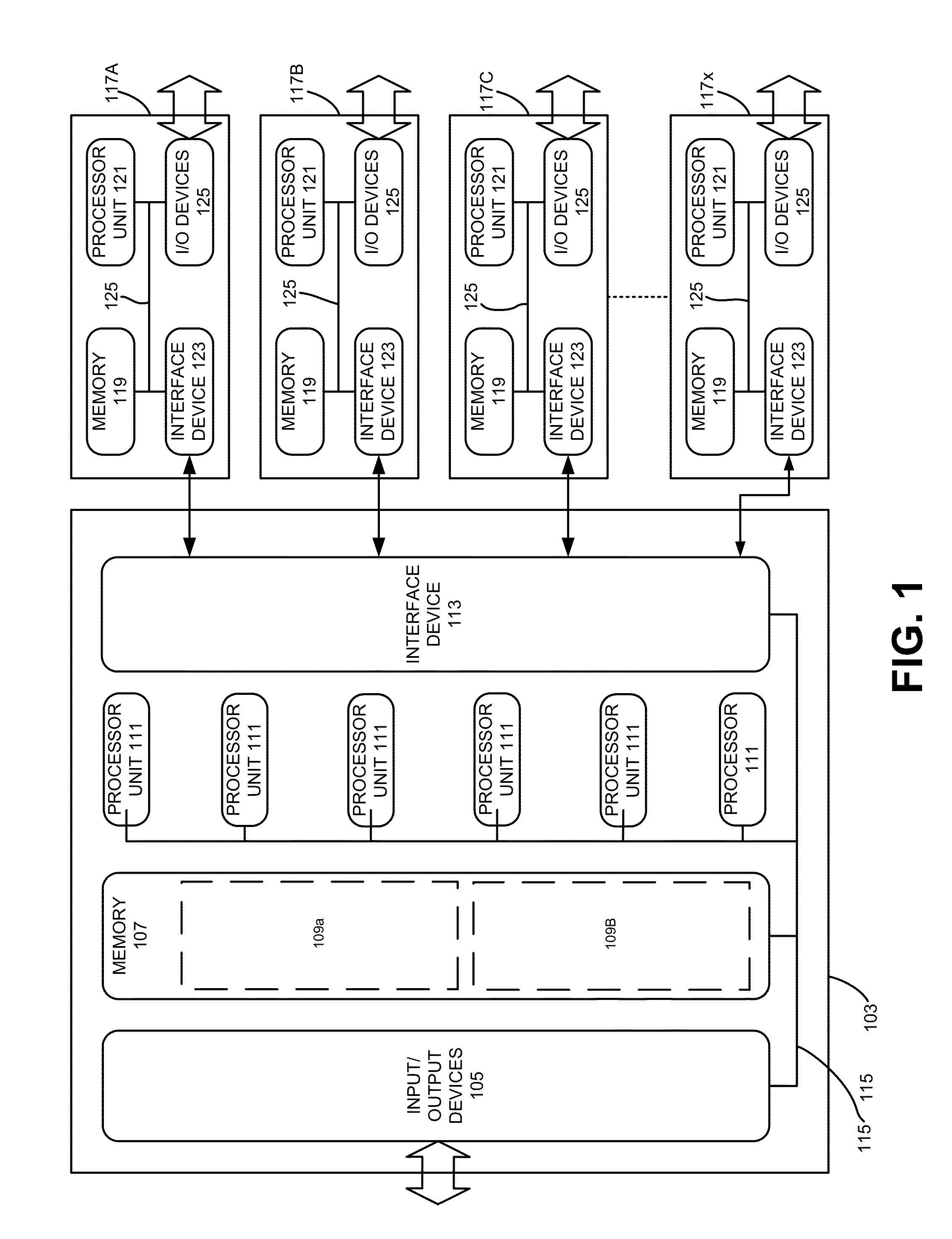
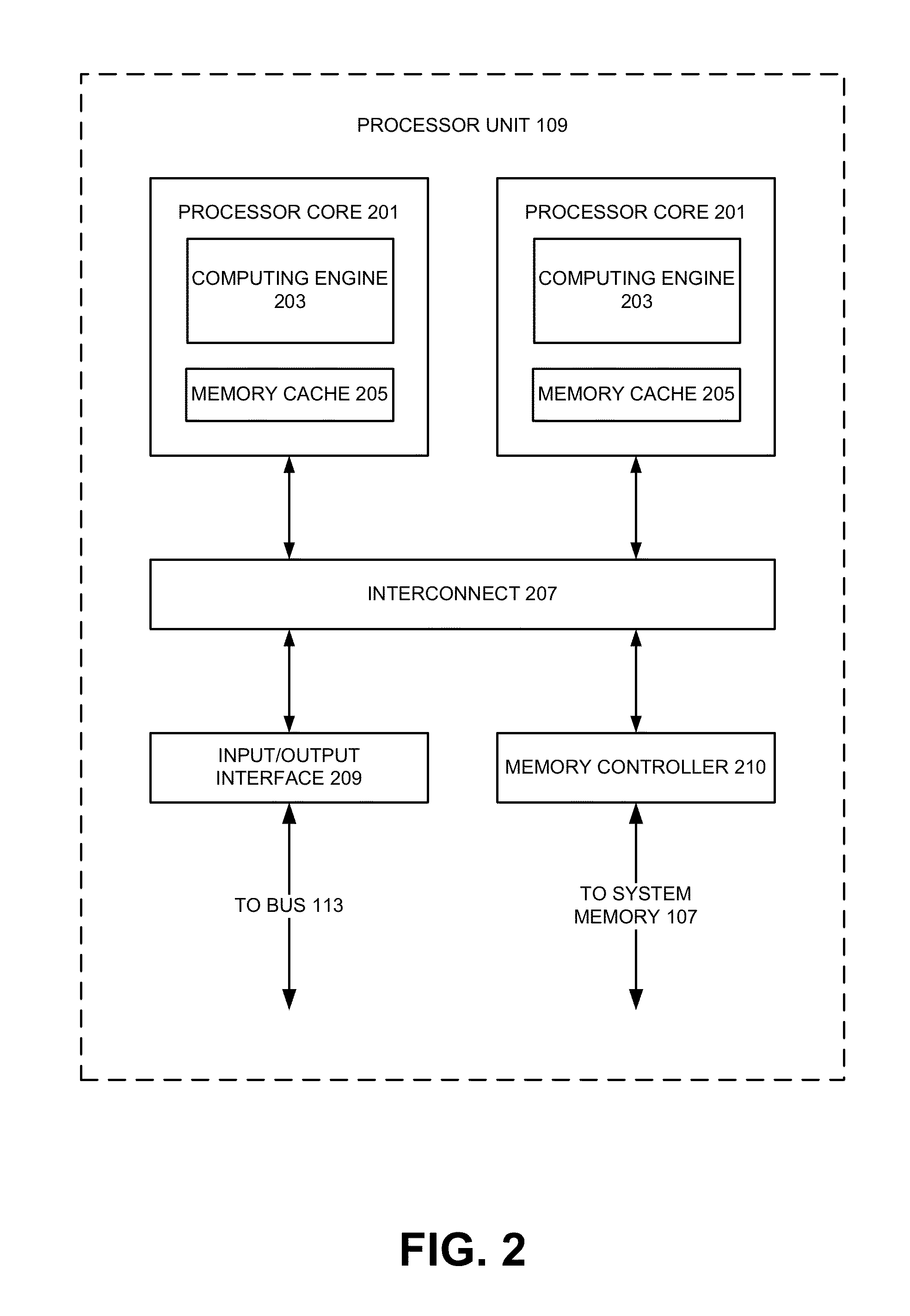
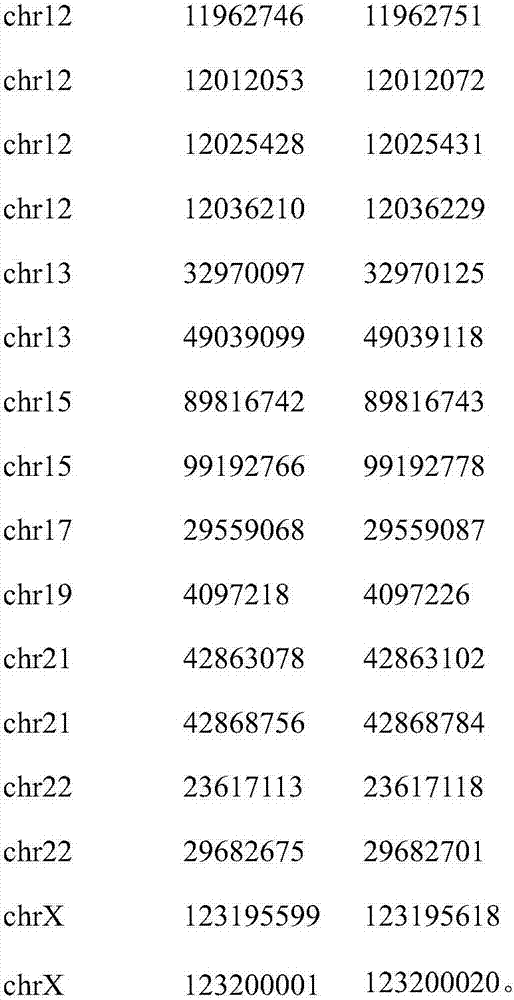
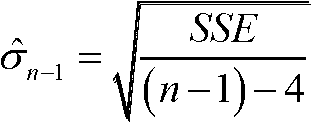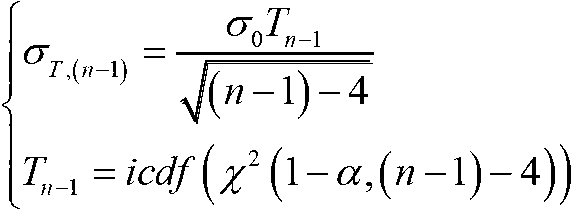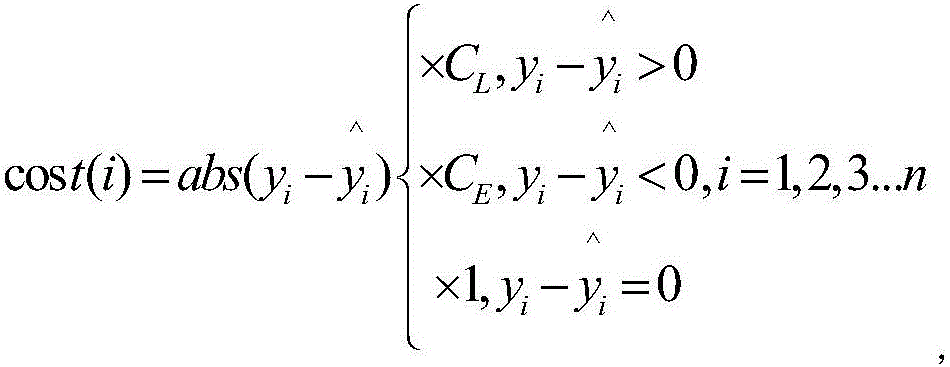Patents
Literature
194 results about "False positives and false negatives" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
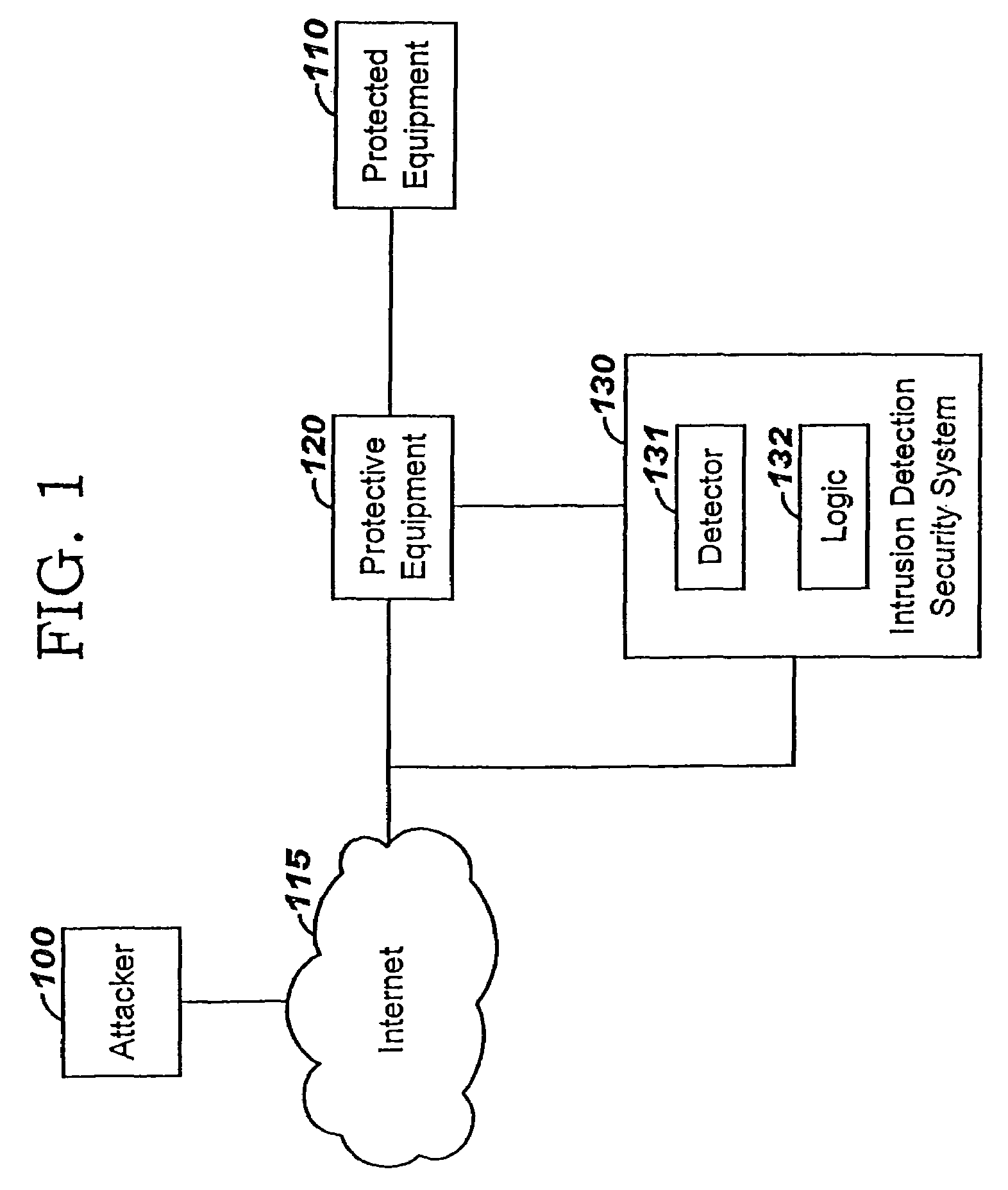
In medical testing, and more generally in binary classification, a false positive is an error in data reporting in which a test result improperly indicates presence of a condition, such as a disease (the result is positive), when in reality it is not present, while a false negative is an error in which a test result improperly indicates no presence of a condition (the result is negative), when in reality it is present. These are the two kinds of errors in a binary test (and are contrasted with a correct result, either a true positive or a true negative.) They are also known in medicine as a false positive (respectively negative) diagnosis, and in statistical classification as a false positive (respectively negative) error. A false positive is distinct from overdiagnosis, and is also different from overtesting.
