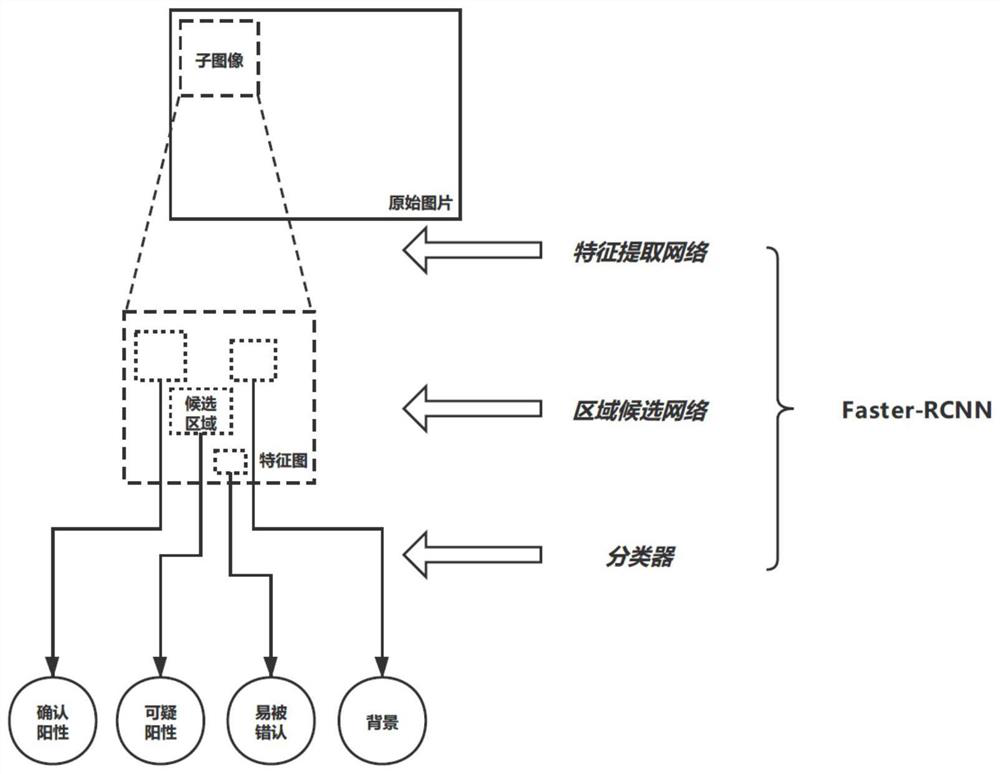
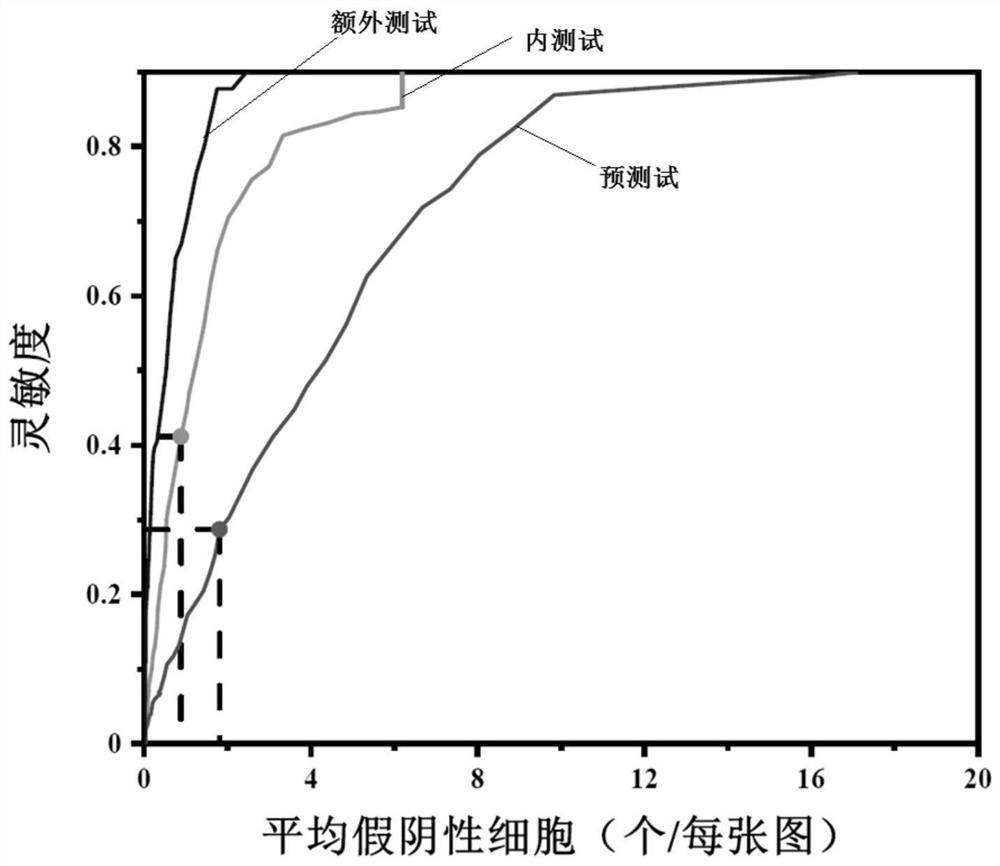
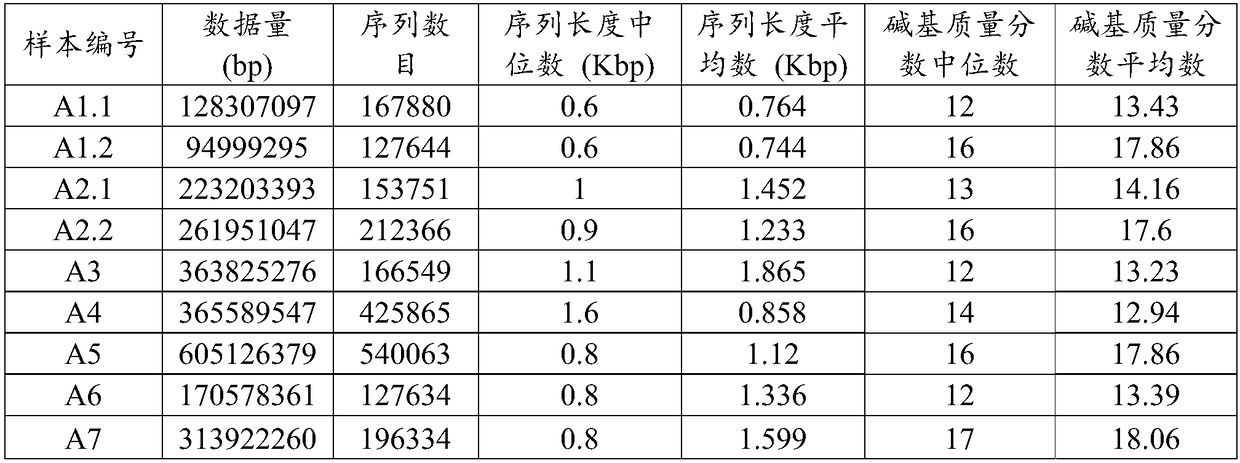
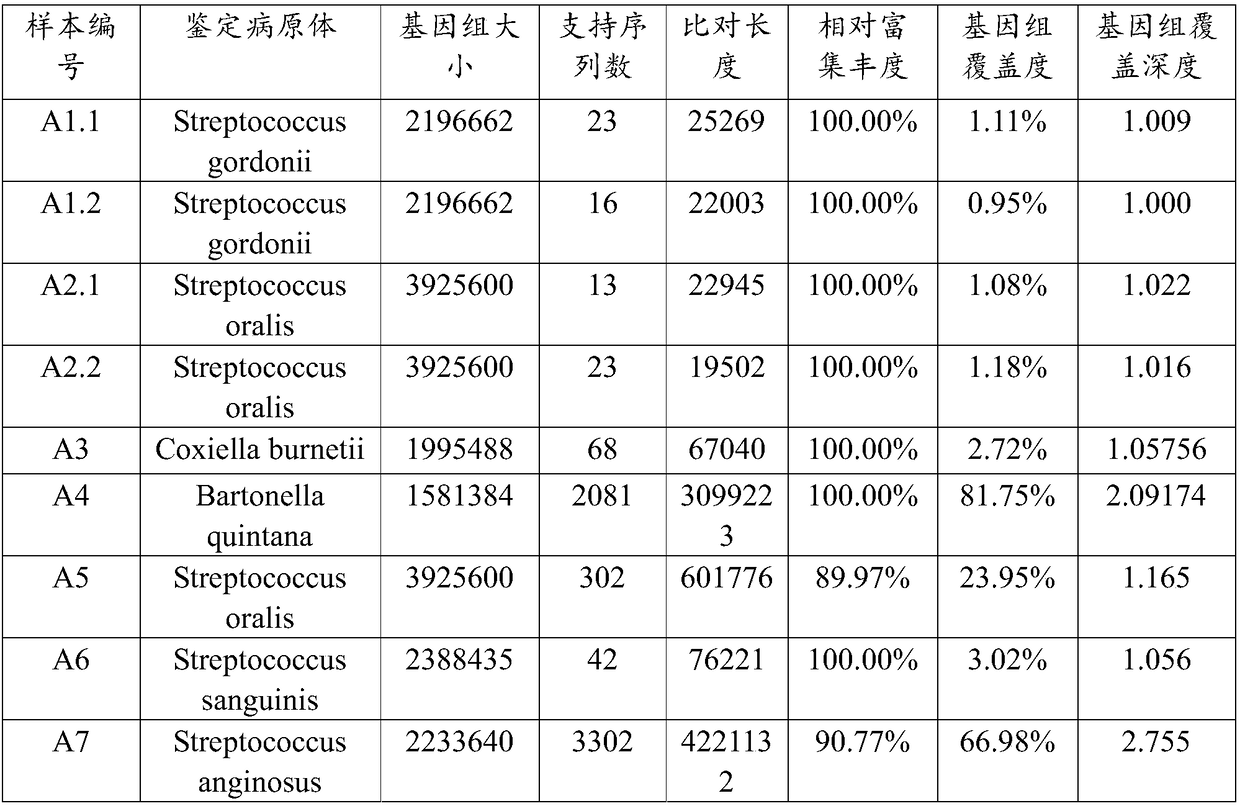
Patents
Literature
70 results about "False positivity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
False pos·i·tive. 1. A test result that erroneously assigns a patient to a specific diagnostic or reference group, due particularly to insufficiently exact methods of testing. 2. A patient whose test results include that person in a particular diagnostic group to which the person may not truly belong.
