Recombination broad-spectrum vaccine specific to Human enterovirus 71
A human enterovirus, virus-like technology, applied in the fields of biomedicine and biology, can solve the problems of high cost and weak immunogenicity of synthetic peptides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
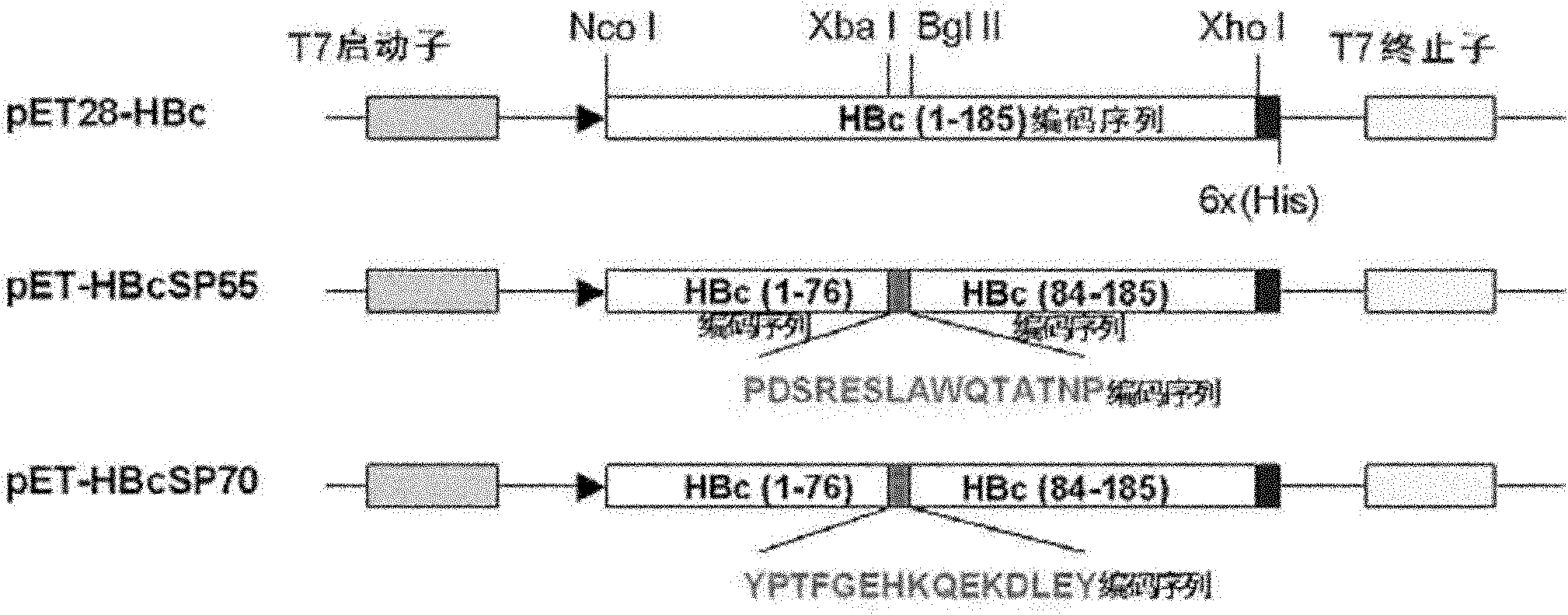
[0095] Example 1. Construction of expression plasmid
[0096] Prepare primers as follows:
[0097] SP55-Xba I-F (SEQ ID NO: 4):
[0098] 5’-CTAGAGCCAGATTCTAGGGAATCTCTTGCATGGCAAACTGCTACTAACCCTGGA-3’,
[0099] SP55-BglII-R (SEQ ID NO: 5):
[0100] 5’-GATCTCCAGGGTTAGTAGCAGTTTGCCATGCAAGAGATTCCCTAGAATCTGGCT-3’,
[0101] SP70-Xba I-F (SEQ ID NO: 6):
[0102] 5’-CTAGAGTACCCAACATTCGGAGAACATAAACAGGAGAAAGACCTTGAATATGGA-3’,
[0103] SP70-BglII-R (SEQ ID NO: 7):
[0104] 5’-GATCTCCATATTCAAGGTCTTTCTCCTGTTTATGTTCTCCGAATGTTGGGTACT-3’,
[0105] HBc-F-NcoI (SEQ ID NO: 8):
[0106] 5’-CTGCCATGGACATTGACCCTTACAAAG-3’,
[0107] HBc-R-XhoI (SEQ ID NO: 9):
[0108] 5’-GGCCTCGAGACATTGAGATTCCCTAGA-3’.
[0109] Take an equal volume of 10uM primers SP55-Xba I-F and SP55-BglII-R, SP70-Xba I-F and SP70-BglII-R and mix them, then denature at 94°C for 4 minutes, and then ice bath for 10 minutes. The product was ligated with pIBT-HBc digested with restriction enzymes Xba I and BglII, and ligated with T4 DNA ligase. The ligati...
Embodiment 2
[0112] Example 2. Expression and purification of fusion protein
[0113] Transform the recombinant plasmids pET28b-HBc, pET28b-HBc-SP55, and pET28b-HBc-SP70 into Escherichia coli BL21, inoculate them on LB agar plates (containing kanamycin 50mg / L), and cultivate them at 37°C. After a single bacteria grows, Pick a single colony and inoculate it in LB culture medium (containing kanamycin 50mg / L), shake culture overnight at 37°C, inoculate it in 500 mL of the same culture medium at a ratio of 1:100 the next day, and continue shaking culture until the OD value of the culture reaches IPTG (final concentration 0.8mmoL / L) was added at 0.6 for induction. After 5h at 37℃, the cells were harvested by centrifugation at 12000rpm for 10min, and the cells were resuspended in 50mL buffer I (0.5M NaCl, 20mM Tris, 10mM imidazole, pH7.9) , Ultrasonic crushing for 5min. Centrifuge at 12000 rpm for 10 min, discard the supernatant, and dissolve the inclusion body pellet with 30 mL buffer II (0.5M Na...
Embodiment 3
[0126] Example 3. Mouse immune program and antibody titer detection
[0127] The purified virus-like particles were diluted with PBS to 0.1 μg / μL, and 100 μL of each was mixed with an equal volume of aluminum adjuvant by shaking for 1 hour. Three experimental groups, each with 6 mice, were injected with 200μL / mouse of BALb / c mice intraperitoneally, and immunized once at 0, 3, and 5 weeks. At the same time, a PBS control group was set up, and PBS and aluminum adjuvant were intraperitoneally administered The mixture is 200μL / only×6. Blood was collected from the orbital vein before and two weeks after immunization, and all serum was stored at -20°C.
[0128] Take the three immunizations (0, 3, and 5 weeks each immunization) two weeks (ie, the 7th week) of the serum for ELISA detection, respectively coat 96-well plate with HBc, SP55, SP70, 100ng / well, 37℃ coating 2h; 2h after sealing with 5% milk. Dilute the tested serum, use the mouse serum of the PBS control group as a negative co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com