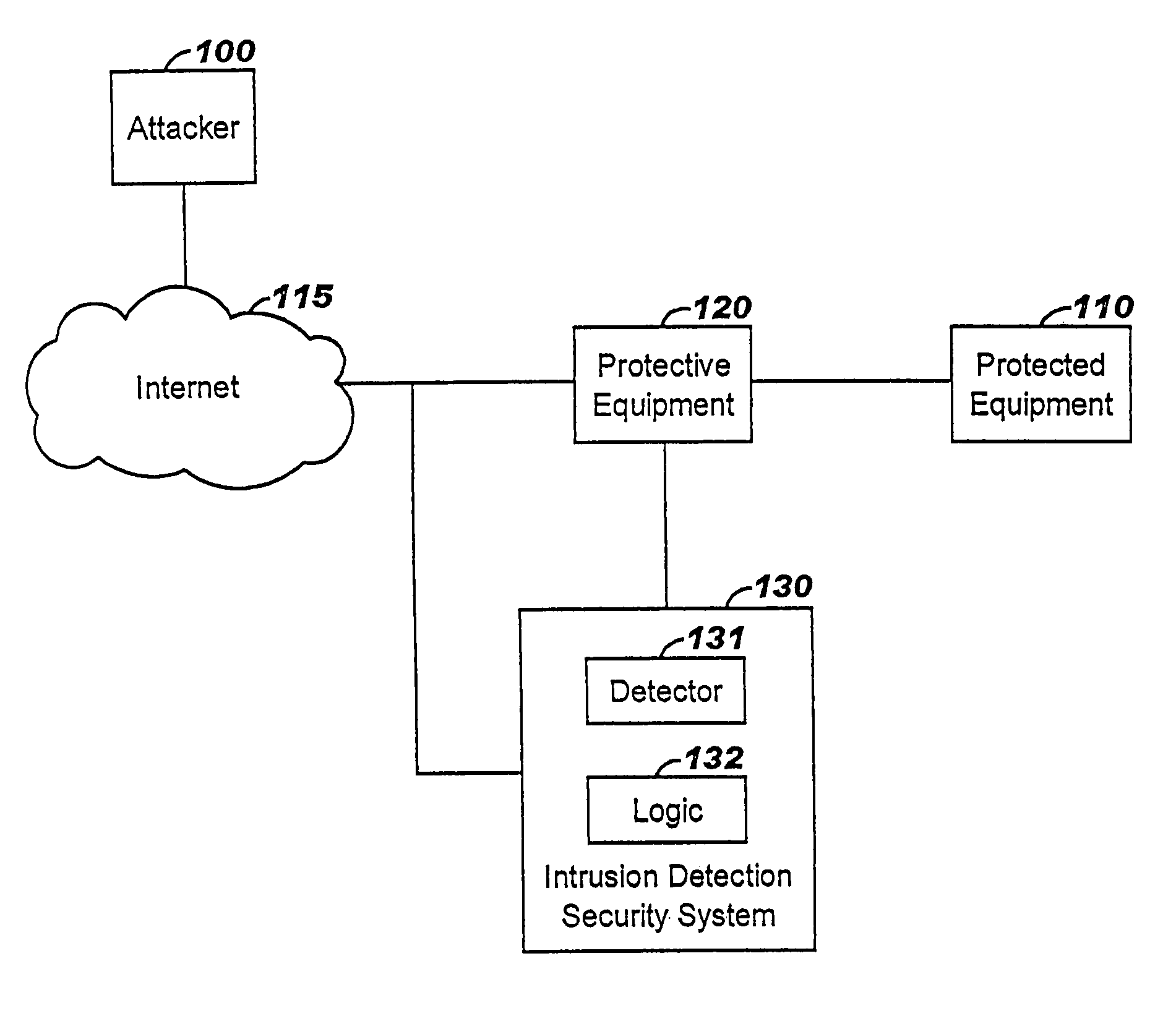
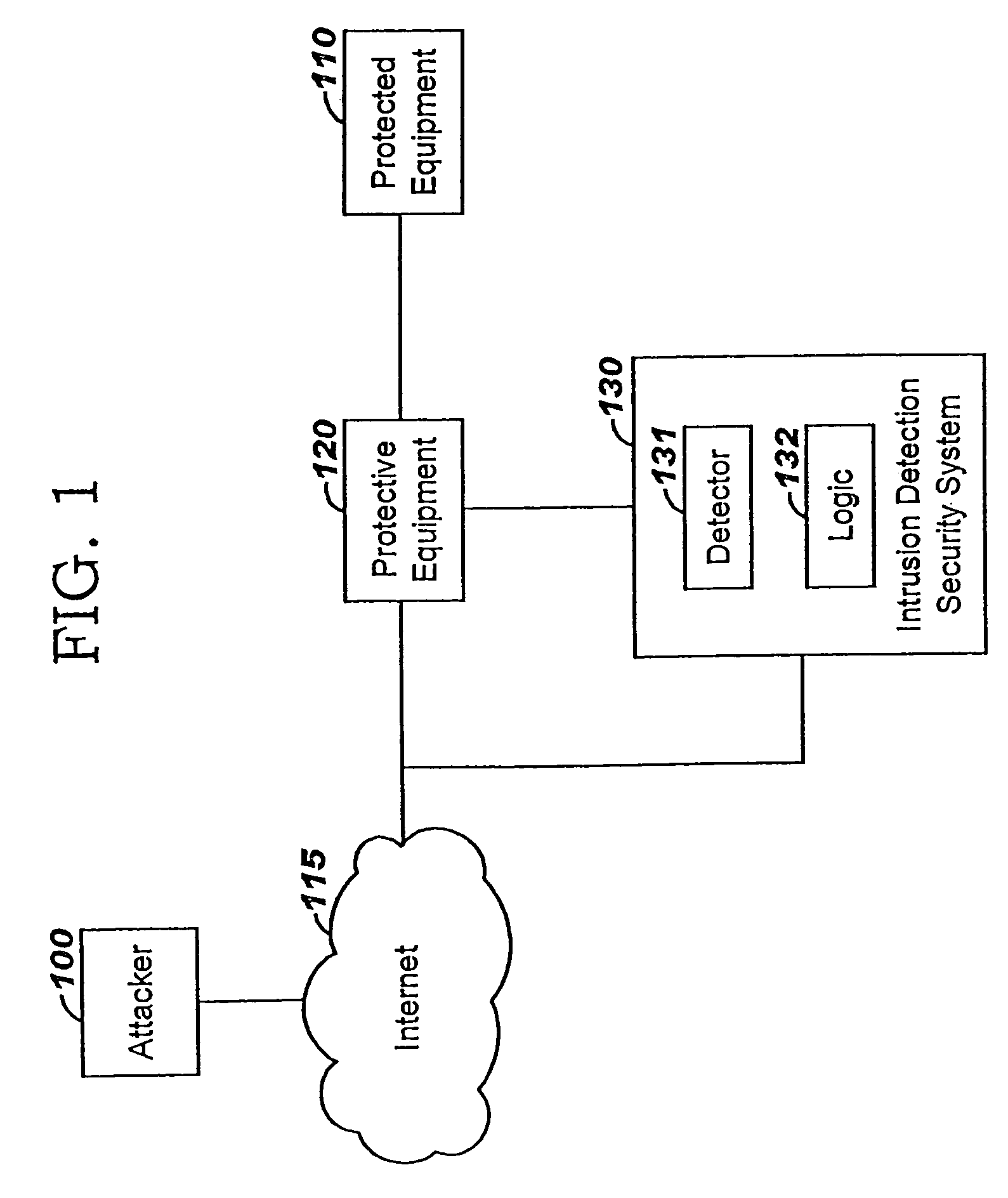
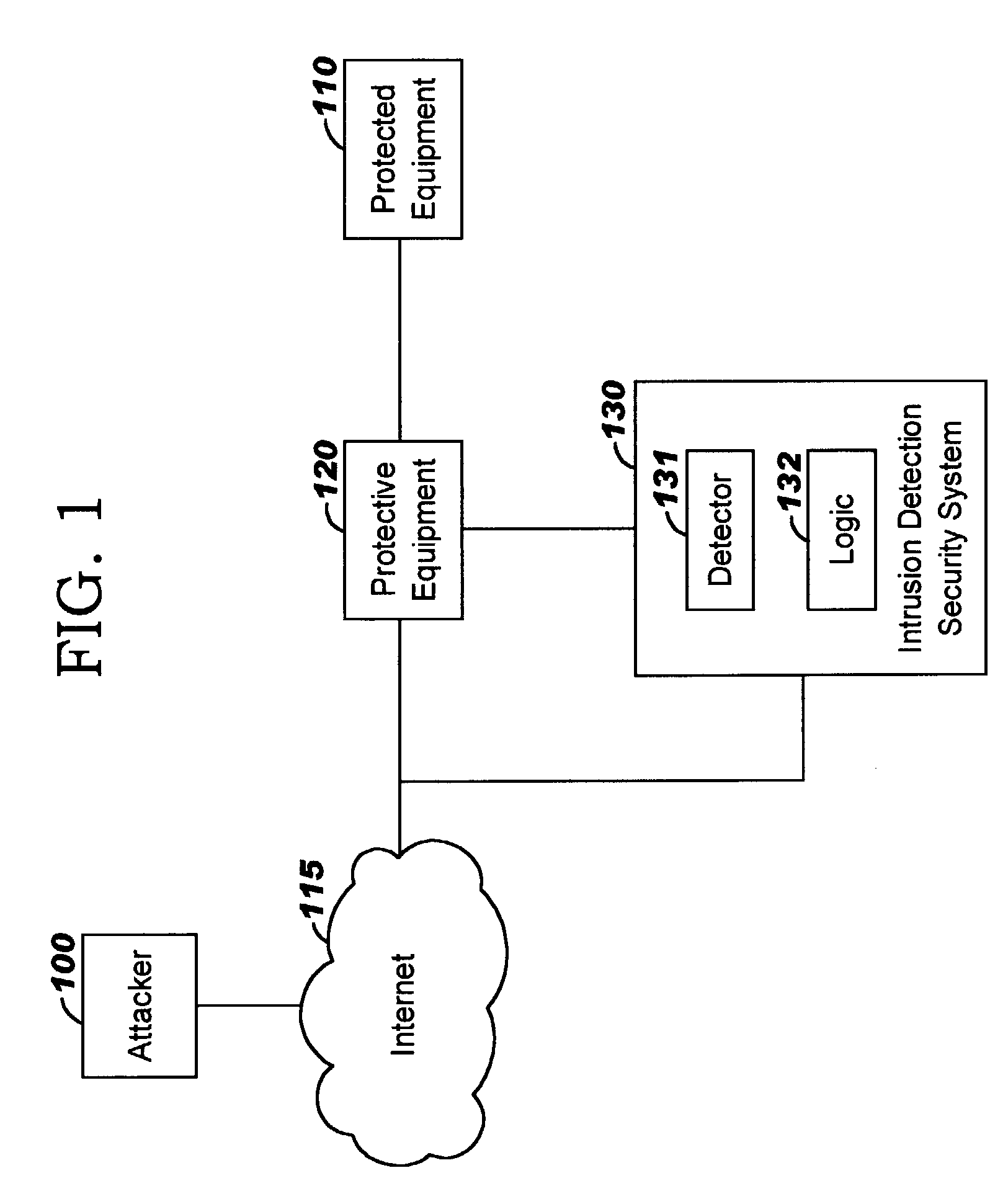
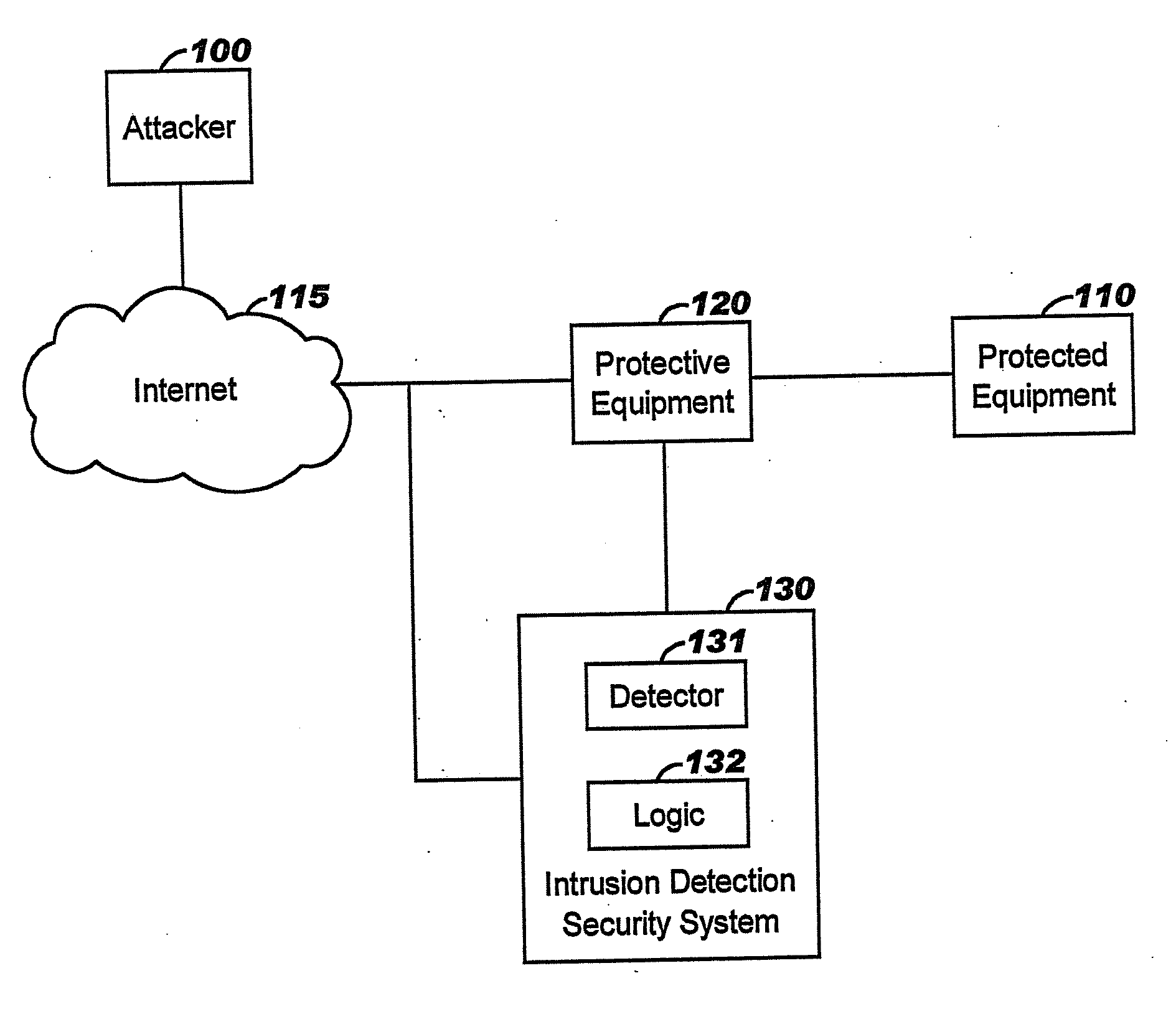
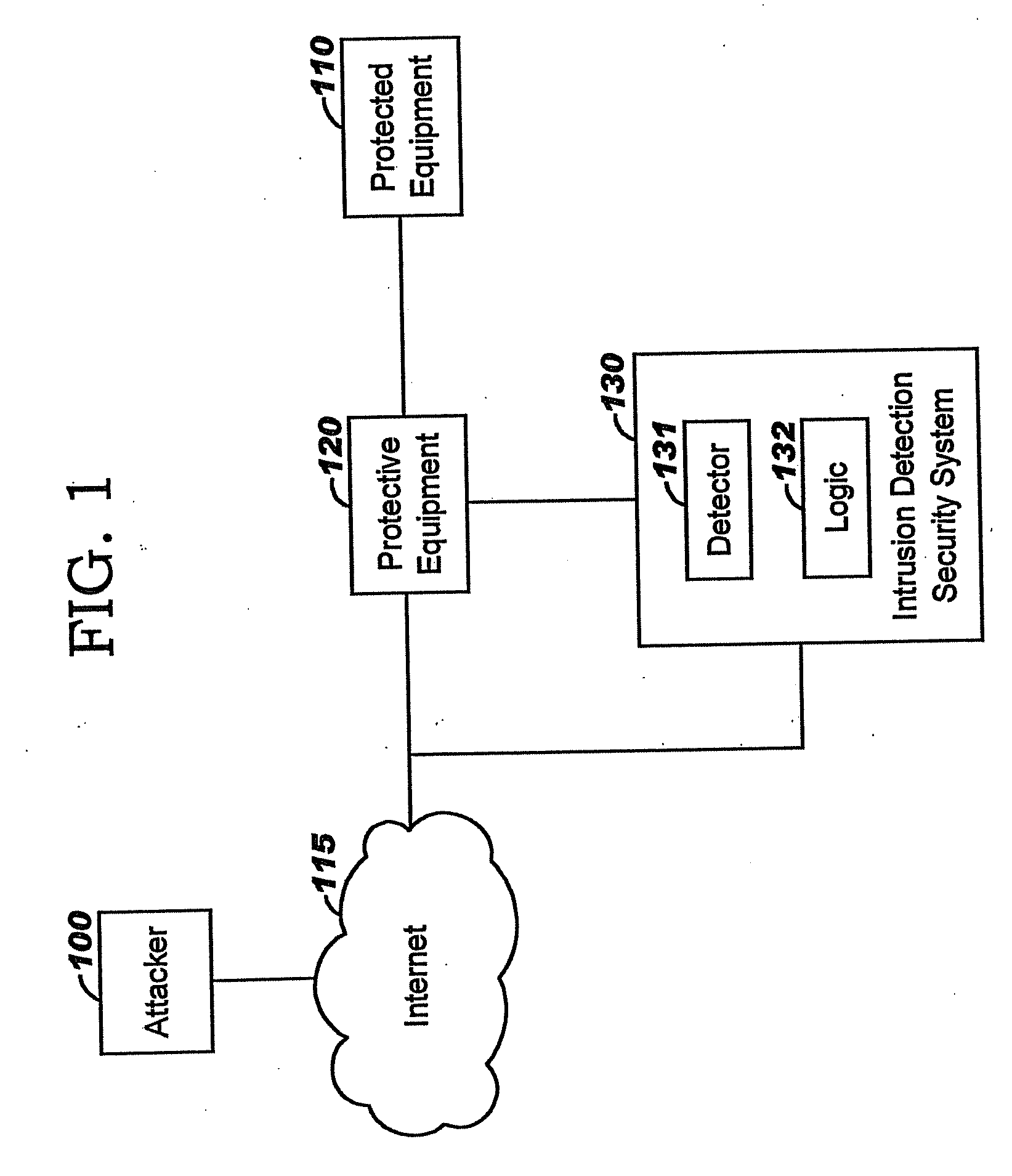
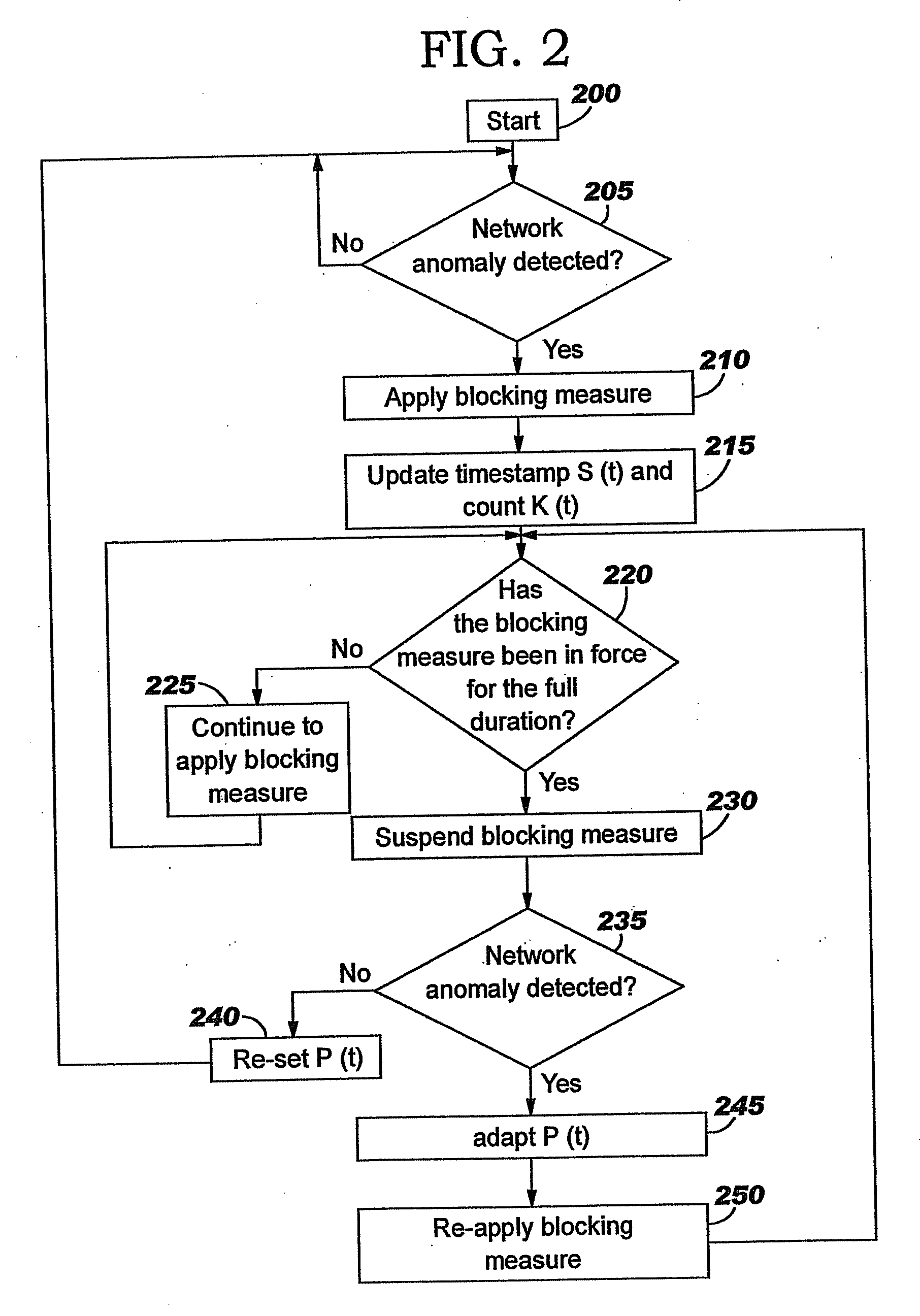
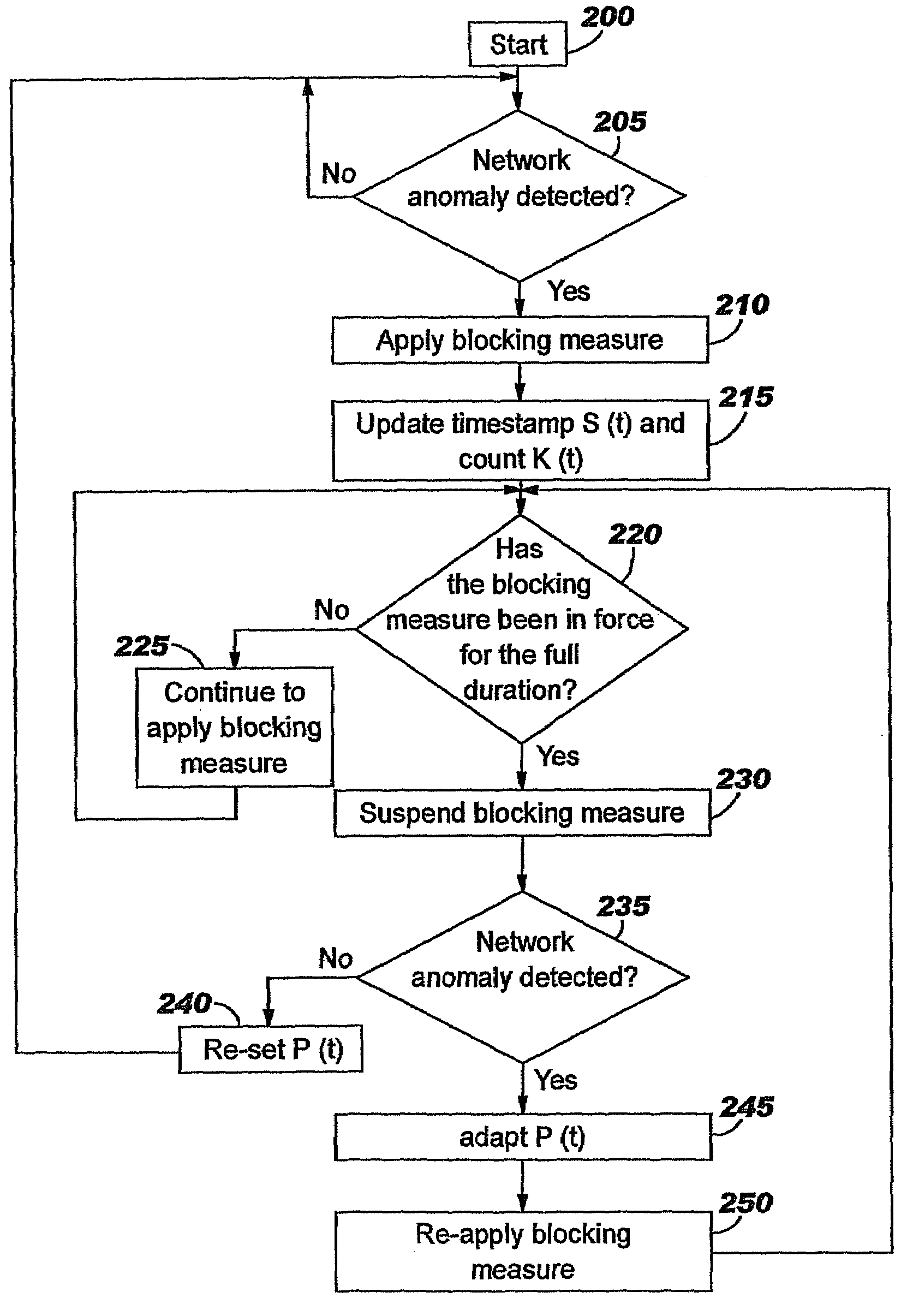
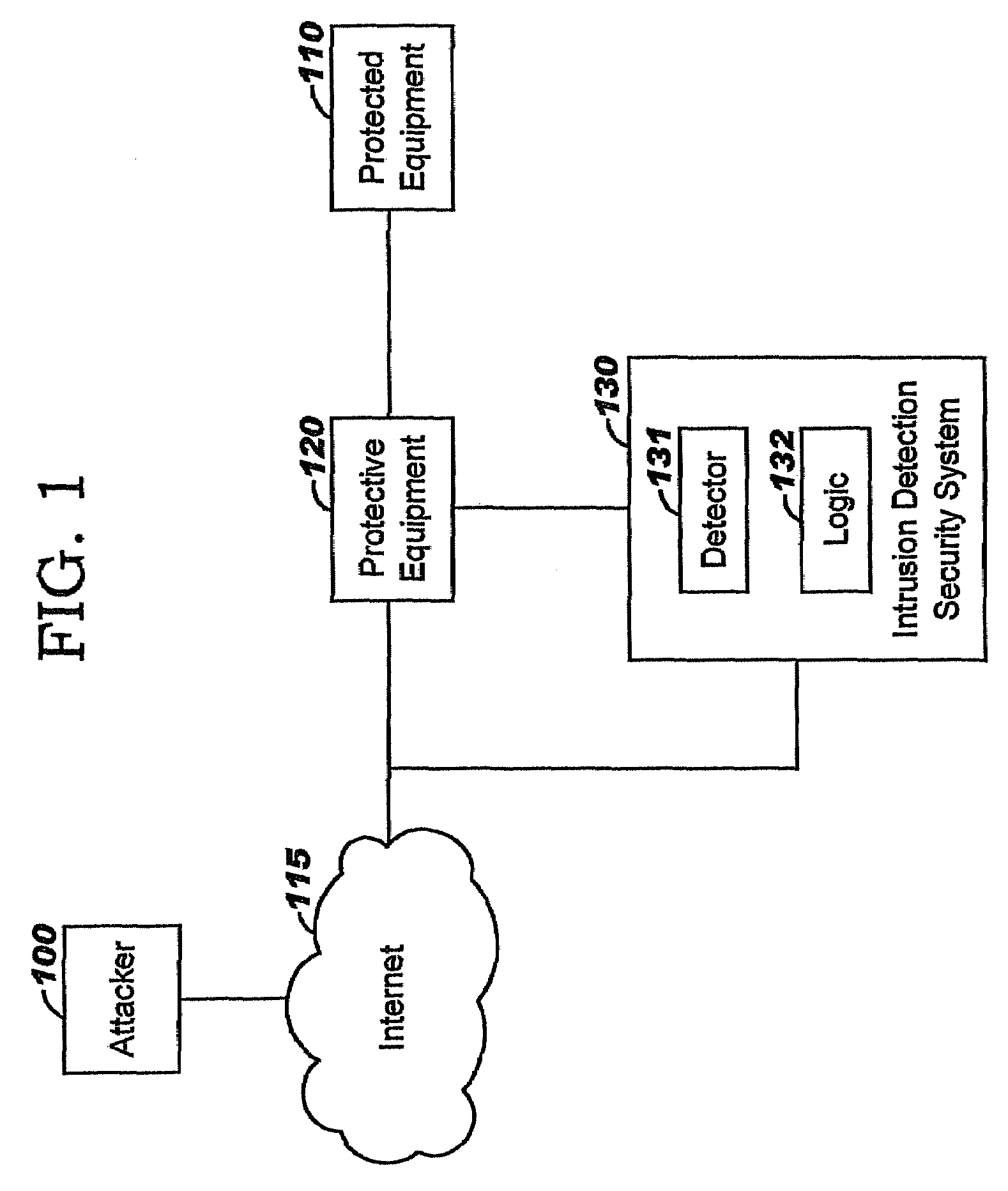
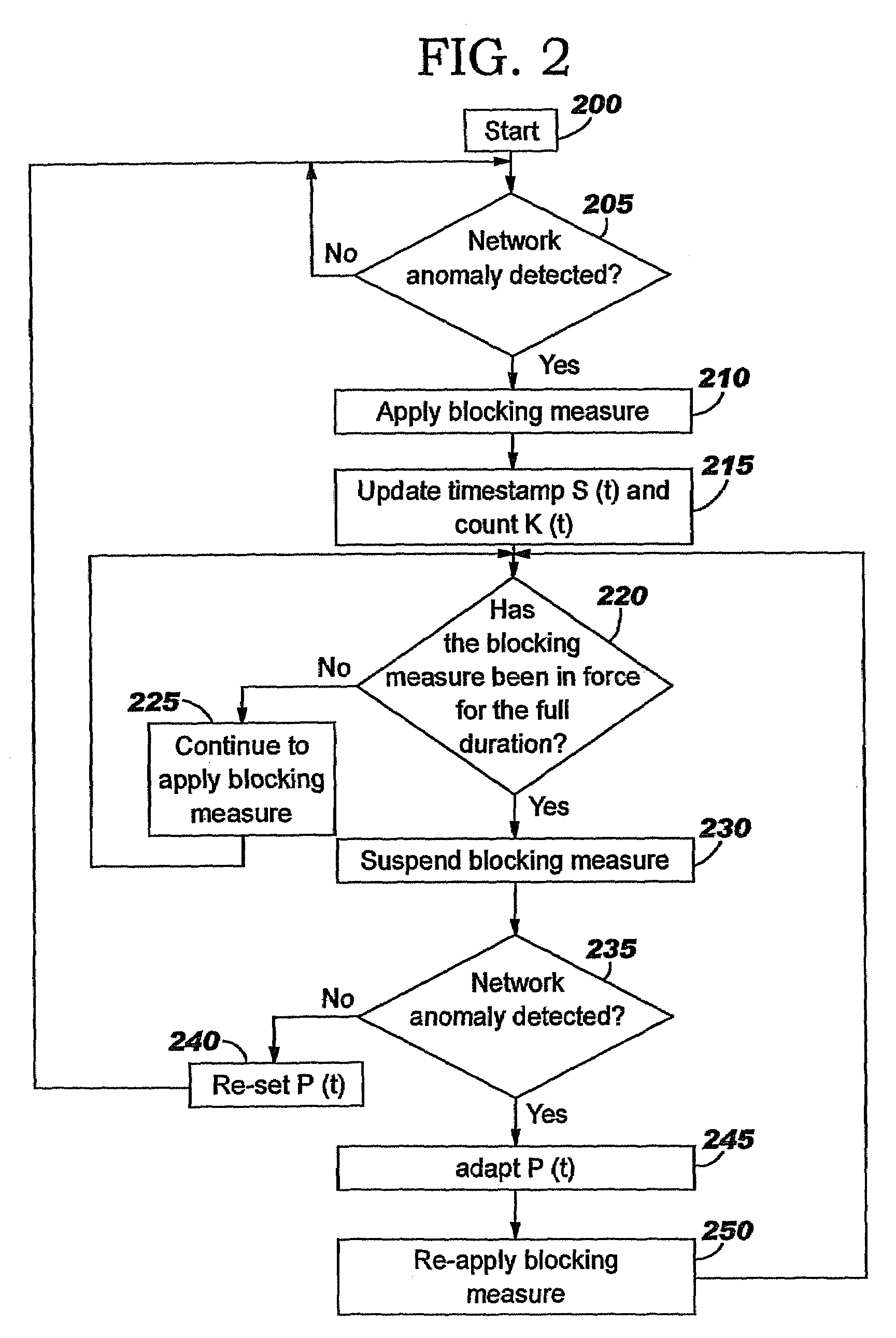
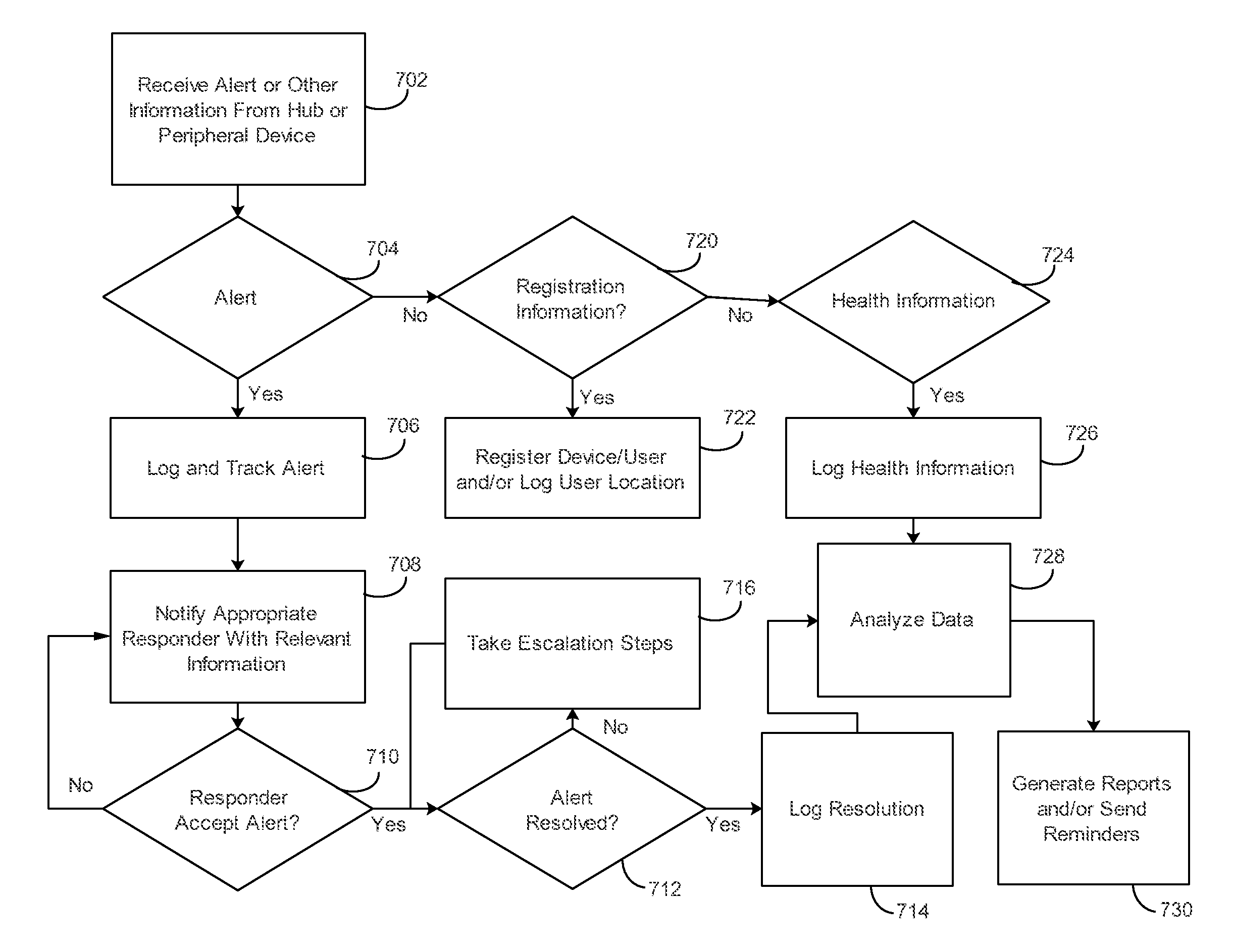