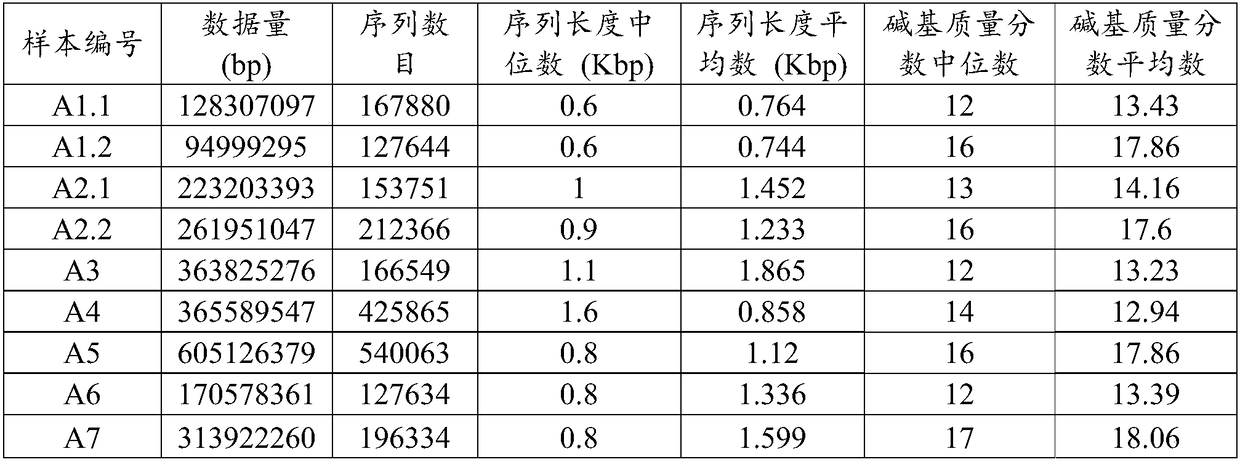
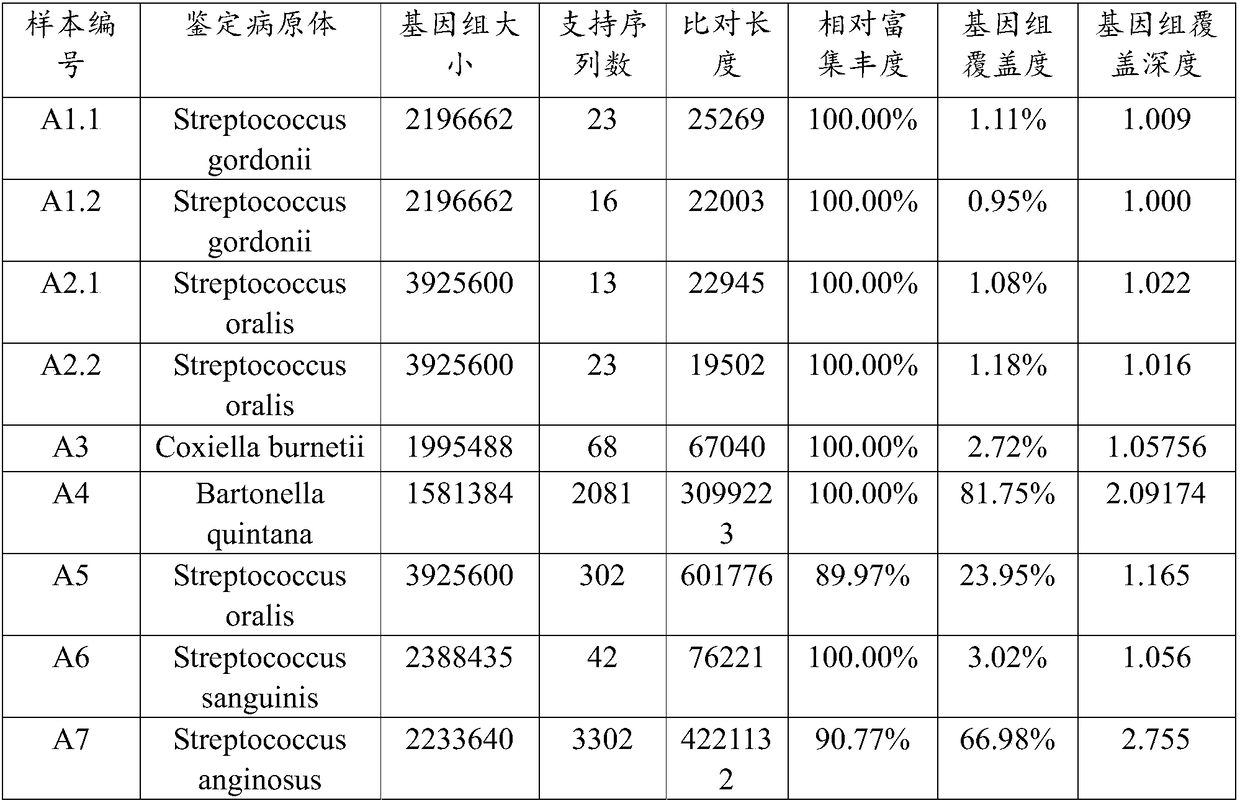
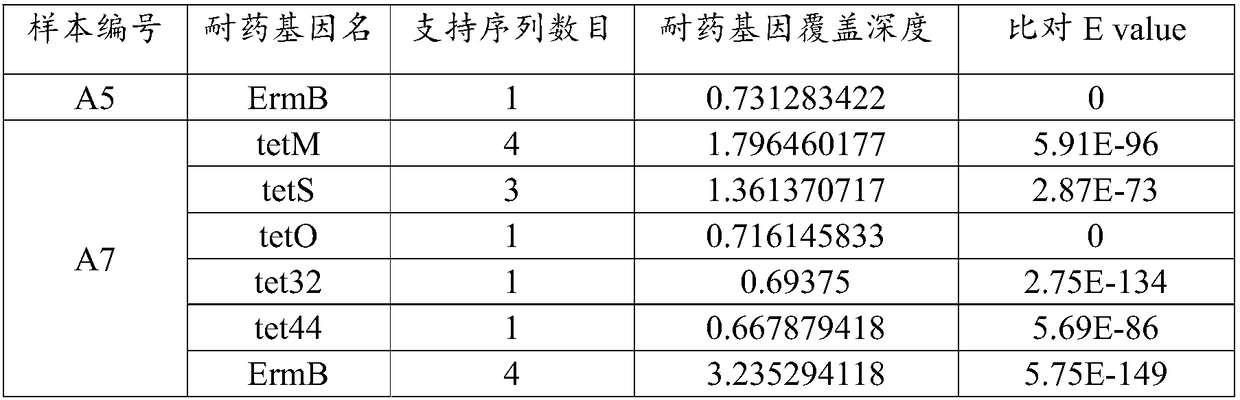
Analysis method and system of metagenome data
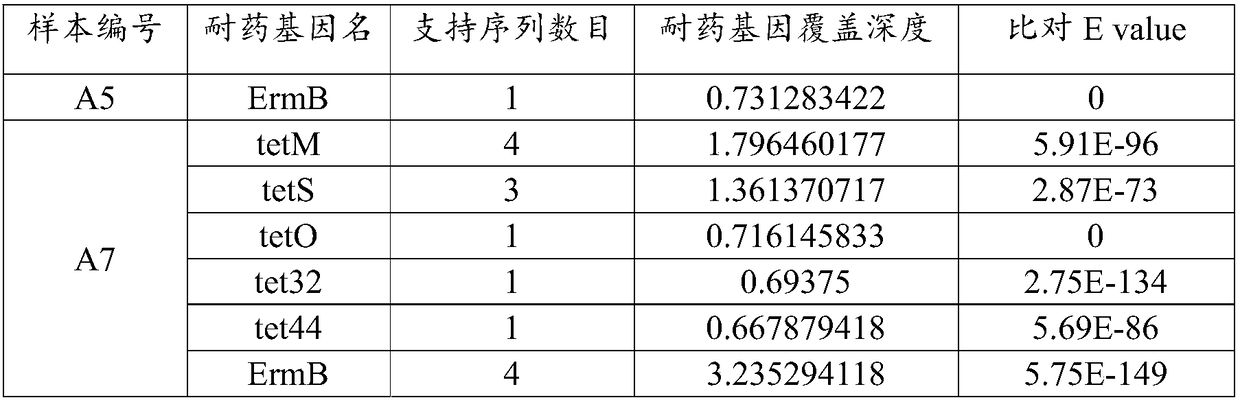
ActiveCN108334750AImprove accuracyReduce processingSequence analysisSpecial data processing applicationsEndocarditisResistant genes
The invention relates to an analysis method and a system of metagenome data. According to the invention, a preliminary species identification result of a sample is obtained on the basis of a k-Mer algorithm, a part or all of supporting sequences are extracted on the basis of the preliminary species identification result, and the preliminary species identification result is verified by using a blast algorithm to judge whether the preliminary species identification result is a reported detected species or not. The method and system disclosed by the invention can lower false positivity, quickly and accurately obtain the reported detected species of the sample in a short time, and are compatible with various mainstream sequencing platforms, thereby being suitable for second-generation sequencing technologies and third sequencing technologies; the method and system of the invention can also accurately identify drug-resistant genes and drug-resistant mutation sites of the sample and map thedrug-resistant genes and the drug-resistant mutation sites of the sample to the reported detected species. Furthermore, the system disclosed by the invention can be used for identifying pathogenic microorganisms, especially endocarditis pathogens to overcome the defect that the endocarditis pathogens are difficultly cultured.
Owner:SIMCERE DIAGNOSTICS CO LTD +2
Method of polymerase chain reaction with ultra-low denaturing temperatures and applications thereof
The invention relates to a method polymerase chain reaction (PCR) and the application thereof A method of PCR performed at ultra-low denaturing temperatures is provided. The denaturing temperatures of the templates adopted are 93-98° C. in the primary 2-3 cycles, and 60-87° C. in the follow-up cycles, those are much lower than 94-96° C., the conventional denaturing temperatures. It is found in the experiment that this method could not only become a universally applied PCR, but also control the reaction specificity by the template selection at ultra-low temperatures. The method possesses unique functions in excluding non-specific amplified products and false-negative results, excluding false-positivity brought about by the contaminants in products and discriminating genomic DNA from cDNA.
Owner:XU DINGBANG +3
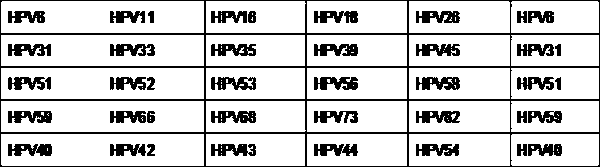
High-throughput sequencing detection method used for HPV typing and integration
PendingCN107739761AImprove accuracyGood repeatabilityMicrobiological testing/measurementSanger sequencingBiology
The invention discloses a high-throughput sequencing detection method used for HPV typing and integration. According to the method, the genes of current HPV subtypes are selected, in combination witha second-generation high-throughput sequencing technology, the type of HPV infected by a patient is detected more comprehensively, and the method overcomes the difficulties that a traditional detection method is low in accuracy rate, high in false positive result, low in repeatability and high in rate of missed diagnosis. In the field of molecular diagnosis, the mostly direct and specific technology is gene sequencing, and the second-generation high-throughput sequencing technology has the advantages of higher detection flux, higher sequencing speed, higher accuracy, lower cost and more abundant information contents compared with a classical Sanger sequencing method mostly adopted at present. According to the method, with the help of the second-generation high-throughput sequencing technology, accurate typing can be carried out on high-risk HPV and low-risk HPV, whether the integration of a human genome occurs is detected, accurate individual assessment is carried out on a detector, and the risk of a disease is prevented, so that the occurrence of a tumor is prevented.
Owner:JIAXING YUNYING MEDICAL INSPECTION CO LTD
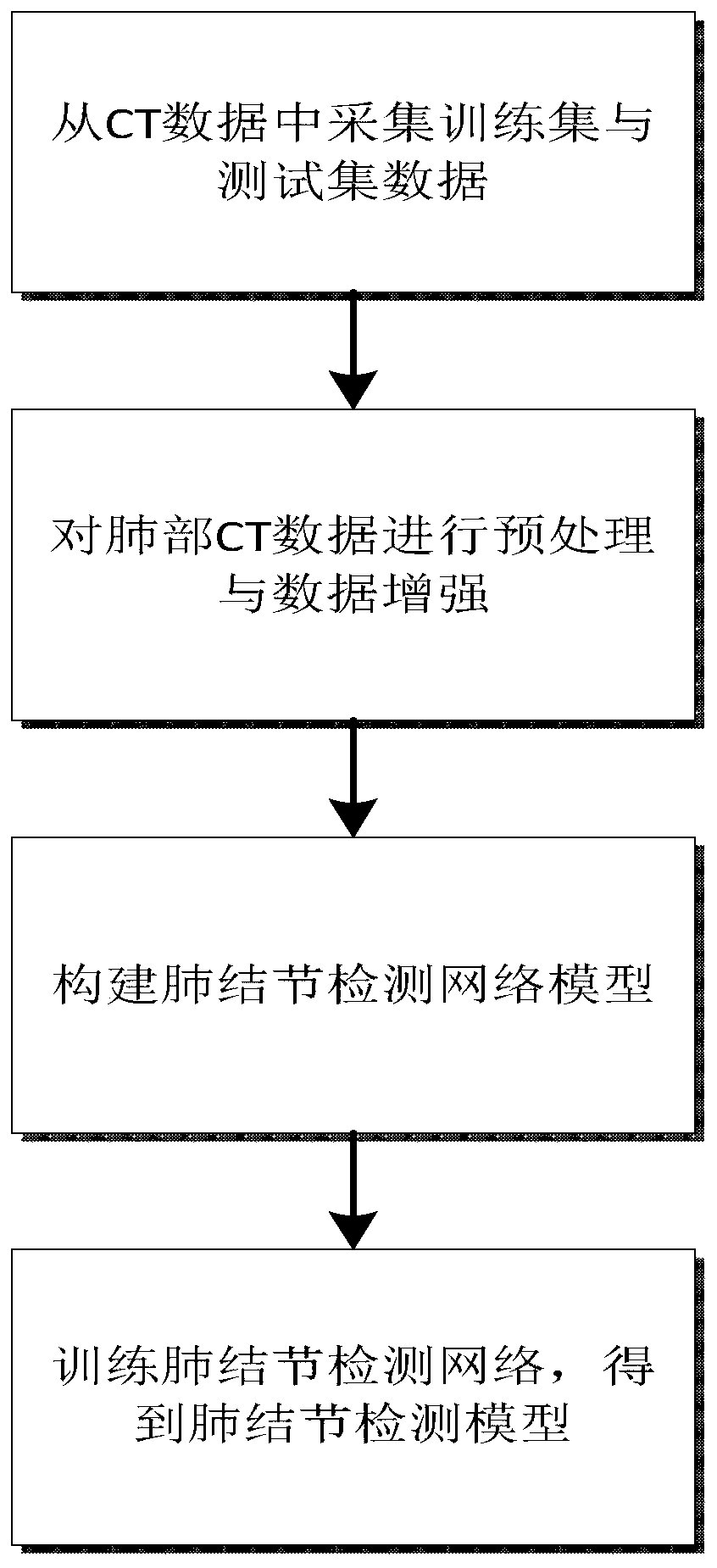
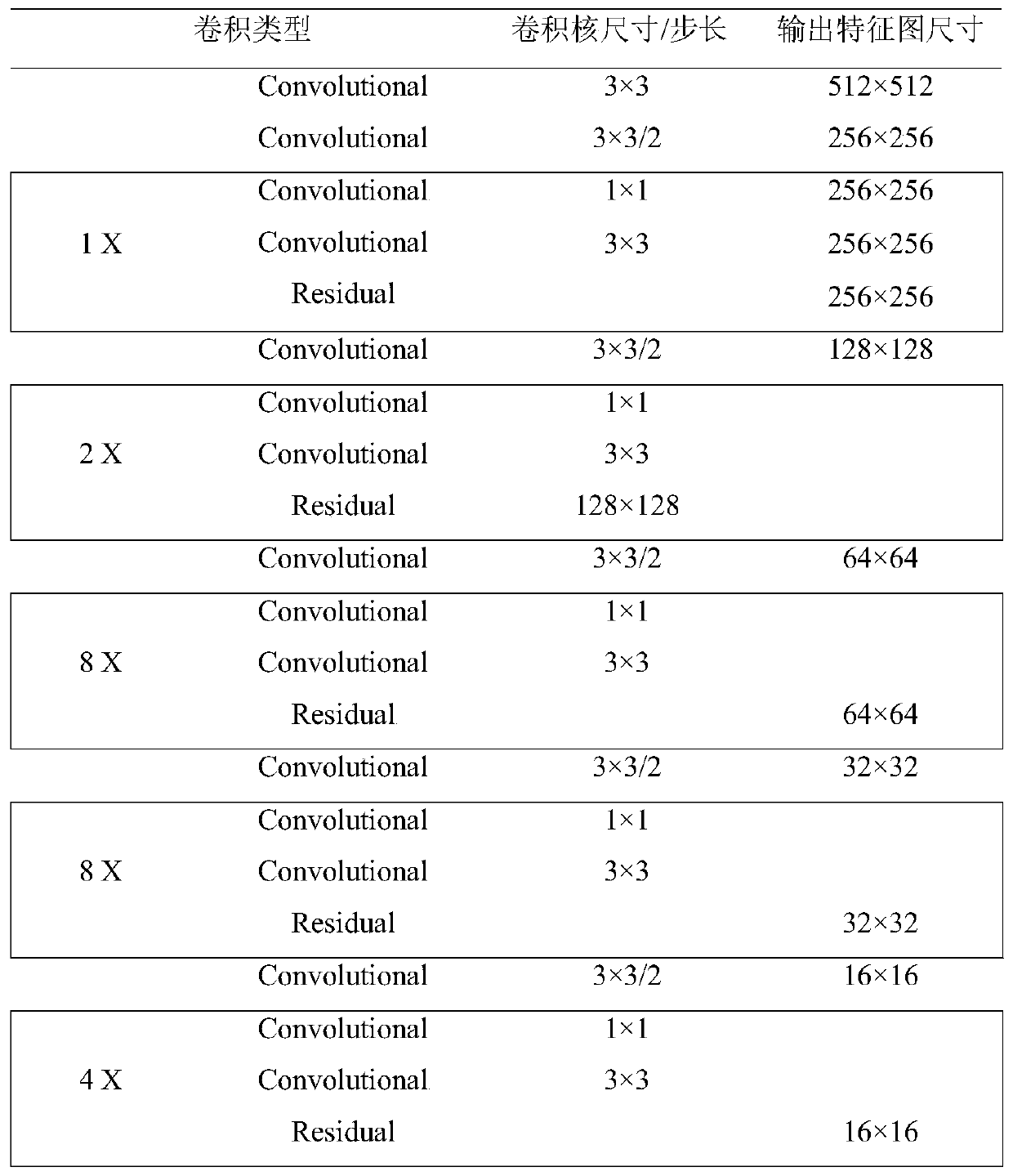
Pulmonary nodule automatic detection method based on CT image
PendingCN110942446AImprove computing efficiencySimple structureImage enhancementImage analysisPulmonary noduleChannel data
