such as human enterovirus 71. The P2X receptor antagonist may be PPADS, iso-PPADS, PPNDS, Suramin, NF023, TNP-ATP, NF279, NF157, Evans Blue, an analog thereof, a derivative thereof, or a pharmaceutically acceptable salt thereof.
Owner:HAINAN HONZ PHARMA
Polypeptide, detection device containing polypeptide and detection kit containing device
The invention relates to a polypeptide, a detection device containing the polypeptide and a diagnostic kit containing the detection device. The polypeptide, the detection device containing the polypeptide and the diagnostic kit containing the detection device provided by the invention are applied in early screening and clinical auxiliary diagnosis of human enterovirus 71, and satisfying effects can be obtained.
Owner:SUZHOU SJ BIOMATERIALS
Application of compound in preparation of drugs for resisting picornavirus
ActiveCN109908127AStrong alcohol resistanceInhibitionOrganic active ingredientsAntiviralsHand-foot-and-mouth diseaseRNA Virus Infections
The invention relates to application of a compound in preparation of drugs for resisting picornavirus. The compound can effectively inhibit the drugs for resisting picornavirus, has a good inhibitingeffect on human enterovirus 71 (EV-A71), can be used for treating various diseases caused by drugs for resisting picornavirus infection, is especially used for treating hand-foot-mouth disease, and isexpected to solve the dilemma that the hand-foot-mouth disease is not targeted clinically. In addition, the compound disclosed by the invention is derived from a natural extract and is high in safety.
Owner:THE SIXTH AFFILIATED HOSPITAL OF SUN YAT SEN UNIV +1
Kit and diagnosis method being capable of diagnosing human enterovirus 71 type infection
The invention provides a kit and a diagnosis method being capable of diagnosing human enterovirus 71 type infection. The kit includes one or more solid carriers, each of which includes a zone I and a zone II which are separated from each other. The zone I comprises a polypeptide collection I which is independently connected to the solid carrier and includes the following polypeptides, independently: a polypeptide represented as the SEQ ID No.1 and a polypeptide represented as the SEQ ID No.2; and the zone II comprises a polypeptide collection II which is independently connected to the solid carrier and includes, independently, the polypeptide represented as the SEQ ID No.1. The kit is used for detecting enterovirus 71 type antibody (IgM and IgG) in human serum, is excellent in accuracy, sensitivity and specificity, is suitable for etiology laboratory auxiliary diagnosis of the enterovirus 71 type, is simple in operation and is suitable for medical treatment and disease control departments at all levels.
Owner:SUZHOU SJ BIOMATERIALS
Application of hsa-miRNA-155-5p for preparing medicine for inhibiting human enterovirus 71
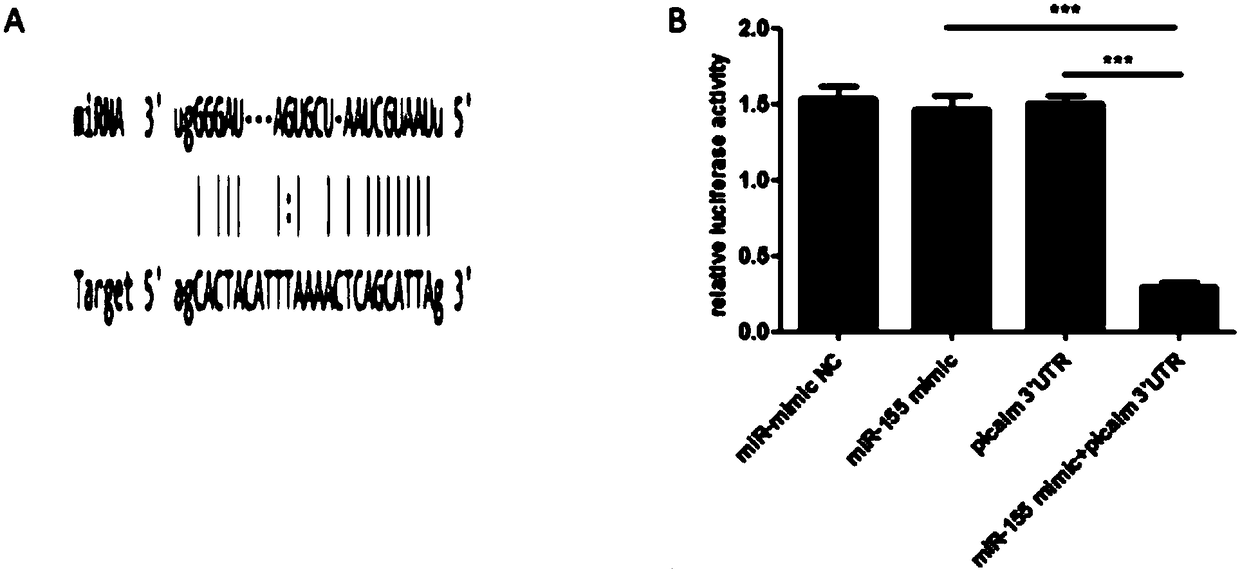
InactiveCN108283646AInhibition of endocytosisInhibition of replicationOrganic active ingredientsAntiviralsHand-foot-and-mouth diseaseDrug development