Applying blocking measures progressively to malicious network traffic
InactiveUS20060075496A1Minimizing adverse consequenceMinimize consequencesMemory loss protectionUnauthorized memory use protectionResponse methodFalse positives and false negatives
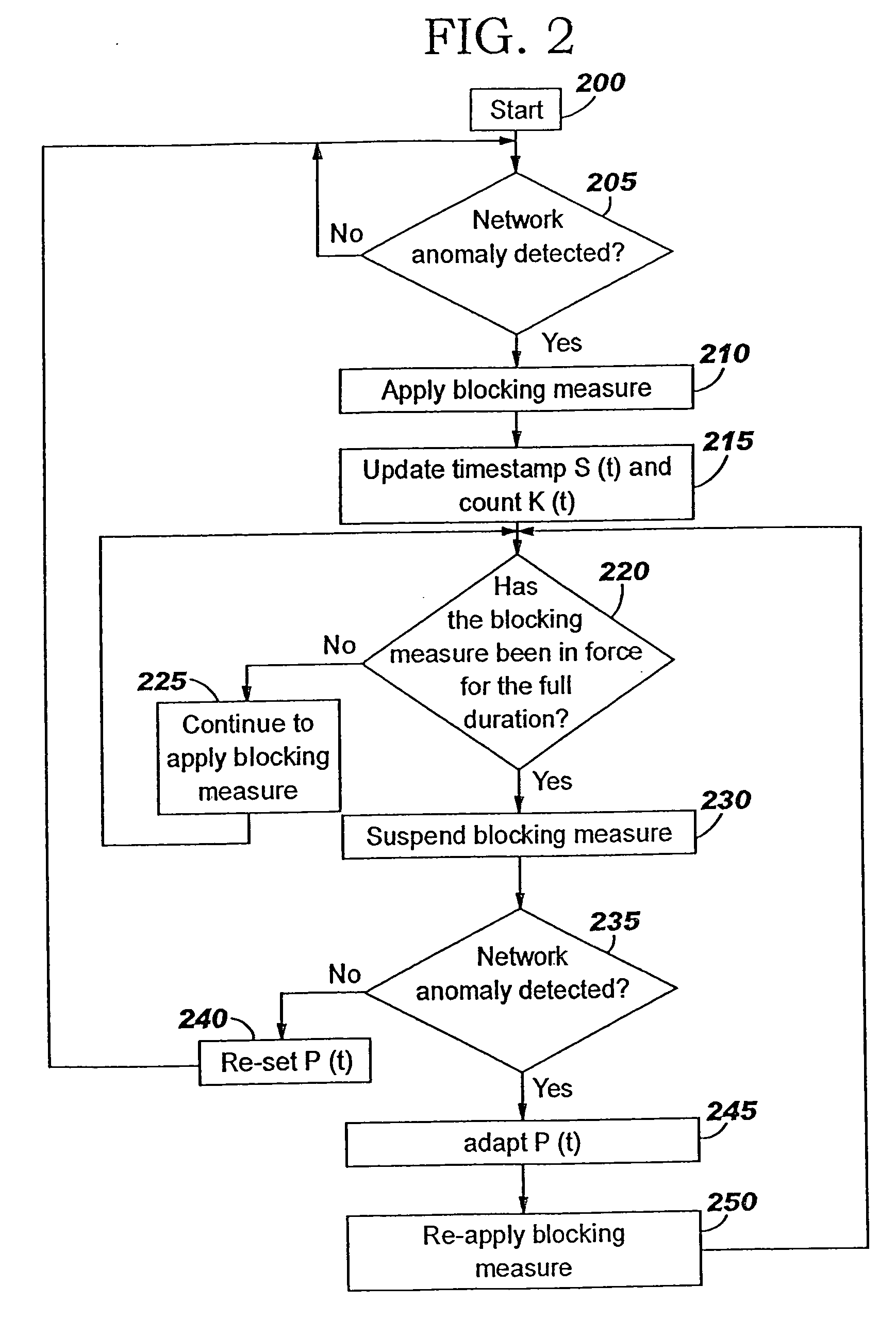
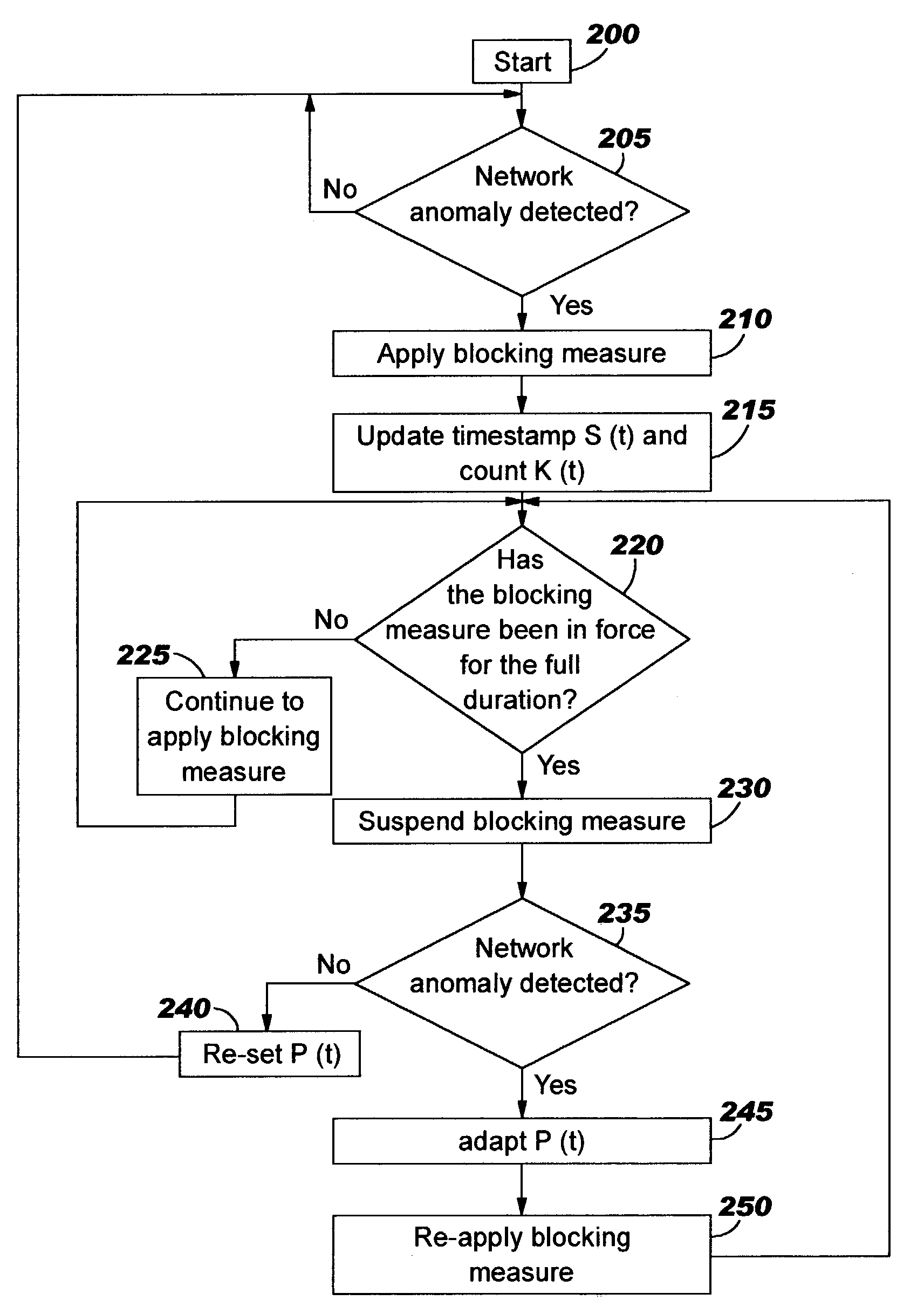
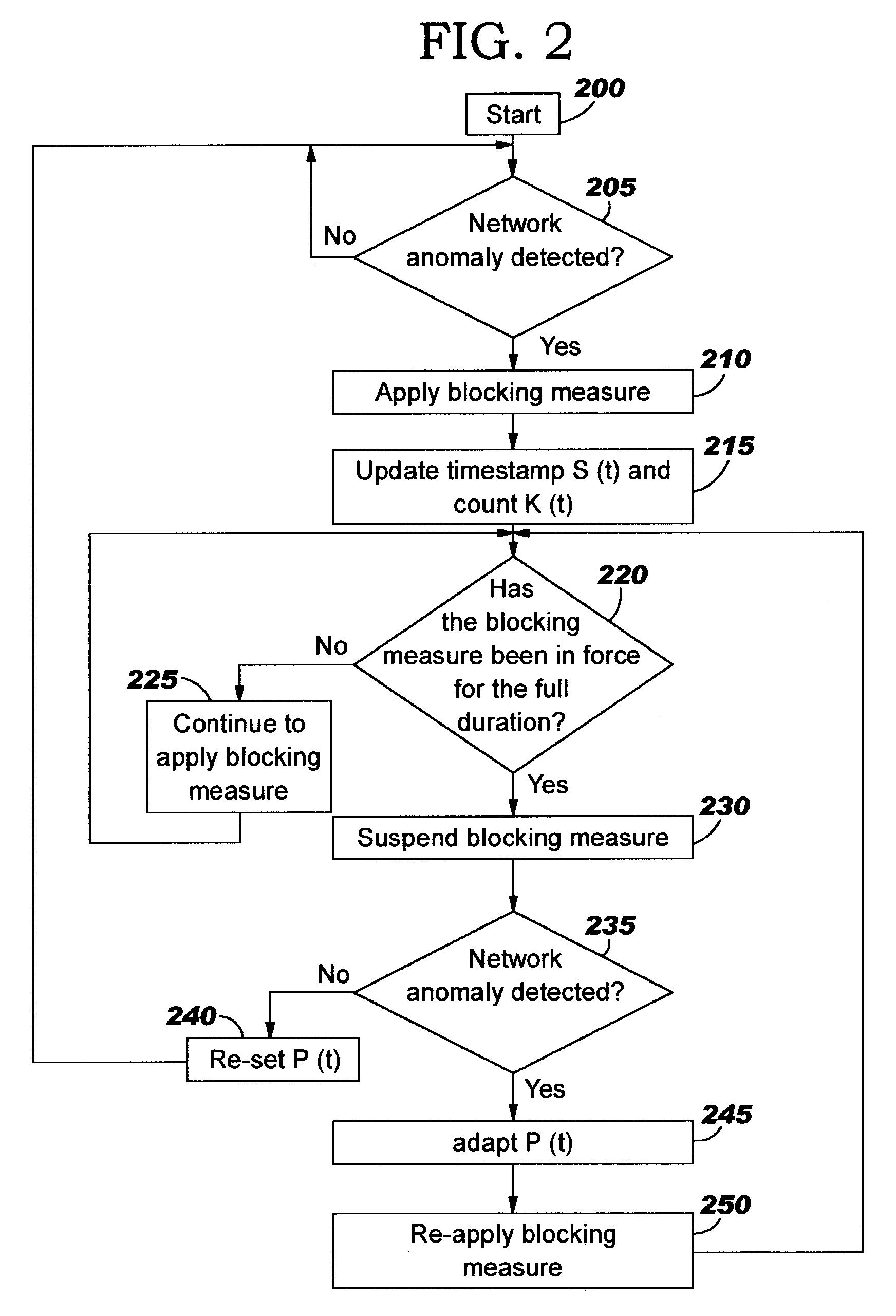
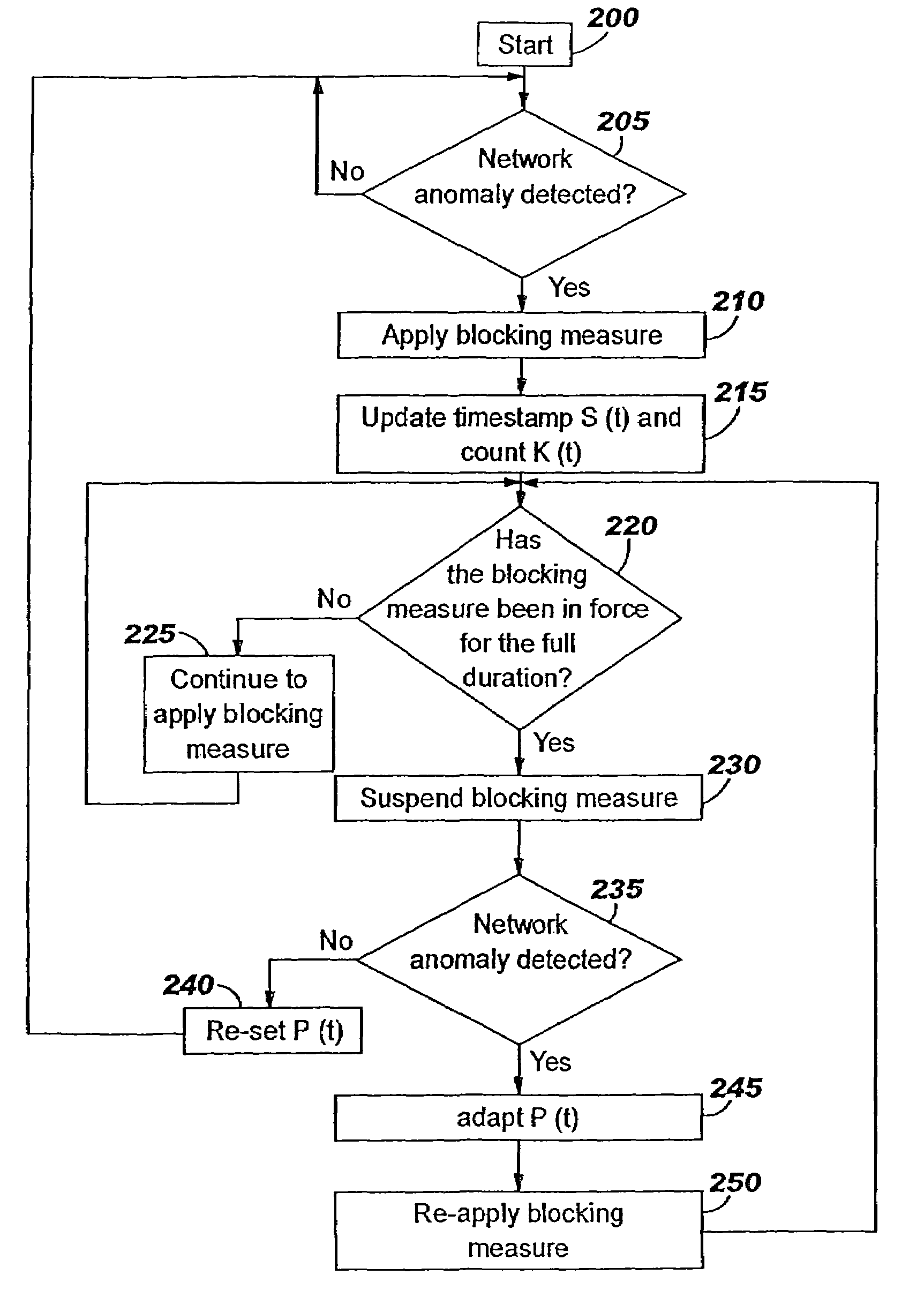
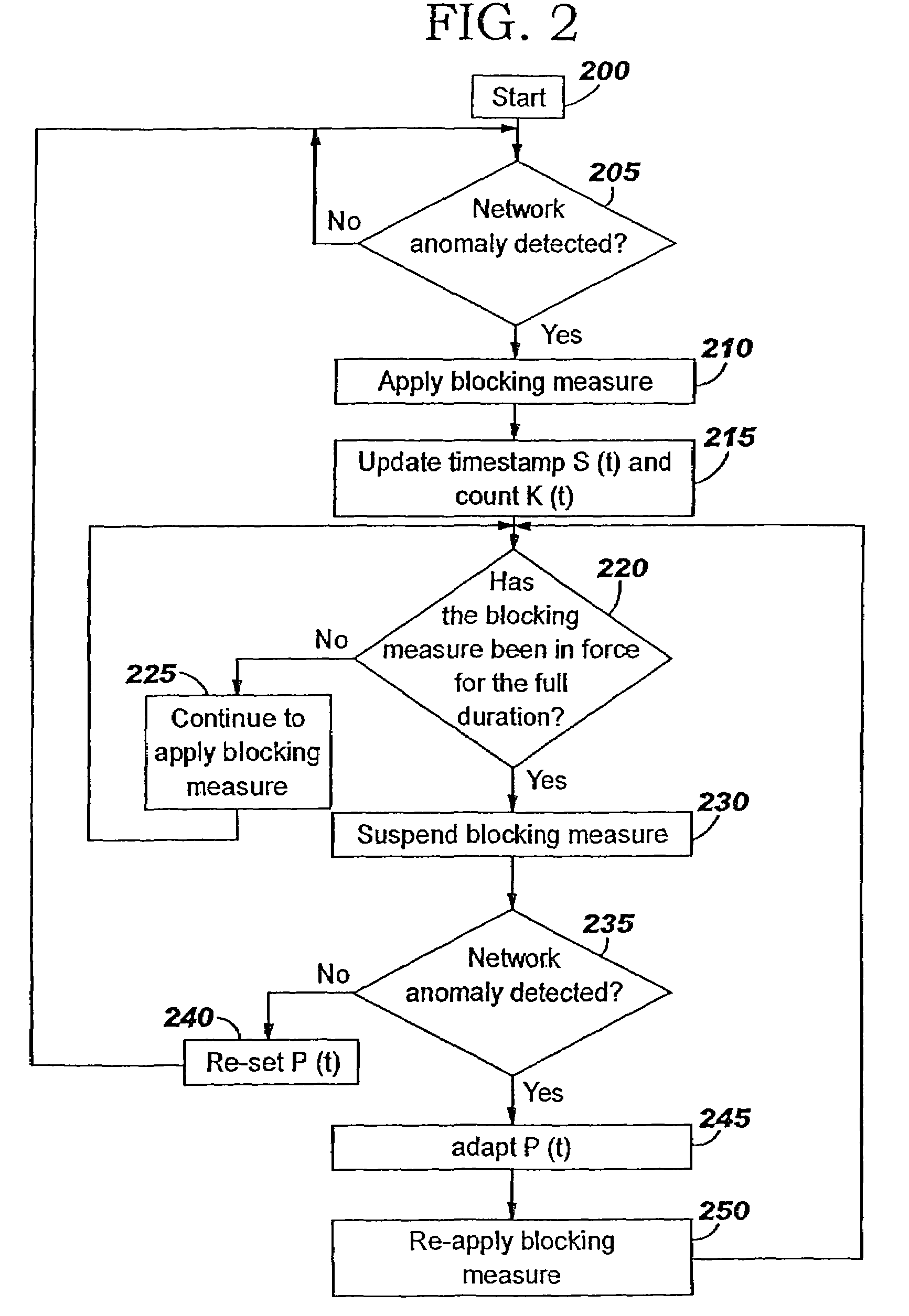
A method of progressive response for invoking and suspending blocking measures that defend against network anomalies such as malicious network traffic so that false positives and false negatives are minimized. When a truncated secure session attack is detected, the detector notifies protective equipment such as a firewall or a router to invoke a blocking measure. The blocking measure is maintained for an initial duration, after which it is suspended while another test for the anomaly is made. If the attack is no longer evident, the method returns to the state of readiness. Otherwise, a loop is executed to re-applying the blocking measure for a specified duration, then suspend the blocking measure and test again for the attack. If the attack is detected, the blocking measure is re-applied, and its duration is adapted. If the attack is no longer detected, the method returns to the state of readiness.