The invention relates to a pulmonary nodule automatic detection method based on a CT image. The method comprises the following steps: 1, establishing a training and testing sample set by utilizing a public lung data set: reading an original file of a CT data set, synthesizing an image where a pulmonary nodule is located and adjacent images of a front layer and a rear layer of the pulmonary noduleinto a group of three-channel data, performing pseudo-colorization processing on the three-channel data, and expanding the sample set by utilizing a data enhancement method; 2, establishing a pulmonary nodule detection network: constructing a feature extraction backbone network, a multi-scale feature layer fusion network and a candidate box regression and prediction network; 3, training the pulmonary nodule detection network by using the training sample to obtain a trained detection model; and 4, verifying the detection model in the test data set, and detecting the pulmonary nodule position. The pulmonary nodule detection network is trained by using the training sample to obtain the trained detection model, false positive is reduced on the premise of ensuring the overall detection rate, and doctors are assisted to improve the diagnosis efficiency.
Owner:付冲 +1
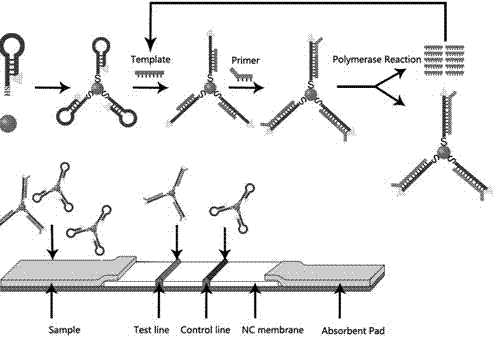
Simple membrane assay method and kit
ActiveUS20070202611A1Highly reliable simple test methodReduce generationBioreactor/fermenter combinationsBiological substance pretreatmentsAnalyteBiology
The present invention is to provide a simple membrane assay method for detecting or quantitating an analyte in a specimen sample using an assay device equipped with a membrane bound with a capture-substance to capture the analyte, comprising the steps of filtering a specimen sample using a filter, dropping the filtrate onto said membrane and detecting the presence of the analyte in said specimen sample, as well as a simple membrane assay kit for detecting the presence of an analyte in a specimen sample, comprising (1) a filter tube, and (2) an assay device equipped with a membrane bound with a capture-substance to capture the analyte. The method or the kit can decrease the occurrence of false positivity and can provide a highly accurate detection of the analyte such as pathogen and antibody in a specimen collected in a medical scene or by an individual.
Owner:DENKA CO LTD
Systems and methods for analyzing nucleic acid
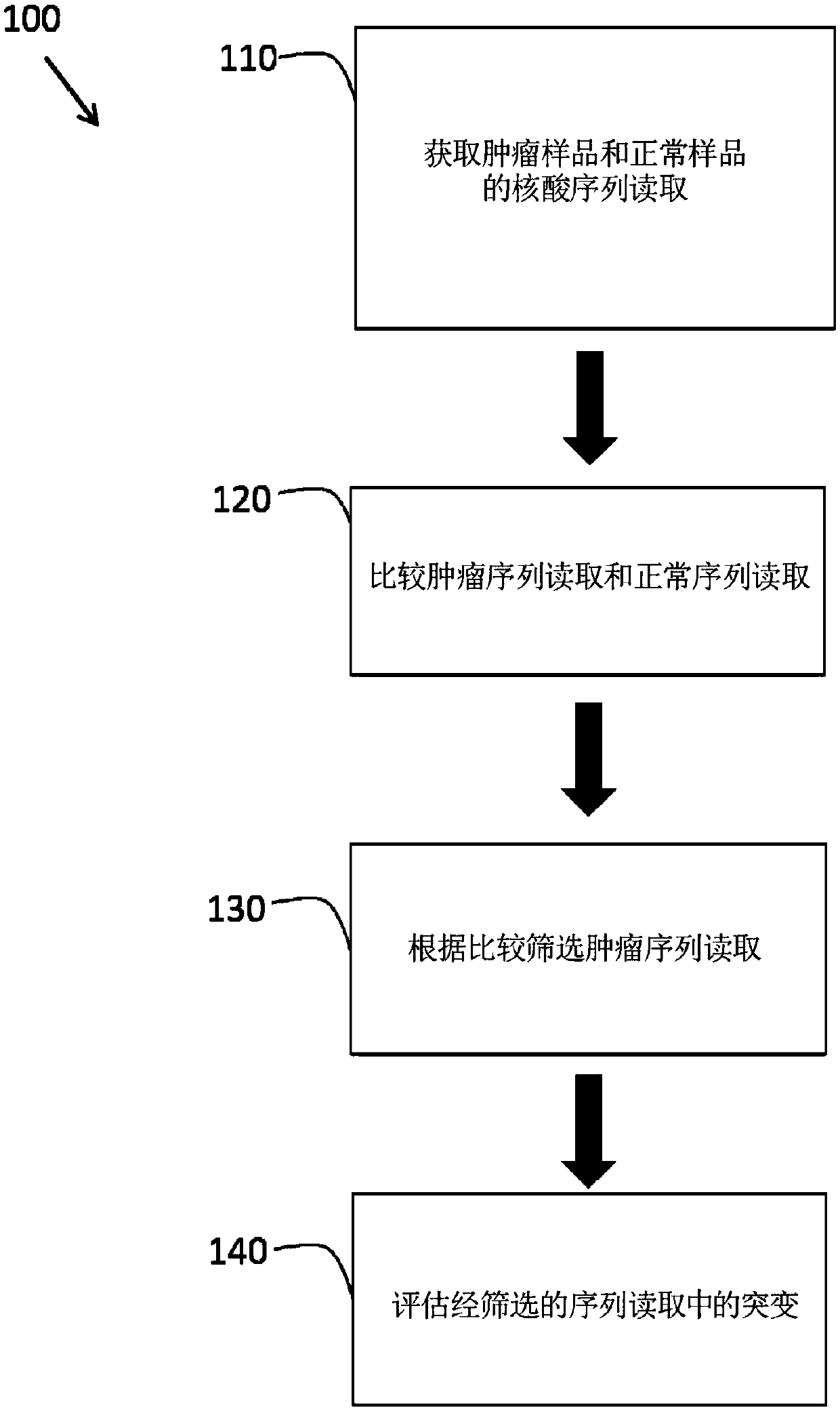
PendingCN107750279AExtensive analysisMicrobiological testing/measurementProteomicsDiseaseFalse positivity
Increased sensitivity and specificity of characterizing patient-specific variations as mutations that are indicative of a cancer or other disease by identifying patient-specific tumor mutations by comparing tumor and normal sequence reads from the patient and filtering for mutations that are unique to the tumor. By comparing tumor sequence to a normal sequence from the same patient, false-positivemutation calls are minimized in the analysis.
Owner:PERSONAL GENOME DIAGNOSTICS INC
Enzyme preparation containing thermostable DNA polymerase, method for producing same, and method for detecting subject organism to be detected
ActiveUS20120094296A1Low production costWithout reducing sensitivityMicrobiological testing/measurementTransferasesMicroorganismMicrobiology
Disclosed is a thermostable DNA polymerase preparation which can illimitably reduce the risk of false positivity in the detection of a subject microorganism utilizing a gene amplification reaction and therefore enables the selective amplification of DNA for detecting the subject microorganism even when the amount of the subject microorganism is small and therefore the amount of DNA collected therefrom is extremely small, and can be produced at a reduced cost. Also disclosed is a method for quantifying or quantifying / identifying a subject organism to be detected rapidly, conveniently and with high sensitivity using the preparation of the present invention.
Owner:HOKKAIDO MITSUI CHEM INC +1
ELISA kit for detecting salmonella antibody
InactiveCN106290918AImprove featuresGood repeatabilityDisease diagnosisBiological testingElisa kitGenus Orthobunyavirus
The invention discloses an ELISA kit for detecting a salmonella antibody. The kit comprises a solid-phase carrier coated with recombinant protein PagC, an enzyme-labeled antibody, salmonella negative serum and positive serum. The ELISA kit and method for detecting the salmonella antibody can be widely applied to salmonella, and application range is wide. The kit has high specificity and repeatability, the influence of temperature on reaction plates is small, and stability is high. Compared with an ELISA method with polysaccharide antigen as detection antigen, the method has the advantages that the occurrence rate of false positivity can be reduced to the maximum, and interference of other bacterial antigens in enterobacteriaceae on detection is avoided. Compared with a slide agglutination antigen detection method mostly adopted clinically, the method has the advantages that sensitivity and accuracy are higher, and detection throughput is increased greatly.