The invention discloses application of hsa-miRNA-155-5p (miR-155 for short) for preparing medicine for inhibiting human enterovirus 71 (EV71 for short). The molecular biological technology is used forimproving expression of miR-155 in cells after the cells are infected with EV71. The miR-155 can assemble protein PICALM through targeting phosphatidylinositol clathrin, and then copying of the EV71is inhibited. The application provides the theoretical and experiment basis for diagnosing and treating diseases such as hand-foot-and-mouth diseases caused by the EV71, and can be applied to development of the medicine for treating EV71 infection.
Owner:ZHENJIANG NO 1 PEOPLES HOSPITAL
Visible recombinant virus of human enterovirus 71 and application thereof
InactiveCN104419712ABiologically consistentStrong representativeFungiBacteriaGenomic DNADouble stranded
The invention discloses a visible recombinant virus of human enterovirus 71 and an application thereof. In the invention, firstly a double-stranded DNA molecule is protected, wherein the double-stranded DNA molecule is obtained by inserting a coding gene labeled by a CCPGCC label into a 3C protease coding area of genomic DNA of the human enterovirus 71. The CCPGCC label is a polypeptide fragment represented as the sequence I in the sequence table. An expression kit, a recombinant vector, a transgenic cell line or a recombinant bacterium are all belongs to a scope of protection in the invention. The invention also protects a recombinant virus of the human enterovirus 71, wherein the coding DNA of a genomic RNA of the recombinant virus is represented as the sequence 4 in the sequence table. The visible recombinant virus of the human enterovirus 71 has an excellent application prospect in fundamental research.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Glucose iodine derivative, preparation method thereof and application thereof in pharmacy
ActiveCN103833799AExact therapeutic effectAntibacterial agentsEsterified saccharide compoundsEscherichia coliStaphylococcus aureus
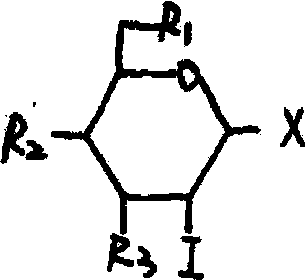
The invention provides a 3, 4, 6-substituted-D-glucose iodine derivative and a preparation method and application thereof. A drug is prepared from the 3, 4, 6-substituted-D-glucose iodine derivative as an active component and a medicinal auxiliary material. Bactericidal tests of the 3, 4, 6-substituted-D-glucose iodine derivative on pseudomonas aeruginosa, staphylococcus aureus and escherichia coli are performed, and results show that the 3, 4, 6-substituted-D-glucose iodine derivative has bactericidal effects on the staphylococcus aureus, pseudomonas aeruginosa and escherichia coli. Antiviral tests of a 3, 4, 6-triacetyl-D-glucose iodine preparation are performed, results show that the 3, 4, 6-triacetyl-D-glucose iodine preparation has a direct inactivation effect on EV71 (Human enterovirus 71) virus, and the therapeutic index is 5.5 times of that of contrast drug ribavirin injection. Therefore, the 3, 4, 6-substituted-D-glucose iodine derivative can be used for the preparation of antibacterial and antiviral drugs, especially can be used for the preparation of drugs for treatment of hand foot and mouth disease, provides a new drug for the treatment of the hand foot and mouth disease, and has a good prospect of clinical application. The present invention provides a preparation method.
Owner:SUZHOU HARMONY BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com