Owner:TREND MICRO INC
Applying blocking measures progressively to malicious network traffic
ActiveUS7308716B2Minimizing adverse consequenceMinimize consequencesMemory loss protectionUnauthorized memory use protectionTraffic capacityFalse positives and false negatives
A method of progressive response for invoking and suspending blocking measures that defend against network anomalies such as malicious network traffic so that false positives and false negatives are minimized. When an anomaly is detected, the detector notifies protective equipment such as a firewall or a router to invoke a blocking measure. The blocking measure is maintained for an initial duration, after which it is suspended while another test for the anomaly is made. If the anomaly is no longer evident, the method returns to the state of readiness. Otherwise, a loop is executed to re-applying the blocking measure for a specified duration, then suspend the blocking measure and test again for the anomaly. If the anomaly is detected, the blocking measure is re-applied, and its duration is adapted. If the anomaly is no longer detected, the method returns to the state of readiness.
Owner:TREND MICRO INC
Applying blocking measures progressively to malicious network traffic
InactiveUS20080072326A1Minimizing adverse consequenceMinimize consequencesMemory loss protectionDigital computer detailsResponse methodFalse positives and false negatives
A method of progressive response for invoking and suspending blocking measures that defend against network anomalies such as malicious network traffic so that false positives and false negatives are minimized. When an anomaly is detected, the detector notifies protective equipment such as a firewall or a router to invoke a blocking measure. The blocking measure is maintained for an initial duration, after which it is suspended while another test for the anomaly is made. If the anomaly is no longer evident, the method returns to the state of readiness. Otherwise, a loop is executed to re-applying the blocking measure for a specified duration, then suspend the blocking measure and test again for the anomaly. If the anomaly is detected, the blocking measure is re-applied, and its duration is adapted. If the anomaly is no longer detected, the method returns to the state of readiness.
Owner:IBM CORP
Applying blocking measures progressively to malicious network traffic
InactiveUS7707633B2Minimizing adverse consequenceMinimize consequencesMemory loss protectionDigital computer detailsTraffic capacityFalse positives and false negatives
A method of progressive response for invoking and suspending blocking measures that defend against network anomalies such as malicious network traffic so that false positives and false negatives are minimized. When an anomaly is detected, the detector notifies protective equipment such as a firewall or a router to invoke a blocking measure. The blocking measure is maintained for an initial duration, after which it is suspended while another test for the anomaly is made. If the anomaly is no longer evident, the method returns to the state of readiness. Otherwise, a loop is executed to re-apply the blocking measure for a specified duration, then suspend the blocking measure and test again for the anomaly. If the anomaly is detected, the blocking measure is re-applied, and its duration is adapted. If the anomaly is no longer detected, the method returns to the state of readiness.
Owner:INT BUSINESS MASCH CORP
Method of responding to a truncated secure session attack
InactiveUS7464404B2Minimizing adverse consequenceMinimize consequencesMemory loss protectionUnauthorized memory use protectionTraffic capacityFalse positives and false negatives
A method of progressive response for invoking and suspending blocking measures that defend against network anomalies such as malicious network traffic so that false positives and false negatives are minimized. When a truncated secure session attack is detected, the detector notifies protective equipment such as a firewall or a router to invoke a blocking measure. The blocking measure is maintained for an initial duration, after which it is suspended while another test for the anomaly is made. If the attack is no longer evident, the method returns to the state of readiness. Otherwise, a loop is executed to re-applying the blocking measure for a specified duration, then suspend the blocking measure and test again for the attack. If the attack is detected, the blocking measure is re-applied, and its duration is adapted. If the attack is no longer detected, the method returns to the state of readiness.
Owner:TREND MICRO INC
Systems and methods for feature detection in retinal images
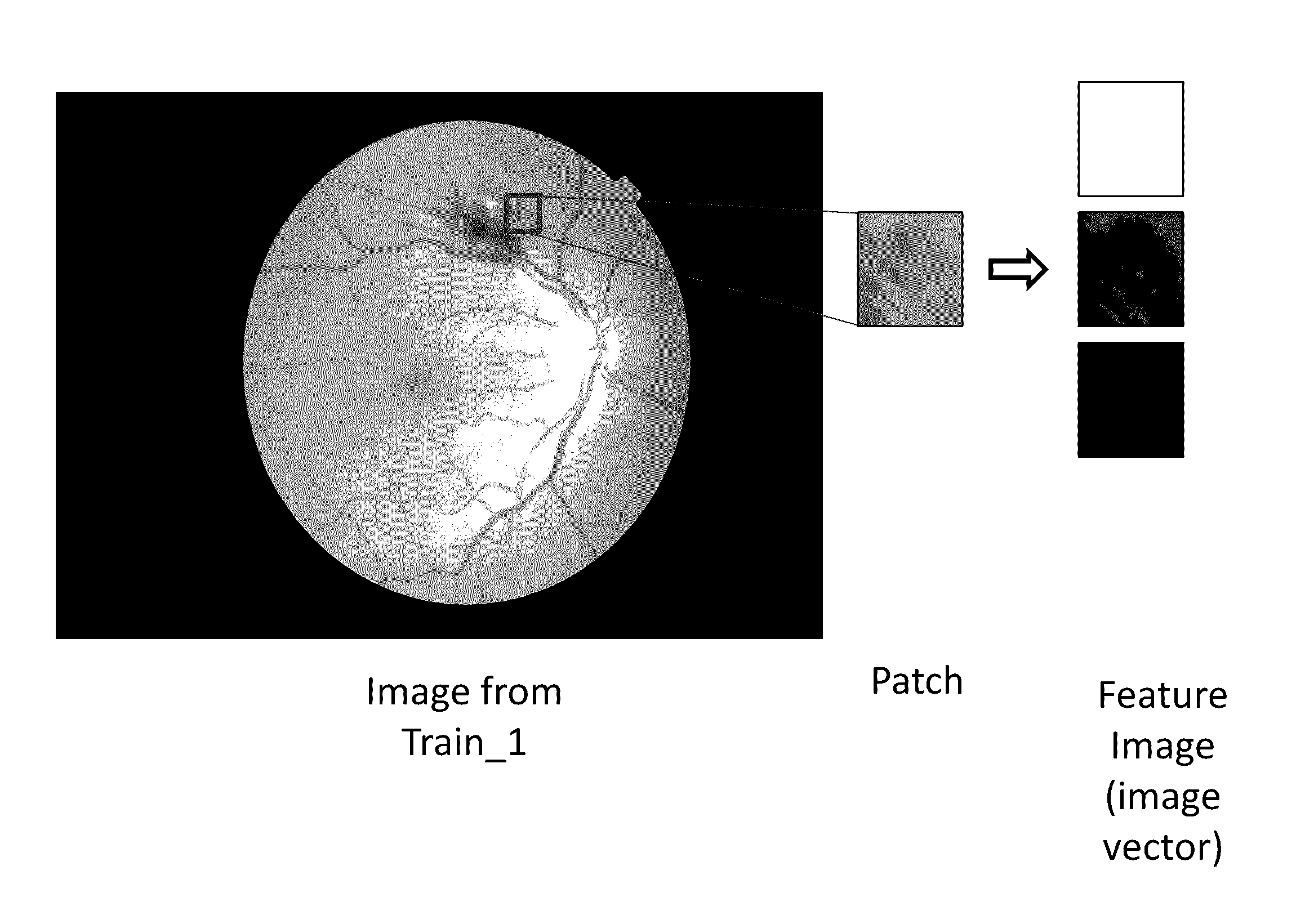
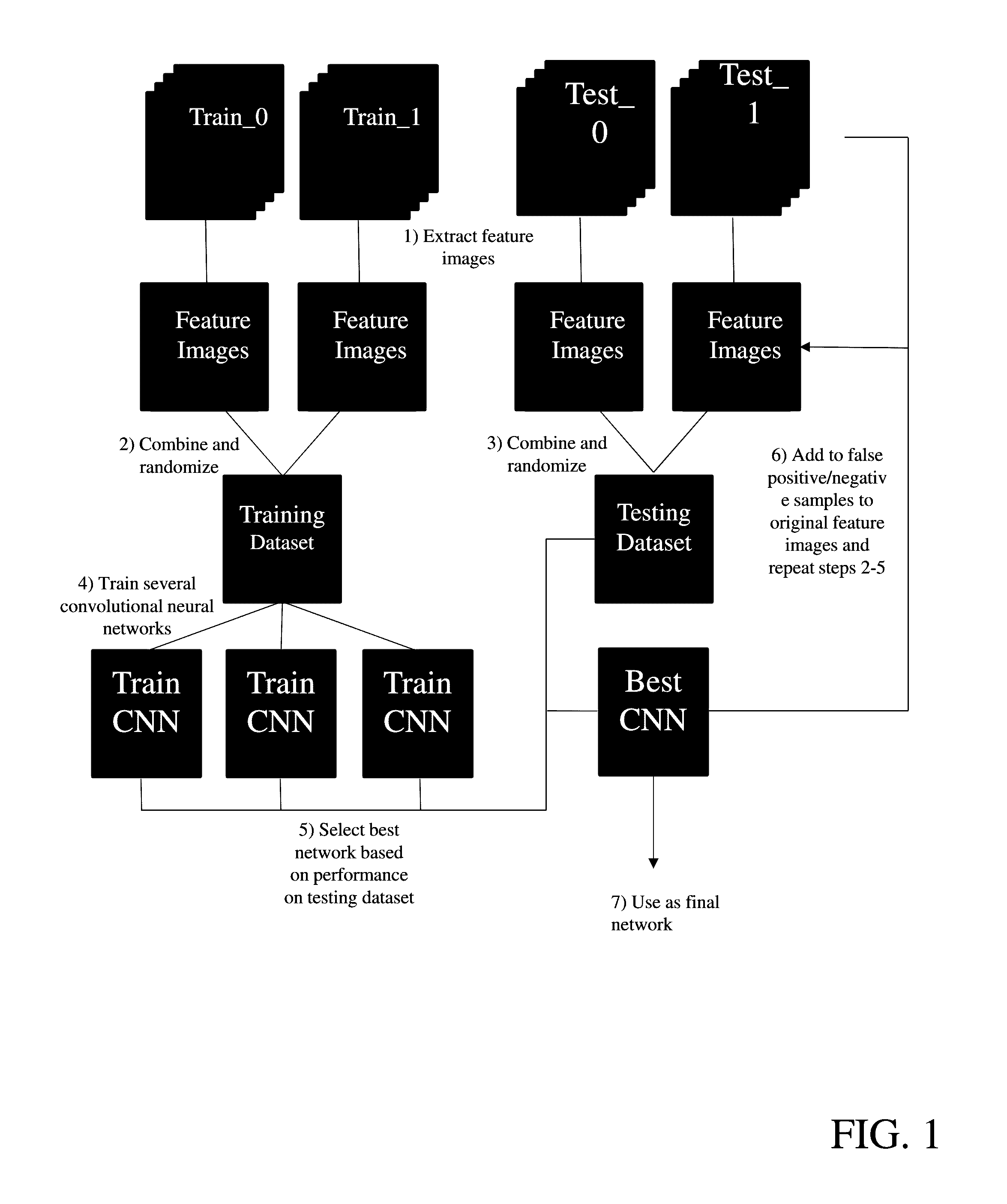
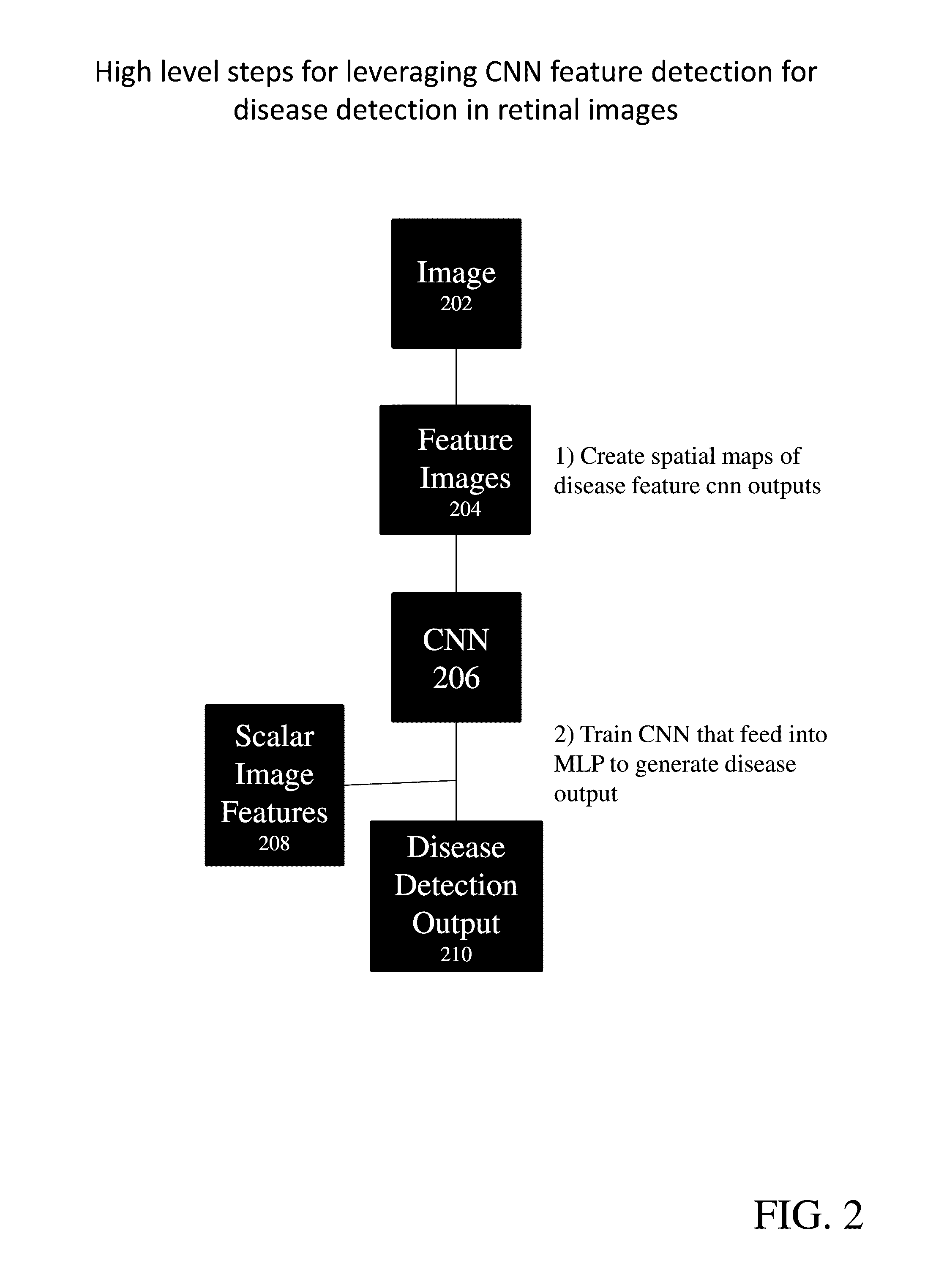
Provide are systems methods and devices for diagnosing disease in medical images. In certain aspects, disclosed is a method for training a neural network to detect features in a retinal image including the steps of: a) extracting one or more features images from a Train_0 set, a Test_0 set, a Train_1 set and a Test_1 set; b) combining and randomizing the feature images from Train_0 and Train_1 into a Training data set; c) combining and randomizing the feature images from Test_0 and Test_1 into a testing dataset; d) training a plurality of neural networks having different architectures using a subset of the training dataset while testing on a subset of the testing dataset; e) identifying the best neural network based on each of the plurality of neural networks performance on the testing data set; f) inputting images from Test_0, Train_1, Train_0 and Test_1 to the best neural network and identifying a limited number of false positives and false negative and adding the false positives and false negatives to the training dataset and testing dataset; and g) repeating steps d)-g) until an objective performance threshold is reached.