Owner:NANJING AGRICULTURAL UNIVERSITY +1
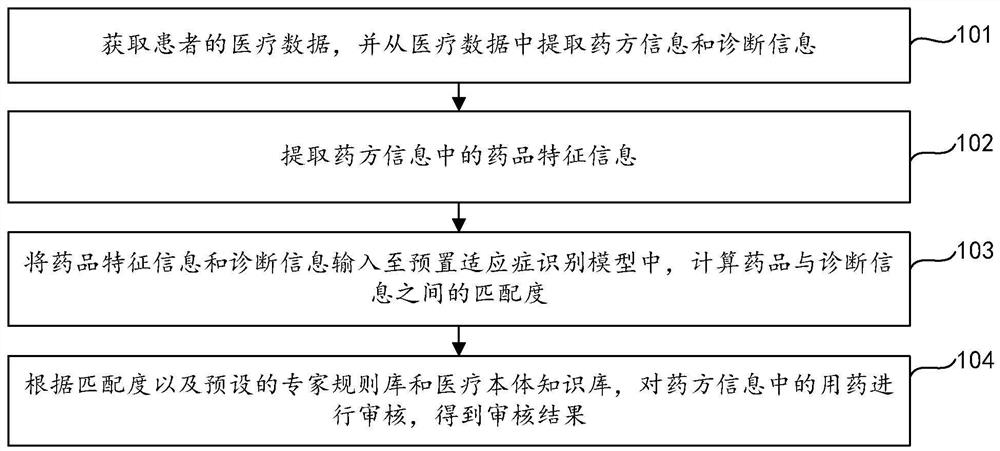
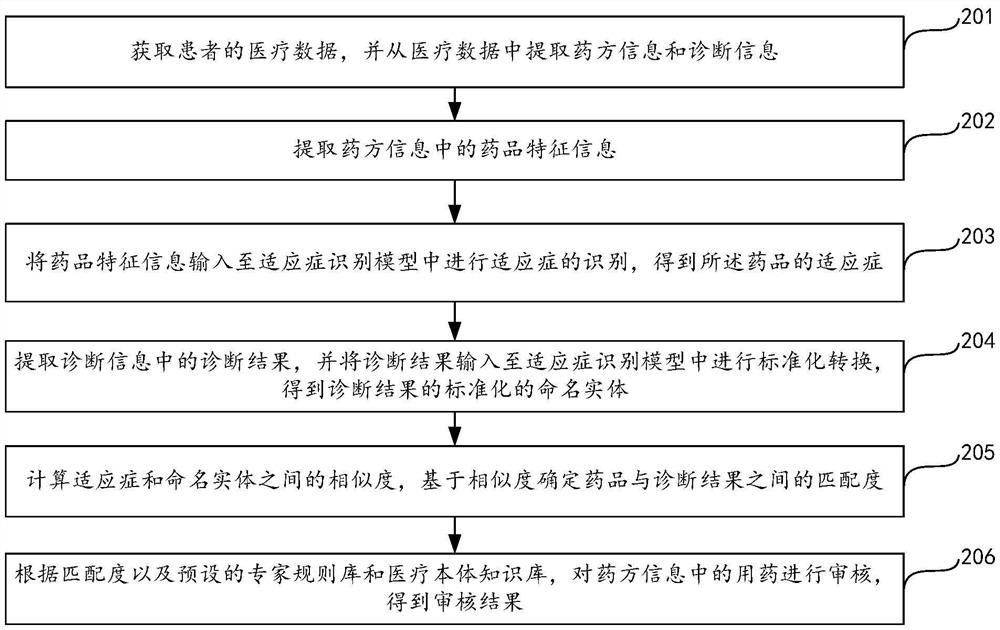
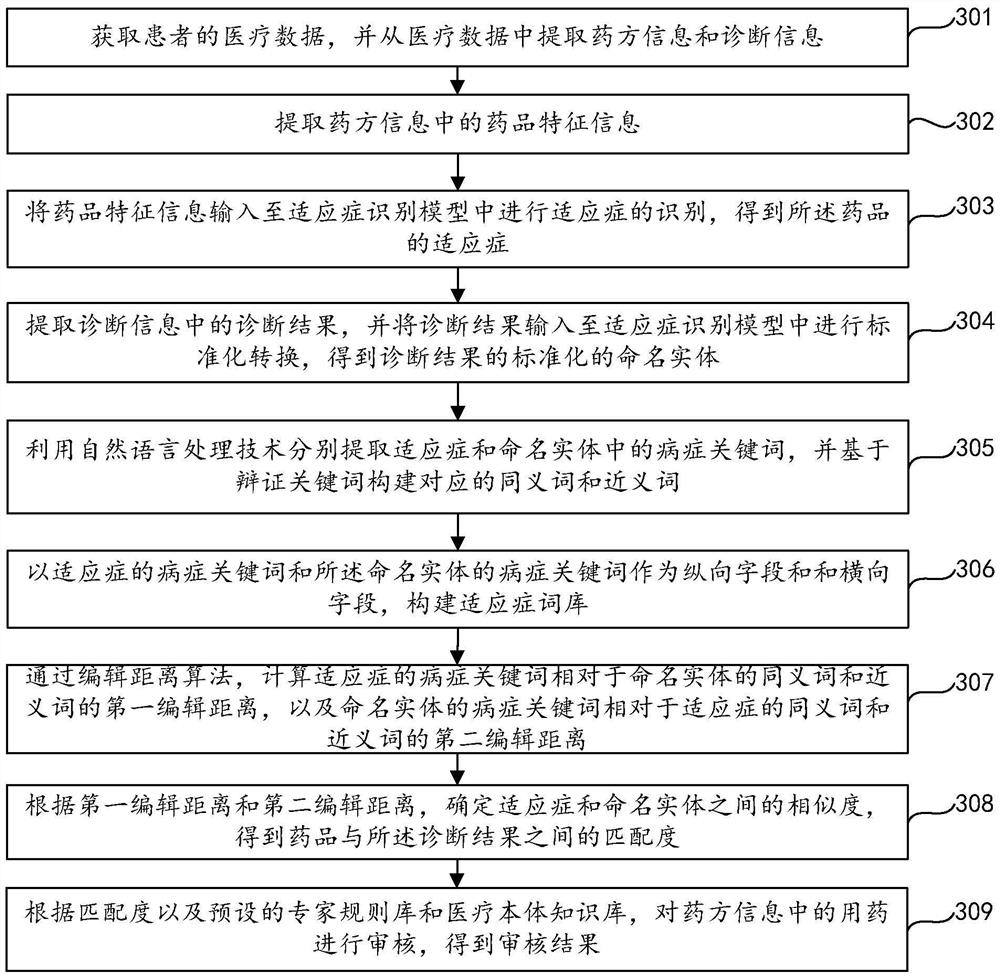
Prescription medication auditing method, device and equipment and storage medium
ActiveCN111986770AIncrease medicinal propertiesIncrease usageDrug and medicationsNatural language data processingDrug utilisationDiagnostic information
The invention relates to the field of data processing, discloses a prescription medication auditing method, device and equipment and a storage medium, and is applied to the field of smart medical treatment. According to the method, prescription information and diagnosis information are extracted from medical data of a patient; medicine feature information is extracted based on the prescription information; based on the medicine feature information and the diagnosis information, the matching degree of a medicine and the diagnosis information is calculated by utilizing an indication identification module; an expert rule base and a medical ontology knowledge base are integrated based on the matching degree; and the medication in the prescription information is audited so as to obtain the auditing result of the medication in the prescription. According to the invention, the detection rate and accuracy of mismatching of the medicine and indications are greatly improved, false negative and false positive prompts are reduced, the influence on normal diagnosis and treatment behaviors of doctors is reduced, complex clinical scenes of hospitals are met, the satisfaction degree of users is improved, medicine taking errors are reduced, and the medicine taking safety of patients is guaranteed. The invention also relates to a block chain technology, wherein the medicine feature information can be stored in the block chain.
Owner:深圳平安医疗健康科技服务有限公司
Isothermal chain multiple detection card of pathogen nucleic acid
ActiveCN102230032AAvoid cross contaminationAvoid false positivesMicrobiological testing/measurementSpiroplasmaQuarantine
The invention discloses an isothermal chain multiple displacement detection card of pathogen nucleic acid. The isothermal chain multiple displacement detection card of the pathogen nucleic acid utilizes the detection card which is prepared through a nucleic acid isothermal chain displacement method and a colloidal gold detection technology to carry out multiple detection on HBV-DNA, HCV-RNA, HIV-RNA, and TP-DNA. The isothermal chain multiple displacement detection card of the pathogen nucleic acid has the characteristics of simple and rapid operation, high sensitivity, and no need of professional equipment. The pathogen nucleic acid to be detected is not amplified in the detection process, so the detection card has the advantages of preventing amplified matter cross contamination in laboratories and preventing false positivity, can be widely used in high sensitivity pathogen nucleic acid detection in the fields of clinical detection, inspection and quarantine, infectious disease control, biological technology and the like, and has a wide application prospect.
Owner:武汉中科志康生物科技有限公司
Human enterovirus four-color fluorescence RT-PCR detection kit and detection method
InactiveCN102851391AEffective monitoringStrong specificityMicrobiological testing/measurementMicroorganism based processesForward primerReverse transcriptase
The invention discloses a human enterovirus four-color fluorescence RT-PCR detection kit and a detection method; the detection kit comprises multiple fluorescence RT-PCR reaction mother liquor, AMV reverse transcriptase liquid, Taq DNA polymerase liquid, a positive control, and a negative control; the detection kit is characterized by further comprising an internal control, a multiple 10*PCR reaction buffer, bovine serum albumin with a concentration of 20 mg / ml, various forward primers and reverse primers with a concentration of 100 muM, and various specific probes with a concentration of 50 muM, wherein sequences of the forward primers, reverse primers, specific probes and the internal control are as shown in SEQIDNO. 1, NO. 2, NO.3, NO.4, NO.5, NO.6, NO.7. NO.8, NO.9, NO.10., NO.11., NO.12., and NO.13. The detection kit of the invention has the advantages of high detection sensitivity, good specificity, accuracy, high speed, and no false positivity.
Owner:NINGBO ACAD OF SCI & TECH FOR INSPECTION & QUARANTINE
Graphene accelerated PCR (polymerase chain reaction) technology
InactiveCN105420364AAvoid pollutionSolve pollutionMicrobiological testing/measurementBiotechnologyGraphene
The invention provides a graphene accelerated PCR (polymerase chain reaction) technology, in particular an integrated novel modified PCR technology integrating a novel material graphene and PCR and being capable of accelerating nucleic acid amplification, and aims to increase contact between nucleic acid molecules, accelerate PCR process and reduce nonspecific amplification in a PCR system by utilizing the characteristics of adsorption and high-specific surface of graphene. The graphene accelerated PCR technology relates to the field of nucleic acid amplification in molecular biology and molecular tests, is a theoretical innovation integrating the novel material graphene and PCR detection, and can be used for increasing the contact probability of nucleic acid molecules by utilizing the characteristics of adsorption and high-specific surface of graphene, integrating a PCR to slowly release one or various ingredients, combining mineral oil or liquid petrolatum to seal and isolate the external environment, and solving the problem of false positivity caused by over-long reaction time of the PCR and increase of nonspecific amplification due to over-long reaction time of the PCR.
Owner:BEIJING LEAGENE BIOTECH CO LTD
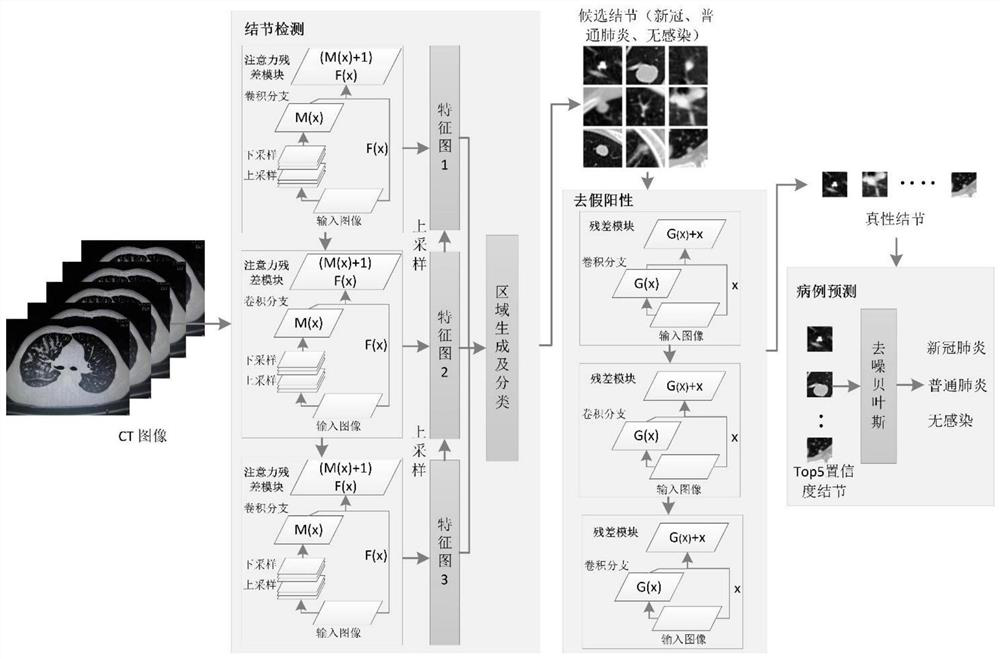
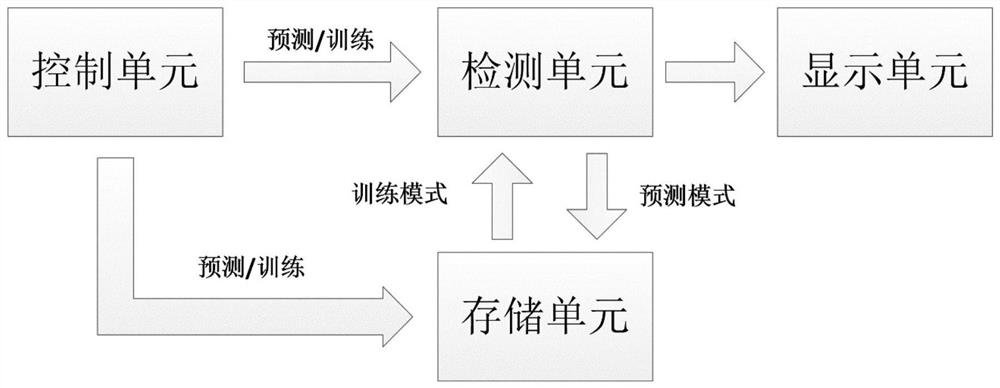
New coronal pneumonia intelligent diagnosis system based on deep learning
ActiveCN112786189ASolve the problem of too small a quantityImprove treatment efficiencyImage enhancementImage analysisControl cellImage manipulation
The invention relates to an intelligent diagnosis system for new coronal pneumonia based on deep learning, and belongs to the field of medical image processing. The system comprises a control unit, an intelligent detection and diagnosis unit, a storage unit and a three-dimensional display unit, the control unit is used for inputting and modifying system data; the intelligent detection and diagnosis unit is used for predicting new coronal pneumonia cases; the intelligent detection and diagnosis unit comprises a data preprocessing module, a focus area detection module, a false positive removal module and a case prediction module. The deep learning network diagnosis system combining three parts of detection, false positive removal and prediction is adopted to output the diagnosis result, the problems that the focus is too small and is not easy to detect, the information of a single local focus is too little, the misdiagnosis rate is too high and the like are solved, the problem that the number of medical samples is too small is solved, the diagnosis efficiency is greatly improved, and the diagnosis accuracy is improved. Therefore, treatment efficiency of a patient can be improved, and clinical data can be accumulated.
Owner:武汉承启医学检验实验室有限公司
Method for eliminating false positive interference of G test
ActiveCN104483495AAccurate detection of β-G concentrationEliminate interferencePreparing sample for investigationBiological testingBlood plasmaTachypleus
The invention relates to the G test detection technical field, and particularly discloses a method for eliminating false positive interference of a G test, that is to say, a buffer solution containing a divalent soluble metal salt and Tris is prepared and is mixed with patient plasma or serum containing an interfering substance to prepare a detection sample, and the detection sample is subjected to the G test. With the use of the method, when the patient plasma / serum containing a nonspecific tachypleus amebocyte lysate is detected, the interference effect can be eliminated, the beta-G concentration in the patient plasma / serum is accurately detected, and patient mental and economic burdens caused by clinical misjudgment are greatly reduced.
Owner:湛江安度斯生物有限公司
Primer, probe and kit for detecting gene locus mutation, and using methods
ActiveCN103571959AQuick checkHigh detection sensitivityMicrobiological testing/measurementDNA/RNA fragmentationFluorescenceBiology
The invention discloses a primer, a probe and a kit for detecting gene locus mutation, and using methods. Primer sequences are shown as SEQ ID NO:1 and SEQ ID NO:2. Probe sequences are shown as SEQ ID NO:3 and SEQ ID NO:4. The kit comprises the primer, the probe, real-time fluorescence quantification PCR Mix reagents, deionized water, bottles or tubes which are used for separating the reagents, and a packaging box which is used for centralized packaging. According to the primer, the probe and the kit, the JAK2 gene V617F locus mutation can be quickly detected, the detection sensitivity is high, the probability of false positive or false negative is small, the detection steps are simple, the time consumption is low, and required clinical data can be obtained immediately.
Owner:北京海思特医学检验实验室有限公司
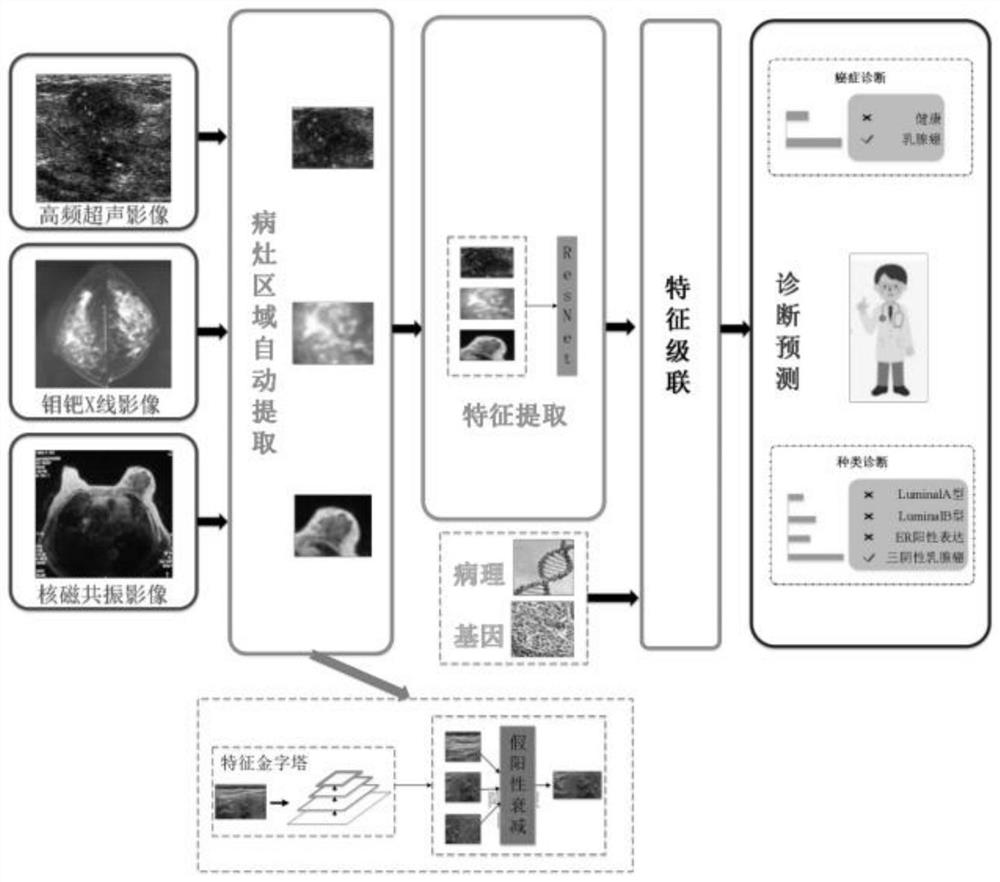
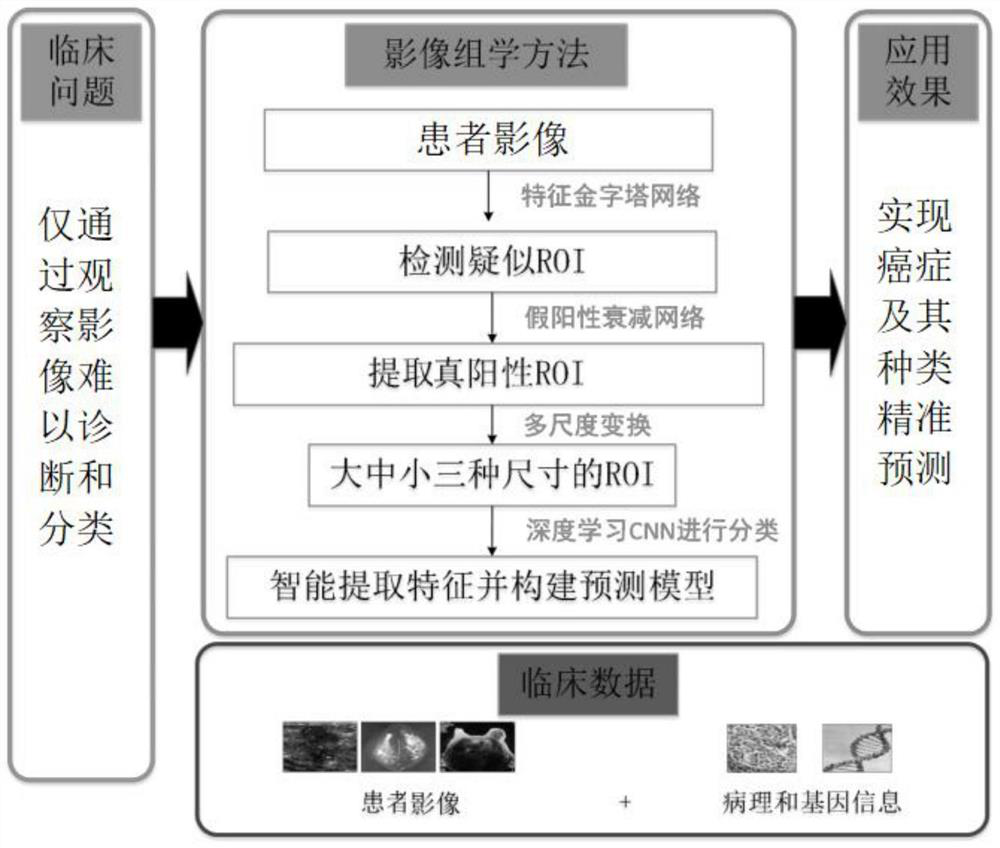
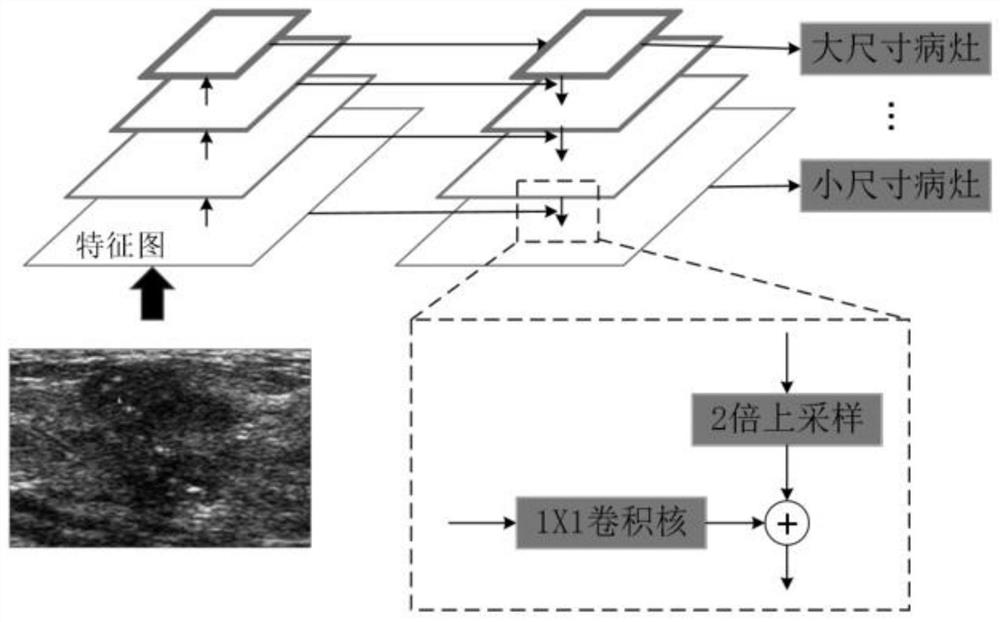
Multi-modal image analysis method and system for cancer diagnosis
PendingCN112489788AStable and accurate predictionImprove screening efficiencyImage enhancementImage analysisCancers diagnosisOncology
The invention provides a multi-modal image analysis method and system for cancer diagnosis, and relates to the technical field of image data analysis. The method comprises the steps: extracting a suspected lesion region through a feature pyramid network, carrying out the classification through a false positive attenuation network based on context features, and extracting a true positive ROI. According to the invention, the classification neural network based on the residual network is adopted, the pathological type is stably and accurately predicted, doctors are assisted in improving the screening efficiency, missed diagnosis and misdiagnosis are effectively reduced, and medical image-based cancer diagnosis and reasonable treatment of primary hospitals are facilitated.
Owner:ZHONGNAN HOSPITAL OF WUHAN UNIV
Simple membrane assay method and kit
ActiveUS7579195B2Avoid it happening againImprove accuracyBioreactor/fermenter combinationsBiological substance pretreatmentsAnalyteMembrane bound
The present invention is to provide a simple membrane assay method for detecting or quantitating an analyte in a specimen sample using an assay device equipped with a membrane bound with a capture-substance to capture the analyte, comprising the steps of filtering a specimen sample using a filter, dropping the filtrate onto said membrane and detecting the presence of the analyte in said specimen sample, as well as a simple membrane assay kit for detecting the presence of an analyte in a specimen sample, comprising (1) a filter tube, and (2) an assay device equipped with a membrane bound with a capture-substance to capture the analyte. The method or the kit can decrease the occurrence of false positivity and can provide a highly accurate detection of the analyte such as pathogen and antibody in a specimen collected in a medical scene or by an individual.
Owner:DENKA CO LTD
Real-time fluorescent quantitative PCR detection method for apple stem pitting virus
ActiveCN104561387ARealize quantitative detectionAvoid pollutionMicrobiological testing/measurementMicroorganism based processesQuantitative determinationTest material
The invention provides a real-time fluorescent quantitative PCR detection method for an apple stem pitting virus, and belongs to the field of virus molecule detection. The method comprises the following steps: with cDNA of an infected material as a template, and ASPV-cp-F2 and ASPV-cp-R2 as primers, carrying out PCR amplification, so as to obtain positive recombinant plasmid standards; with copy number concentration of the positive recombinant plasmid standards and positive recombinant plasmid standards with the concentration as templates, building a standard curve by a Ct value of real-time fluorescent quantitative PCR employing specific primers and probes; carrying out real-time fluorescent quantitative PCR on a tested material according to the same condition; and achieving quantitative detection of the apple stem pitting virus of the tested material by comparing the Ct value with the standard curve. According to the method, quantitative determination of the apple stem pitting virus is achieved; the detection result can be directly read out through computer software; and the problems of false positivity of the detection result and environmental pollution are overcome.