Owner:DIGITAL DIAGNOSTICS INC
Methods of improving performance of automotive intersection turn assist features
ActiveUS20170113683A1Reduce false positive warning falseReduce false warning false negativeExternal condition input parametersDriver/operatorCollision analysis
A system and method for warning a vehicle driver of a potential collision when turning left or right at or near an intersection, where the system and method provide additional analysis to limit false positive and false negative warnings based on specialized circumstances. The method includes determining if the host vehicle is likely to turn at or near the intersection, and obtaining speed, velocity and position data of the host vehicle and any relevant remote vehicles. The method determines a predicted path of the host vehicle and the remote vehicles based on the speed, velocity and position data, and issues a warning to a driver of the host vehicle if the host vehicle and one of the remote vehicles may collide based on the predicted paths. If the host vehicle is in a specialized circumstance, the method provides additional collision analysis to reduce false positive warnings and / or false negative warnings.
Owner:GM GLOBAL TECH OPERATIONS LLC
Intelligentize lung cancer early cell pathological picture recognition processing method
InactiveCN101226155AHigh simulationAvoid situations with low classification accuracyImage analysisMaterial analysis by optical meansSmall-cell carcinomaSquamous Carcinomas
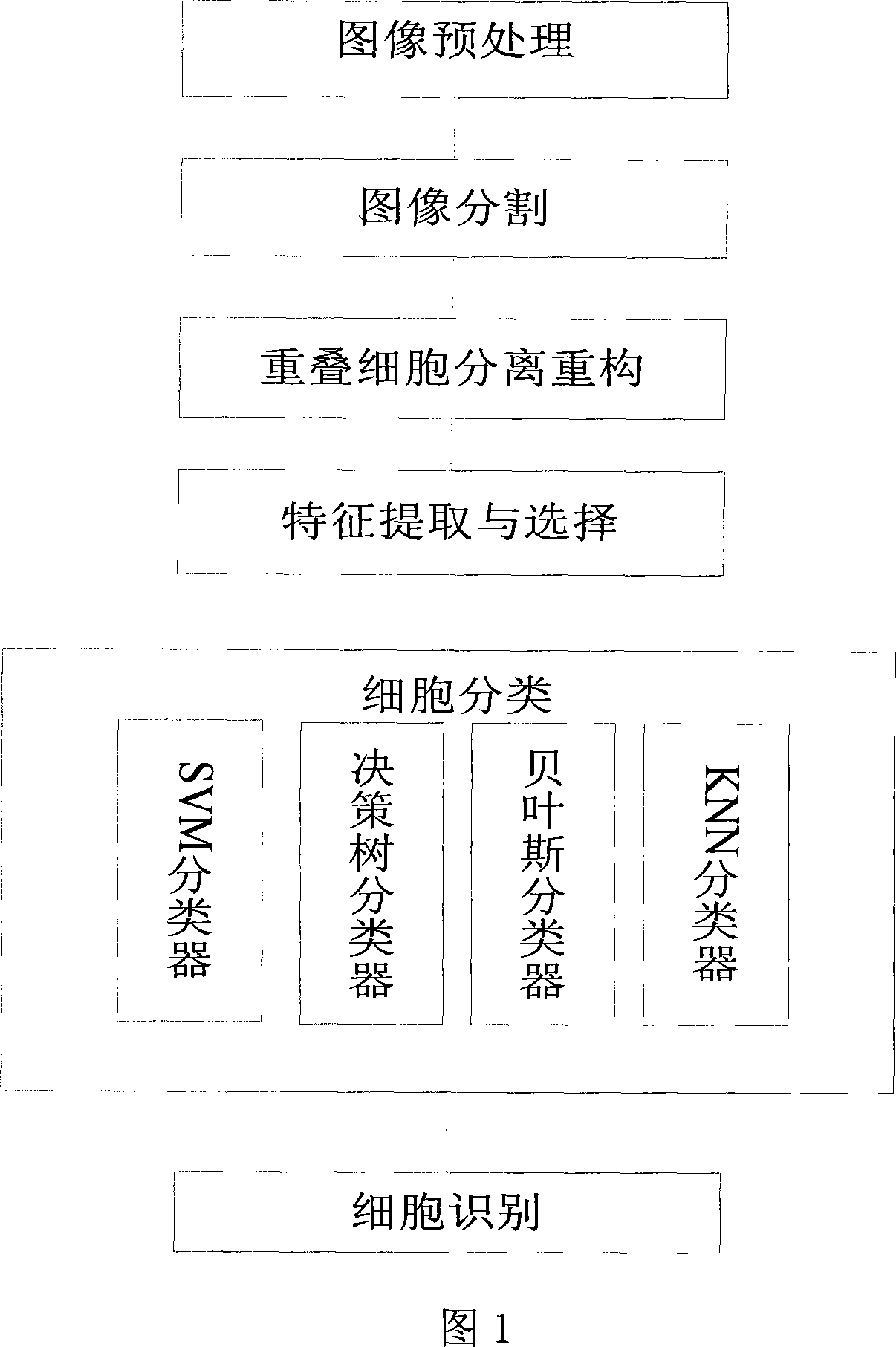
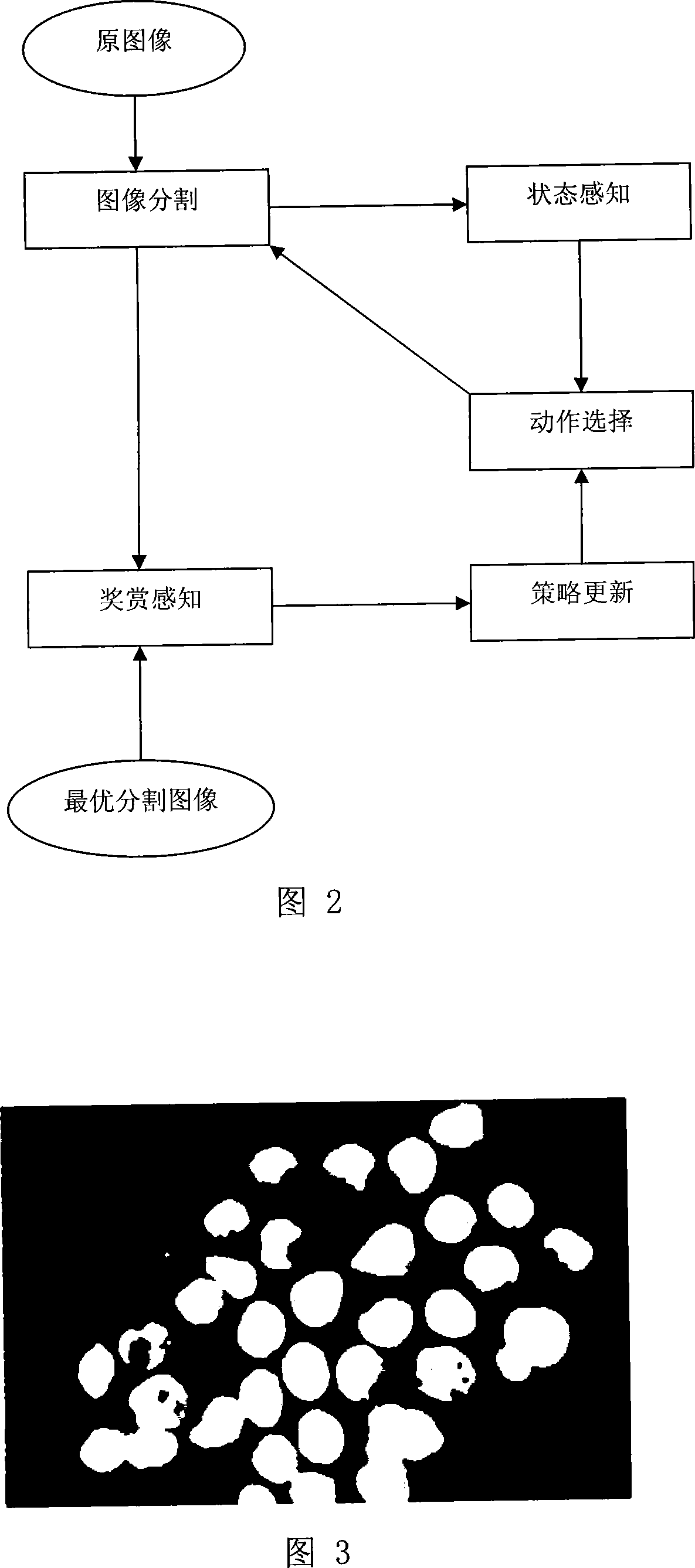
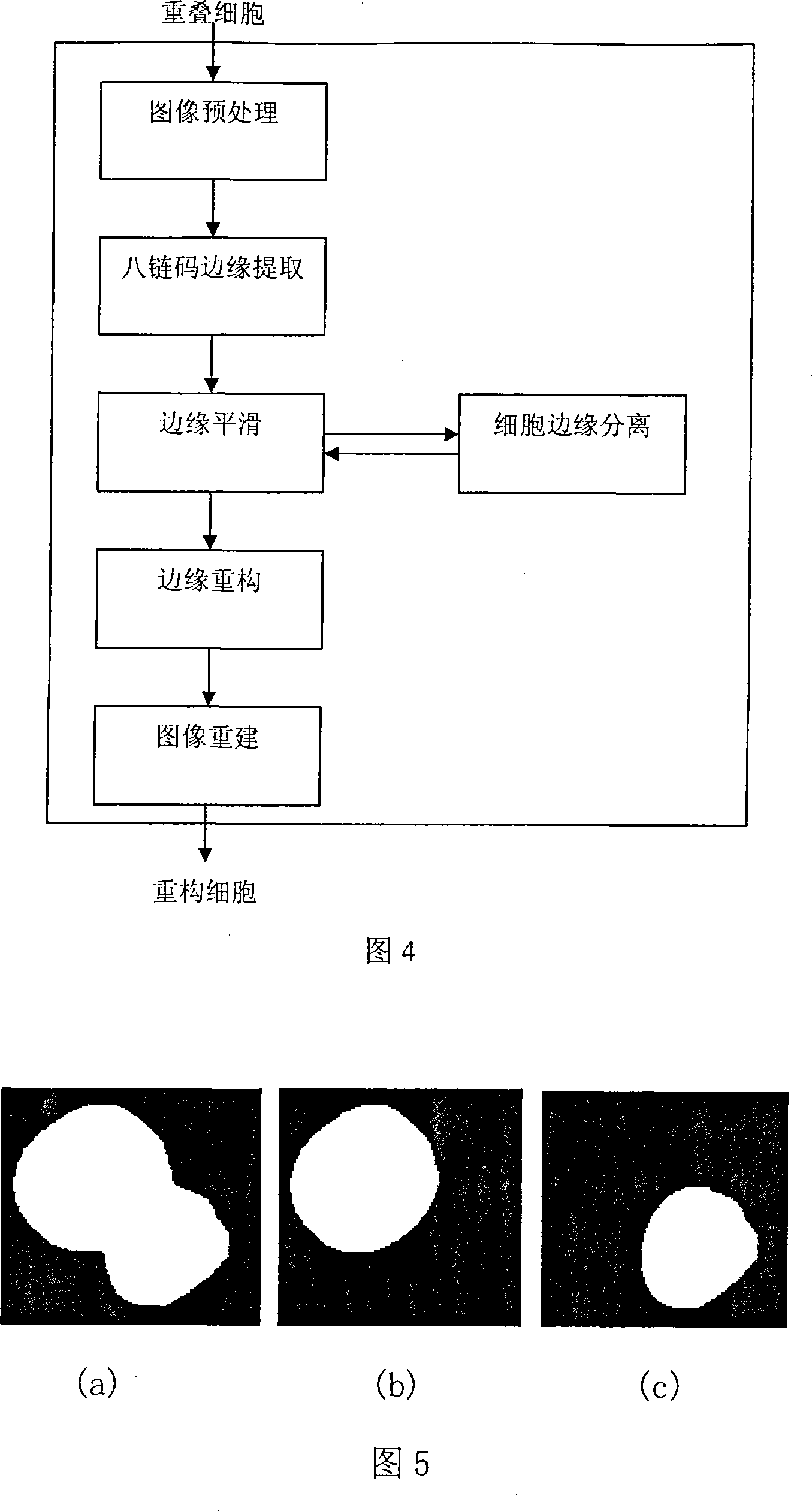
The invention relates to an intelligent lung cancer early cell pathological image recognition processing method, which comprises image pretreatment, image segmentation, laminate cell separation and reconstruction, cell character extraction and cell classification. The invention has the advantages that the image segmentation based on reinforcement learning uses incremental learning and continuous interaction with environment to search for optimized segmentation threshold value to obtain the segmentation effect which average value is 91%, the laminate cell separation and reconstruction uses B spline and modified deBoor-Cox method to simulate true cell edge better, the classifier uses general vote method to avoid low classifying accuracy of single classifier and improve total classifying accuracy, the application of two-stage classifier can reduce the possibility of false positive and false negative. Tests prove that the classifying accuracy of cancer or no cancer can average reach 93.8%, the classifying accuracy of squamous cell carcinoma, adenocarcinoma and small cell carcinoma average reaches 75%, and the false positive and negative average reach 4-6%.