Owner:HEBEI NORMAL UNIVERSITY OF SCIENCE AND TECHNOLOGY
ROS1 fusion gene ARMS fluorescent quantitative PCR typing detection kit
ActiveCN105506140AIncrease TM valueExperimental optimization steps are simpleMicrobiological testing/measurementFluorescenceTrue positive rate
The invention discloses a ROS1 fusion gene ARMS fluorescent quantitative typing detection kit. The kit comprises a positive primer for detecting a ROS1 fusion body variant, a common reverse primer and a fluorescence probe, wherein the positive primer is at least one of ten single-chain DNAs as shown in SEQ ID NO.1 to SEQ. ID NO. 10; the common reverse primer is at least one of three single-chain DNAs as shown in SEQ ID NO.11 to SEQ ID NO.13; and the fluorescence probe is at least one of three single-chain DNAs as shown in SEQ ID NO.14 to SEQ ID NO.16. The invention further discloses a method for detecting the ROS1 fusion gene variant. The specific primers and fluorescence probe are designed for the ROS1 fusion gene variant, so that the sensitivity and specificity for ROS1 fusion gene variant detection are improved, and the false positivity is low.
Owner:ANHUI DAJIAN MEDICAL TECH CO LTD
Multi-fluorescent PCR kit and method for detecting clostridium difficile genes and toxin genes
ActiveCN110129458AAvoid false negativesPollution is fully degradedMicrobiological testing/measurementDNA/RNA fragmentationBacteroidesClostridium difficile (bacteria)
The invention belongs to the technical field of bacterial detection, and specifically discloses a multi-fluorescent PCR kit and method for detecting clostridium difficile genes and toxin genes, wherein the kit and method not only can identify clostridium difficile but also can detect toxin B genes. The kit mainly comprises a specific primer group and a probe, the primer group and the probe are composed of a primer pair and probe for detecting clostridium difficile, a primer pair and probe for detecting toxin B, and an internal primer pair and probe. By design of the specific primers and probes, the clostridium difficile genes and the toxin genes can be detected from samples with complex components. The kit has the advantages of simple operation, short reporting time, high specificity, highsensitivity and good accuracy; PCR false negativity is avoided by introducing internal standard products, false positivity caused by contamination of amplified products is reduced, and the kit is suitable for pathogenesis screening of patients with diarrhea of unknown clinical cause.
Owner:GUANGZHOU SAGENE BIOTECH
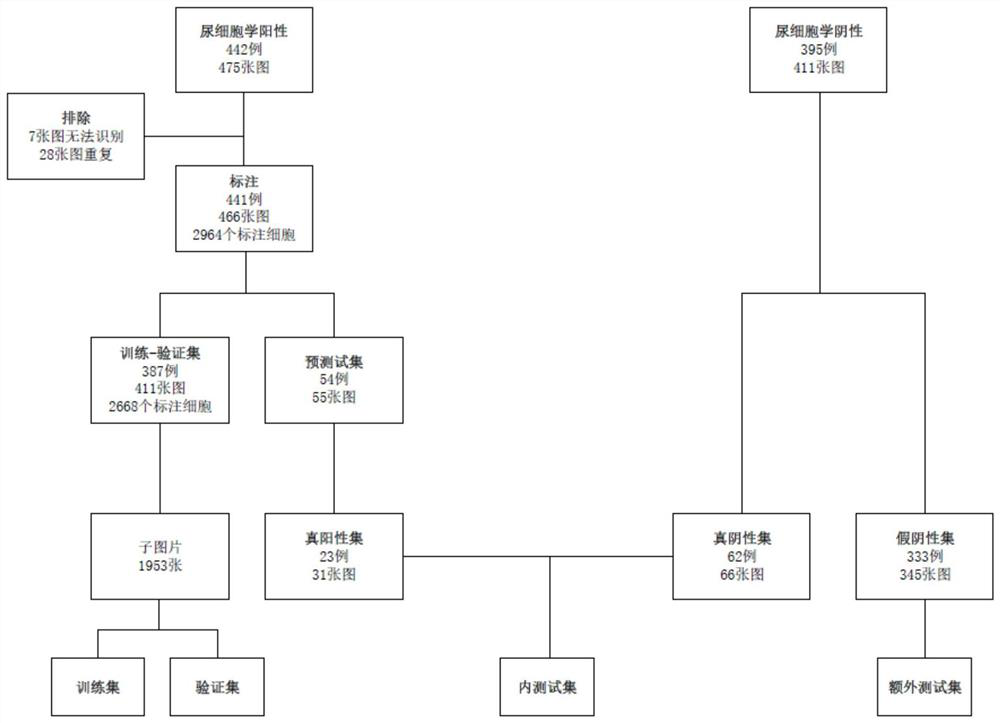
Urinary cytology artificial intelligence urinary tract epithelium cancer identification system
The invention relates to a urinary cytology artificial intelligence urinary tract epithelium cancer identification system. The system includes: a data classification module used for classifying urinary cytology data acquired in advance to obtain a urinary cytology positive data set and a urinary cytology negative data set, and matching the urinary cytology positive data set and the urinary cytology negative data set based on an operation pathology result to obtain a true positive data set, a false positive data set, a true negative data set and a false negative data set; a data grouping module which is used for grouping various data sets obtained by the data classification module to obtain a training-verification set and a test set; a model training module which is used for training a pre-constructed model to obtain a final model; a model testing and auditing module which is used for performing cell level testing and tissue level testing on the obtained final model; and an identification module which is used for identifying the to-be-identified urinary cytology data to obtain an identification result. The system can be widely applied to the field of cytology pathology recognition.
Owner:PEKING UNIV FIRST HOSPITAL
A method and system for analyzing metagenomic data
The invention relates to a metagenomic data analysis method and system. The data analysis method and system of the present invention obtains the preliminary species identification result of the sample based on the k-mer algorithm, and based on the preliminary species identification result, extracts part or all of the supporting sequences, uses the blast algorithm to verify the preliminary species identification result, and judges Whether the preliminary species identification result is a reported detected species. The method and system of the present invention can reduce false positives, quickly and accurately obtain the reported species of the sample in a short period of time, and are compatible with a variety of mainstream sequencing platforms, and are suitable for second-generation sequencing technology and third-generation sequencing technology; The method and system of the present invention can also accurately identify and map the drug-resistant genes and drug-resistant mutation sites of samples to report detection species. Furthermore, the system of the present invention can be used to identify pathogenic microorganisms, especially endocarditis pathogens, and overcome the defect that they are difficult to cultivate.
Owner:SIMCERE DIAGNOSTICS CO LTD +2
Real-time fluorescent quantitative PCR detection primer probe set, kit and method of porcine circovirus type 3
PendingCN111621596AImprove accuracyStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationVirus detectionFalse positivity
The invention belongs to the technical field of virus detection, and specifically relates to a real-time fluorescent quantitative PCR detection primer probe set, kit and method of porcine circovirus type 3. The detection accuracy is enhanced by designing primers on PCV3 cap protein in the invention for the first time; a fully closed tube operation is achieved in the detection method, the occurrence of false positive phenomenon is reduced, and at the same time, the high specificity of TaqMan probe technology and the high sensitivity of spectroscopy technology not only overcome the shortcoming of conventional PCR technology that can only achieve qualitative detection, but also solve the shortcoming of an SYBR Green method with poor specificity, and the detection method is more suitable for clinical testing; and the method has high sensitivity, good specificity and good reproducibility, the lowest detection limit is 11.8 copies / [mu]L, and the detection method is more sensitive than otherfluorescent quantitative PCR detection methods.
Owner:ZHAOQING INST OF BIOTECHNOLOGY CO LTD
Method for rapidly detecting rice aspergillus mating type genes through multiple PCR
InactiveCN106048046AReduce pollution interferenceImprove featuresMicrobiological testing/measurementMicroorganism based processesAspergillusGenomic DNA
The invention belongs to the field of crop pathogenic bacterial generative propagation type identification and particularly relates to a method for rapidly detecting rice aspergillus mating type genes through multiple PCR. The method is specially used for detecting rice aspergillus generative propagation mating type genes and comprises the steps of rice aspergillus isolated culture, rice aspergillus genomic DNA preparation and storage for use, PCR, gel electrophoresis detection and the like. The method has the advantages that operation is easy and rapid, and sensitivity is high; the accuracy of rice aspergillus generative propagation mating type gene detection and mating type identification is effectively improved, amplification specificity is guaranteed, amplification efficiency is improved, and false positivity and nonspecific positivity cannot be caused.
Owner:INST OF PLANT PROTECTION SICHUAN ACAD OF AGRI SCI
Toxoplasma gondii tandem multi-epitope gene ELISA detection kit
InactiveCN106556693ABiosafetyNo risk of poisoningBiological material analysisAgainst vector-borne diseasesSerum igeChromogenic Substrates
The invention relates to a Toxoplasma gondii tandem multi-epitope gene ELISA detection kit. The kit is characterized in that serum to be detected is diluted with a sample, 100 [mu]l of the diluted serum to be detected is added to a 96-well ELISA plate<(1)> and is incubated for at 37 DEG C for 1 h, and positive control, negative control and blank control are arranged; the incubated serum to be detected is washed with a washing liquid for 3 min every time and is washed 5 times; a rabbit anti-sheep IgG-HRP conjugate is added, and acts at 37 DEG C for 1 h, the obtained material is washed with the washing liquid for 3 min every time and is washed 5 times; and a chromogenic substrate solution is added, a substrate A and a substrate B are mixed according to a ratio of 1:1, 100 [mu]l of the obtained substrate mixture is added to the ELISA wells, shady coloration is carried out for 15 min, 50 [mu]l of a stopping solution is added, and the OD490 value is detected; and the inhibition rate PI = (OD negative control - OD sample) / OD negative control * 100%, the sample is positive if the PI is not less than 50%, and the sample is negative if the PI is less than 50%. The kit has the advantages of mature method, high repeatability, and effective reduction of false positivity and non-repeatability in the detection of the Toxoplasma gondii, and can be operated by general researchers.
Owner:吉林省畜牧兽医科学研究院
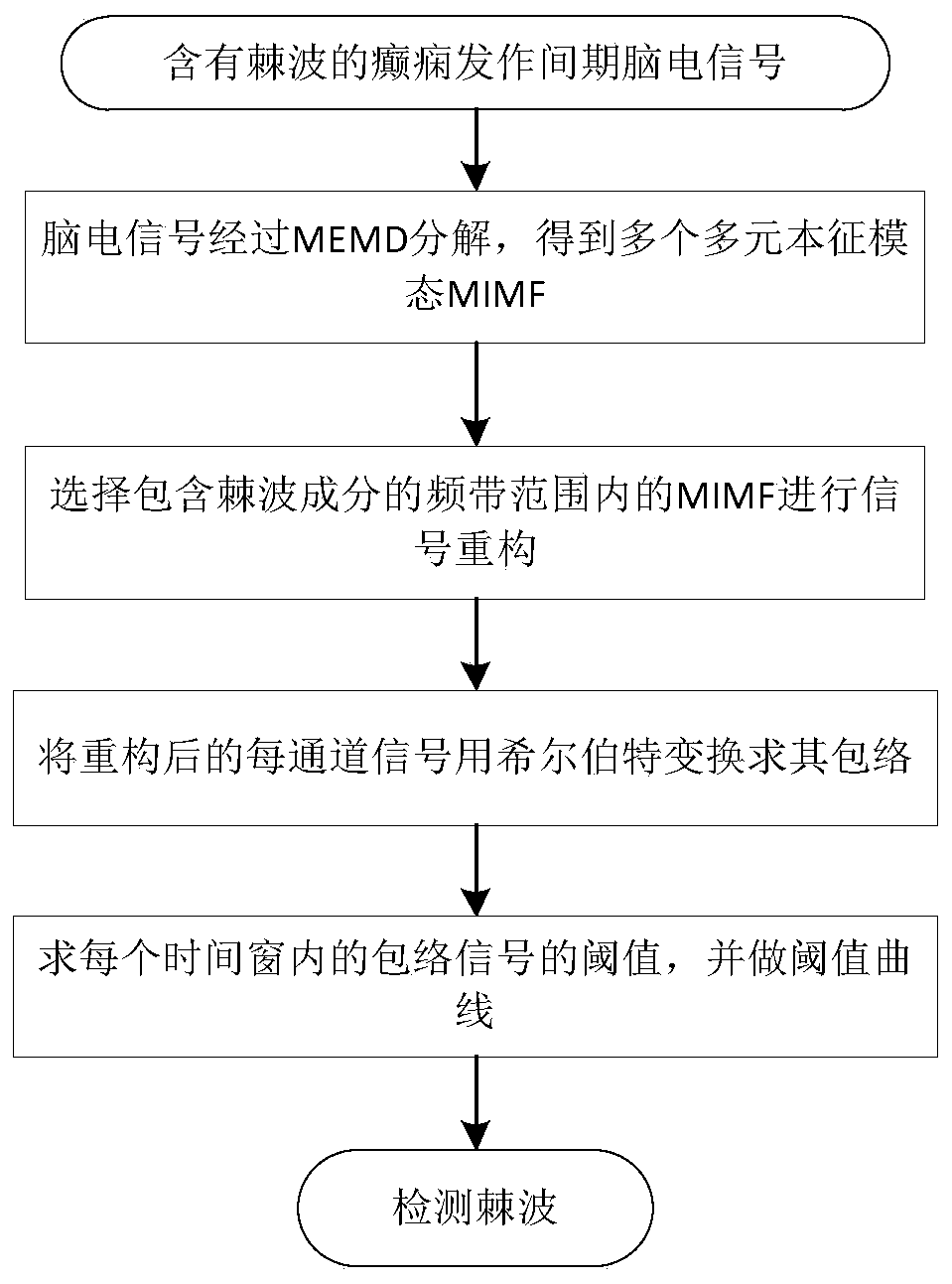
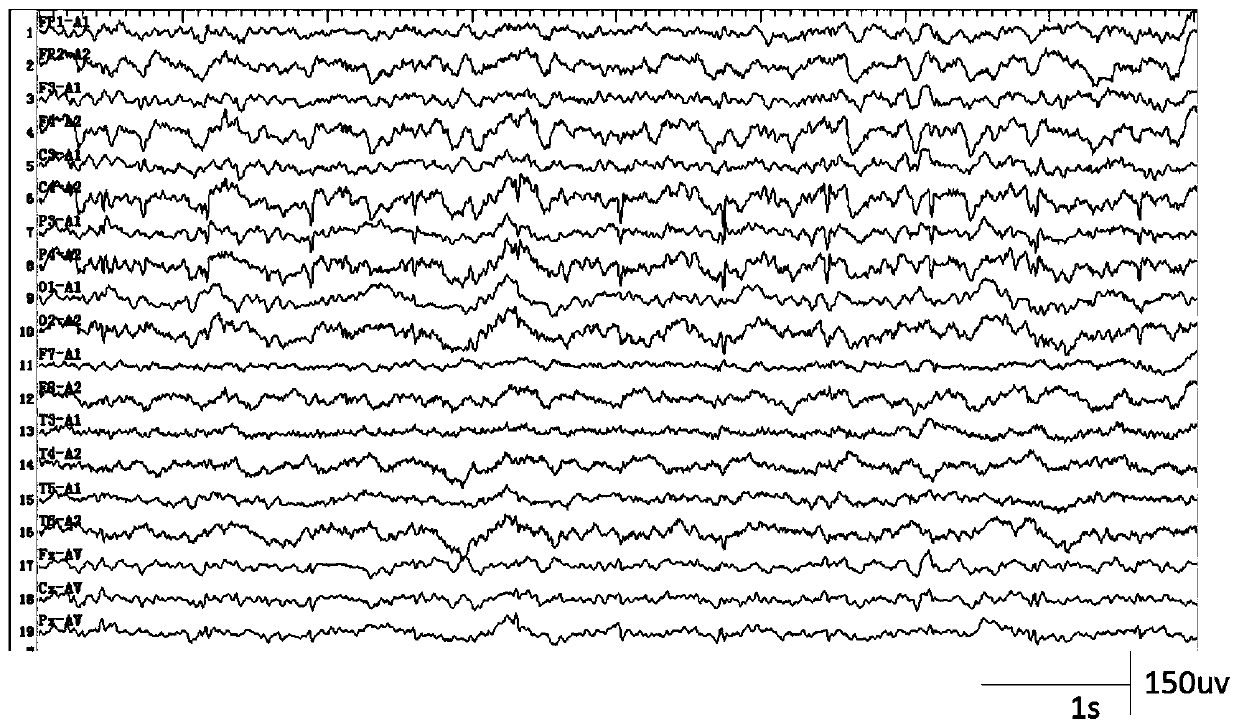
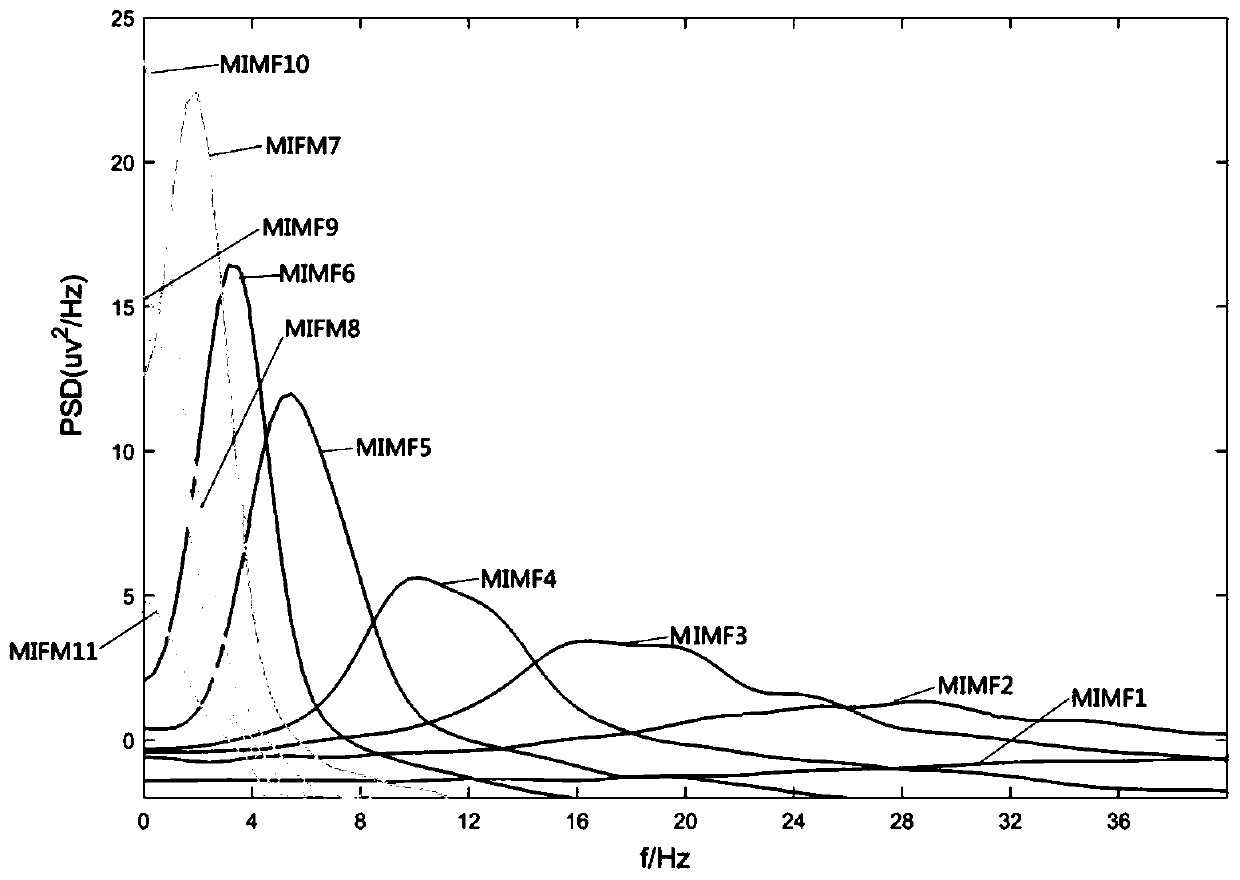
A method for automatic detection of epileptic spikes
ActiveCN108577834BImprove accuracyReduce false alarm rateDiagnostic recording/measuringSensorsAdditive ingredientSignal envelope
A method for automatic detection of epileptic spikes. MEMD extracts the components of spikes from the scalp EEG of epileptic patients. After calculating the signal envelope and dynamic threshold curve, it can locate the location of the spikes; The advantage of the present invention is that it can self-adaptively adjust the frequency band range according to the EEG, and accurately locate the location of the spikes in the interval between epileptic seizures; it provides a new idea for reducing the burden on clinicians to identify spikes with human eyes, and realizes the epileptic seizures. Through comparison, it is found that this method is superior to its method based on the signal envelope distribution model in terms of sensitivity and false alarm rate, and can have better spike detection results.
Owner:杭州瑞尔唯康科技有限公司
Imaging system for screening and diagnosis of breast cancer
PendingUS20200008682A1Screening and diagnosis of breast cancer more sensitiveMore rapid, non-invasiveDiagnostic recording/measuringSensorsNon invasiveOxygen metabolism
This invention provides a non-invasive diagnosis system that is not only capable of producing high-resolution, three-dimensional images of abnormalities of tissue growth inside the body but, it can also detect the type of abnormalities and their location using multispectral imaging techniques. It is possible to provide a portable, non-invasive device that is handheld and with which women may use to screen themselves for early detection of breast cancer without the need to visit a physician. As the present invention uses broadband sources and / or multiple coherent sources, secondary factors such as oxygen metabolism or blood volume associated with the cancer tissues could also be detected to provide further verification of the type. This invention would raise the accuracy of diagnosis and reduce the rate of false positives and false negatives.
Owner:BANPIL PHOTONICS
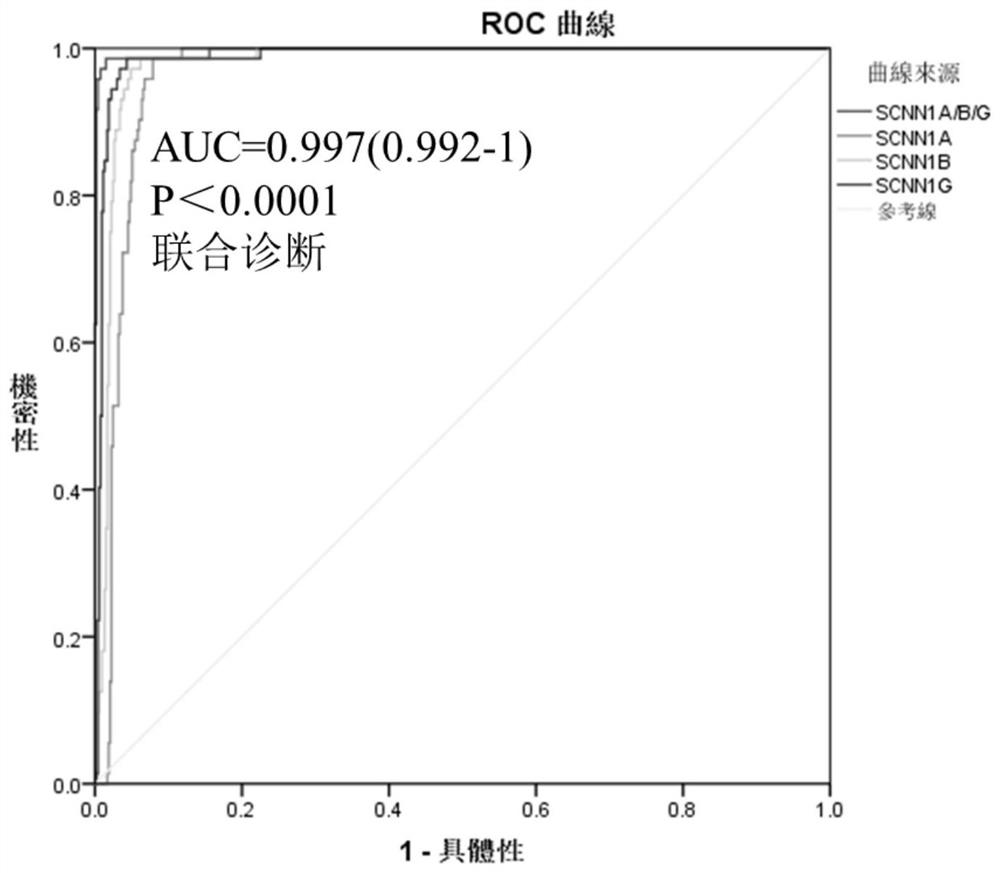
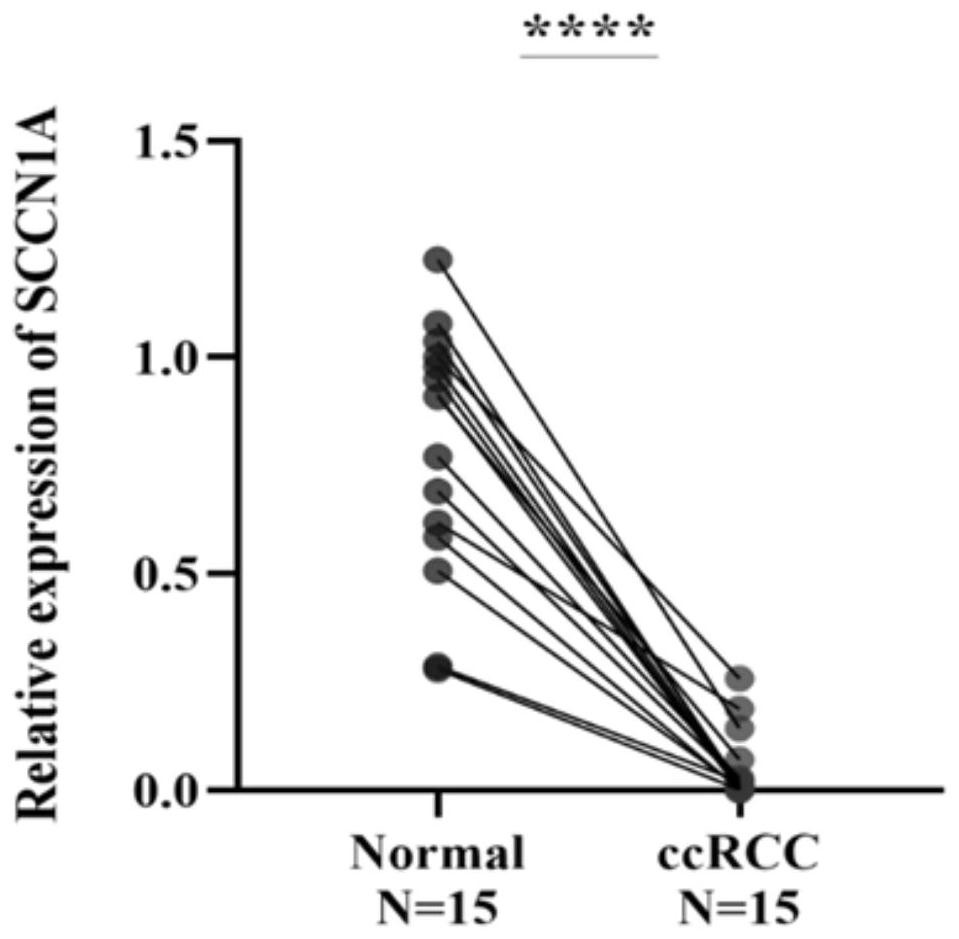
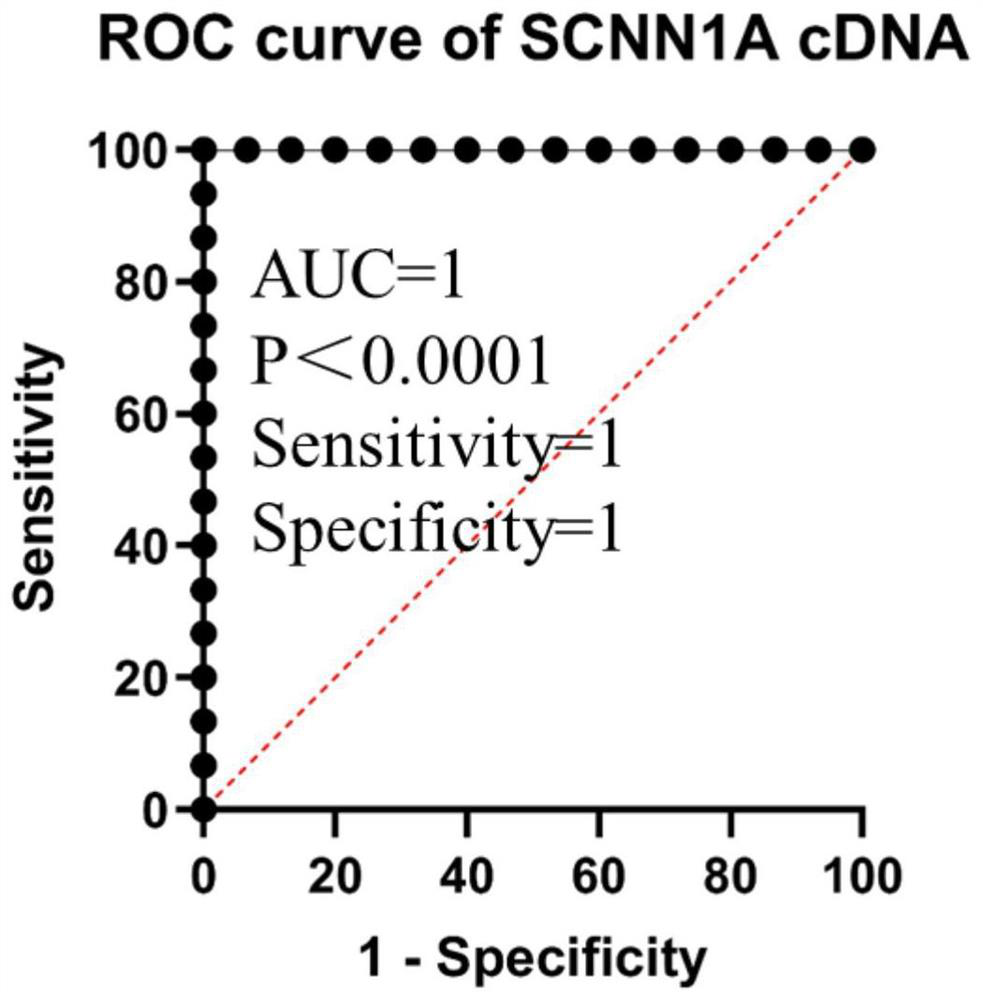
SCNN1 primer and diagnostic kit for clear cell nuclear cell carcinoma and application of SCNN1 primer
ActiveCN112553341AStrong specificityFast and good diagnosticsMicrobiological testing/measurementDNA/RNA fragmentationForward primerNucleotide
The SCNN1 primer for rapidly diagnosing clear cell nuclear cell carcinoma provided by the invention comprises an internal reference primer pair which is GAPDH, mRNA sequence forward primers and reverse primers of SCNN1A genes with nucleotide sequences shown in SEQ ID NO: 1 and SEQ ID NO: 2, mRNA sequence forward primers and reverse primers of SCNN1B genes with nucleotide sequences shown in SEQ IDNO: 3 and SEQ ID NO: 4, and mRNA sequence forward primers and reverse primers of SCNN1G genes with nucleotide sequences shown in SEQ ID NO: 5 and SEQ ID NO: 6, which take human GAPDH as an internal reference control, and forward primers and reverse primers, having nucleotide sequences shown as SEQ ID NO: 7 and SEQ ID NO: 8. A corresponding kit is also prepared. The kit comprises the following reagents: a tissue lysis solution Trizol, an SYBRGreen Master Mix reaction solution, a reverse transcription reaction solution, a negative control and a positive control. The primer and the kit can eliminate false negative and false positive of clinical sample detection, and are safe, reliable, high in accuracy and suitable for clinical popularization.
Owner:GUANGXI MEDICAL UNIVERSITY
Enzyme preparation containing thermostable DNA polymerase, method for producing same, and method for detecting subject organism to be detected
ActiveUS9243272B2Low production costWithout reducing sensitivityMicrobiological testing/measurementTransferasesMicroorganismMicrobiology
Disclosed is a thermostable DNA polymerase preparation which can illimitably reduce the risk of false positivity in the detection of a subject microorganism utilizing a gene amplification reaction and therefore enables the selective amplification of DNA for detecting the subject microorganism even when the amount of the subject microorganism is small and therefore the amount of DNA collected therefrom is extremely small, and can be produced at a reduced cost. Also disclosed is a method for quantifying or quantifying / identifying a subject organism to be detected rapidly, conveniently and with high sensitivity using the preparation of the present invention.
Owner:HOKKAIDO MITSUI CHEM INC +1
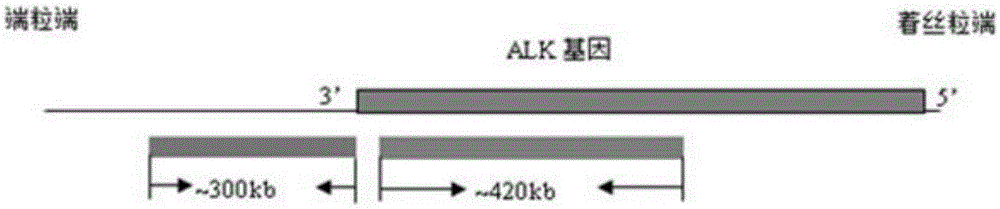
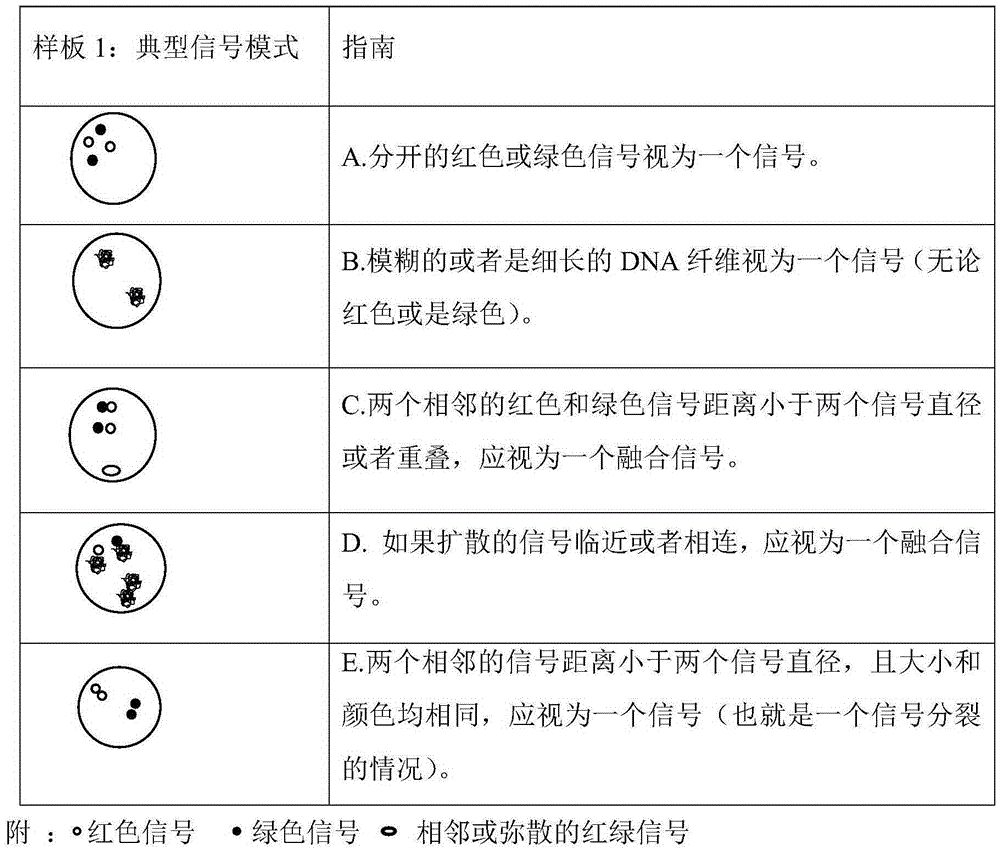
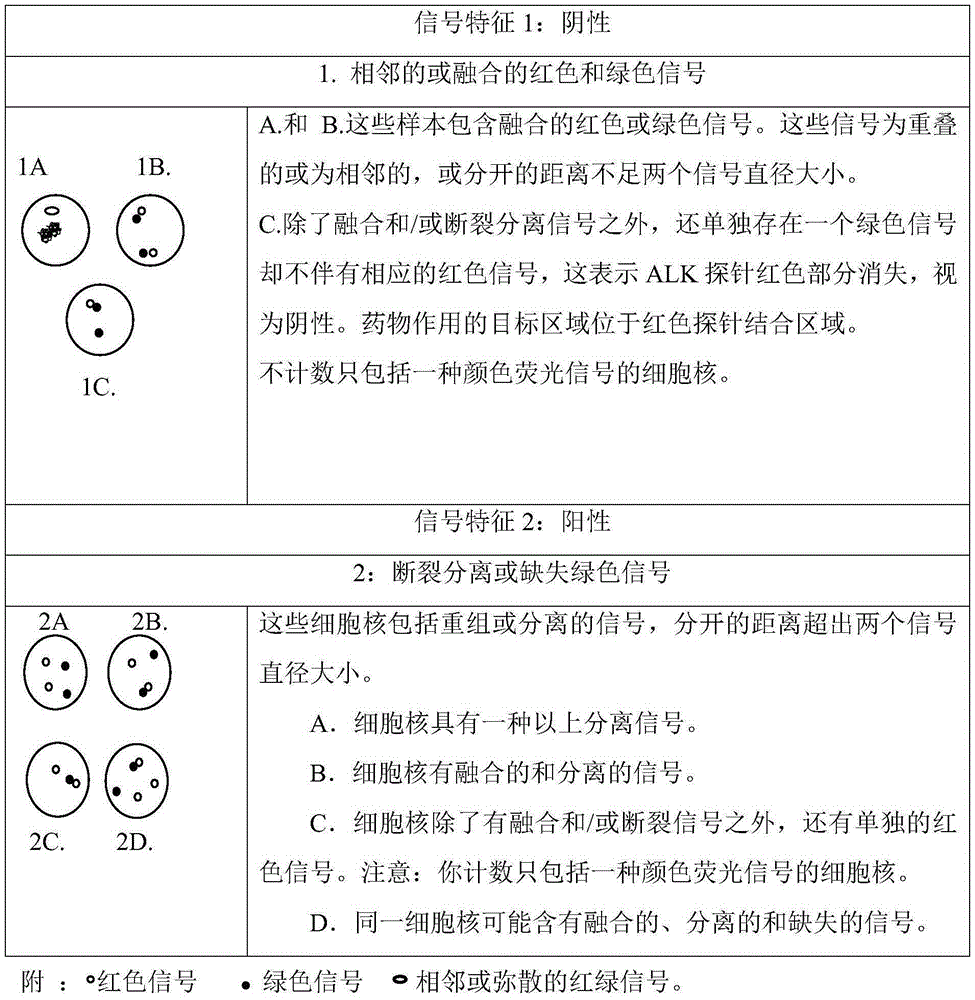
Fluorescence probe for rapid detection of lung cancer ALK gene rearrangement and preparation method
InactiveCN105368962AQuick checkAccurate detectionMicrobiological testing/measurementDNA preparationHybridization probeFluorescence
The invention belongs to the technical field of biology, and particularly relates to the technical field of rapid detection of lung cancer related genes. An obtained fluorescence probe for rapid detection of lung cancer ALK gene rearrangement does not contain repeated sequences, can avoid cross reactions and has the advantages of being high in accuracy, good in specificity and low in false positivity, a rapid fluorescence in situ hybridization is utilized for shortening the hybridization time which is originally 16 h to be 2 h, the diagnosis efficiency is greatly improved, and time is saved.
Owner:河南赛诺特生物技术有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com