Owner:中国人民解放军第八一医院 +1
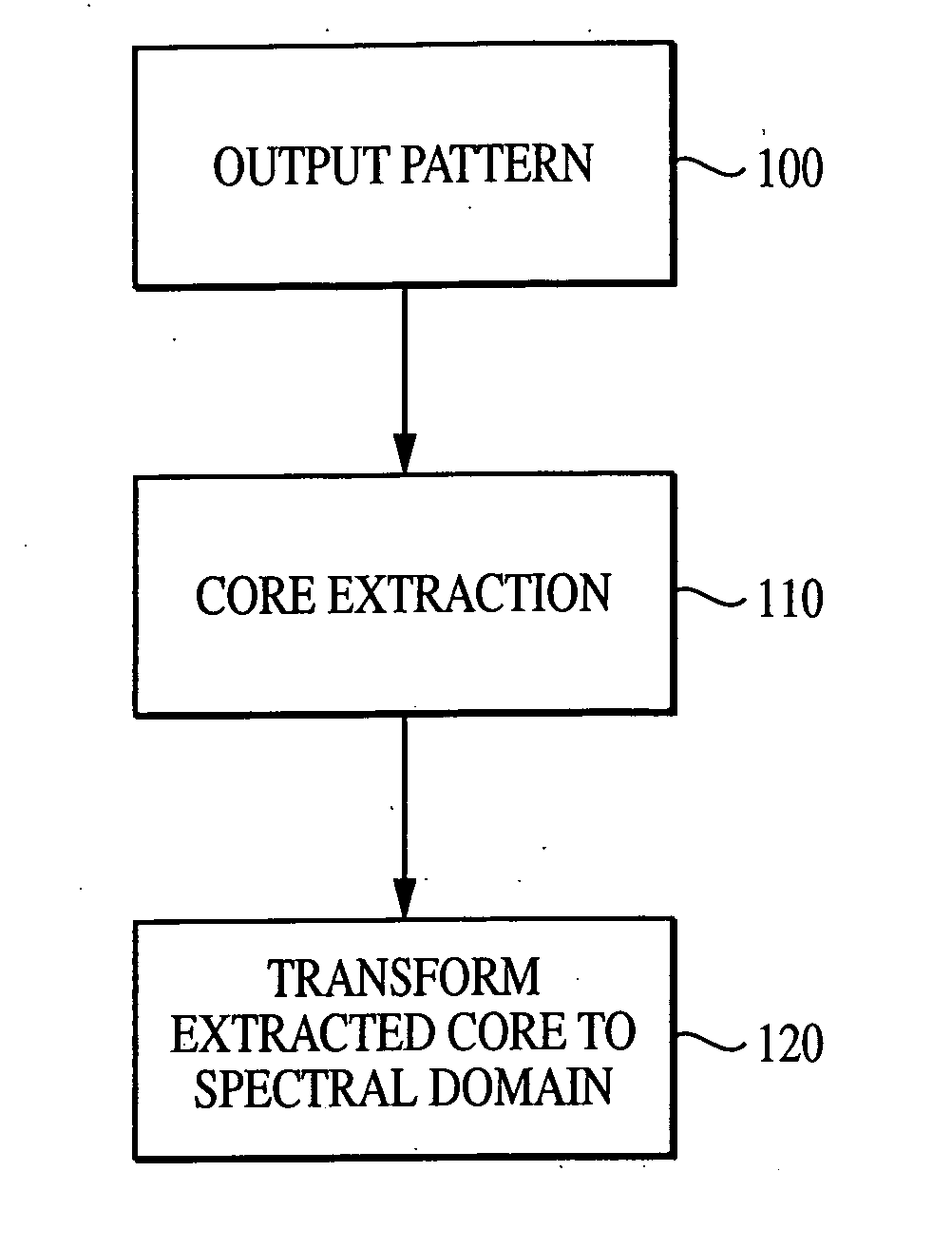
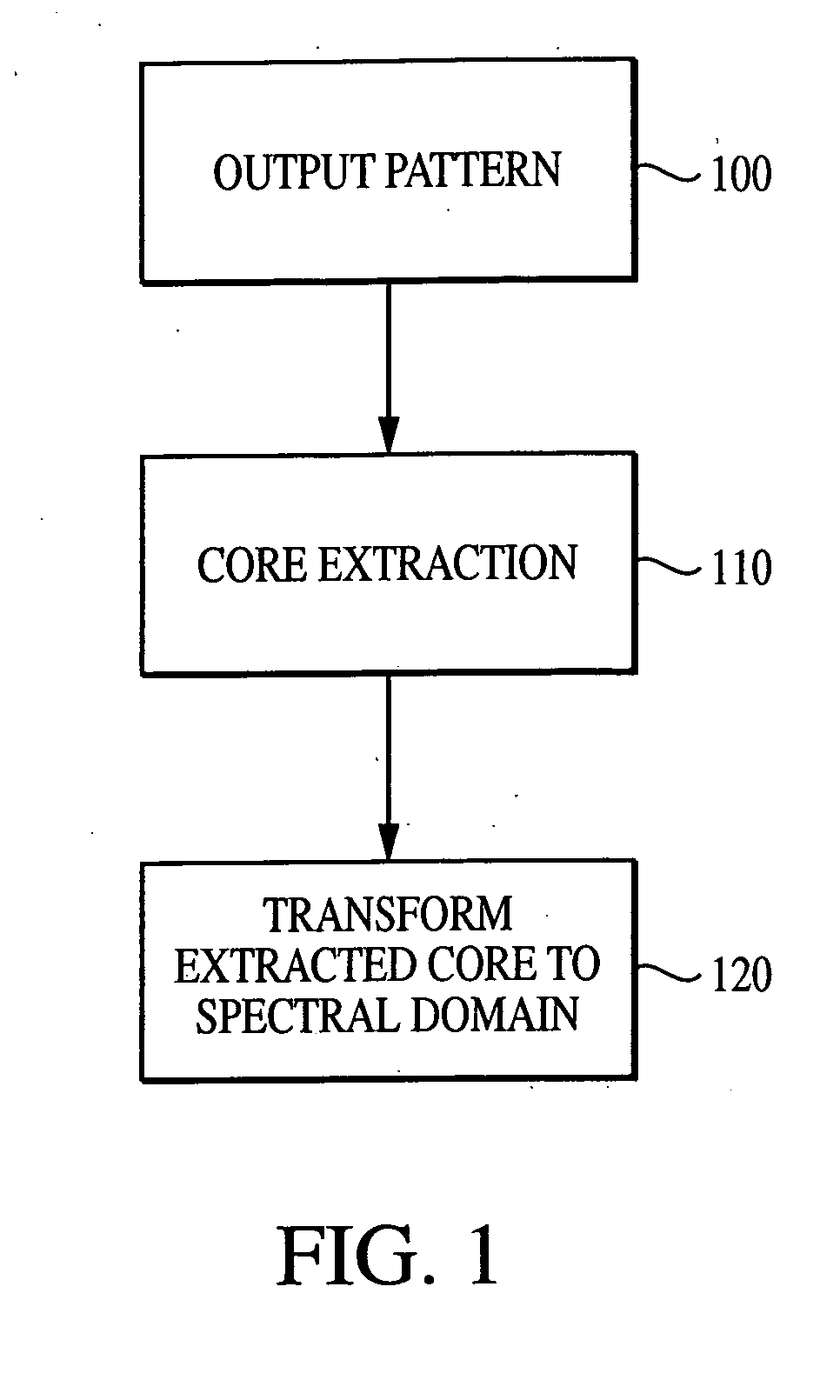
System and method for characterizing microarray output data
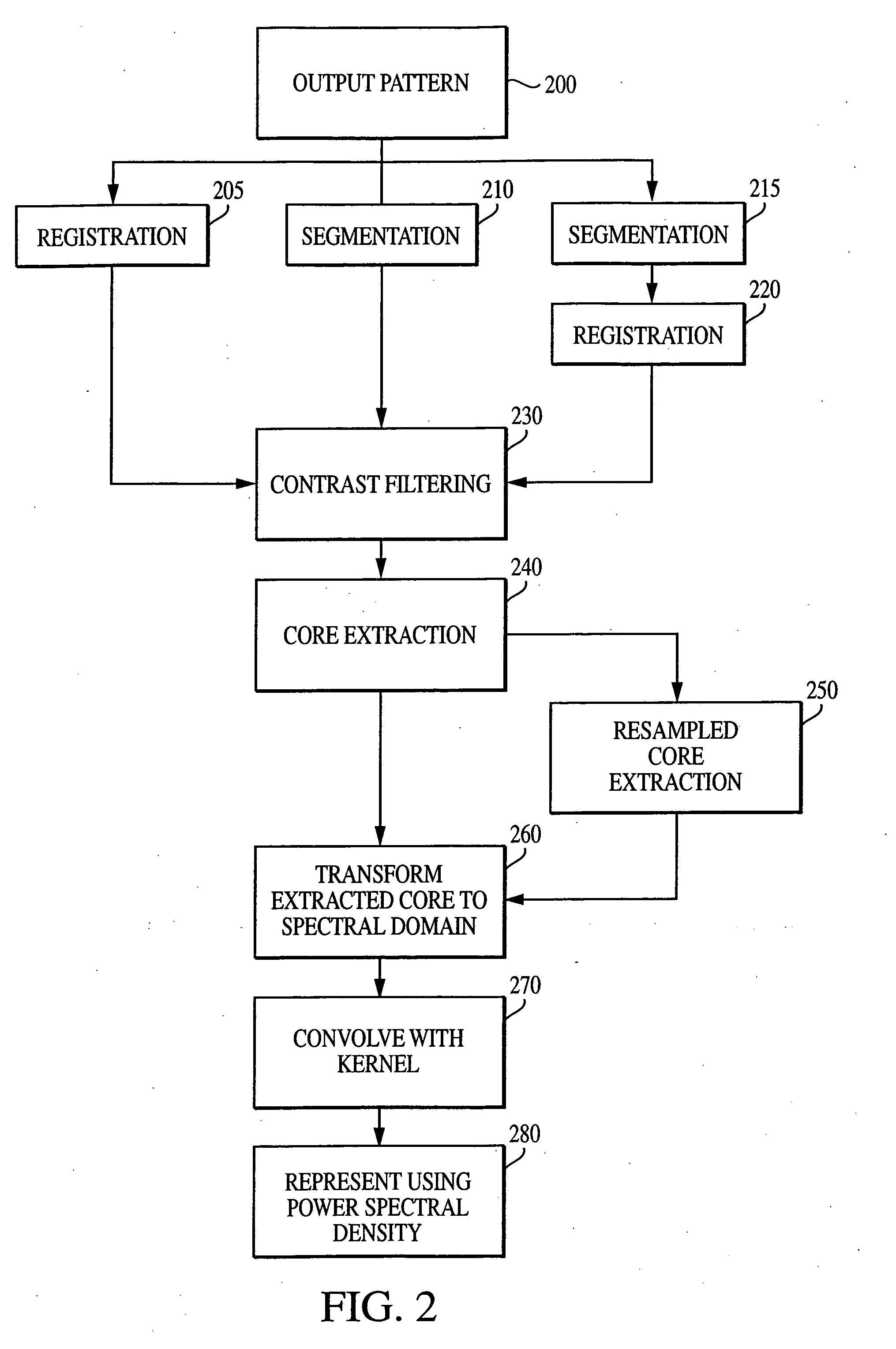
InactiveUS7006680B2Increase contrastImprove spatial resolutionImage enhancementImage analysisFrequency spectrumSpectral transformation
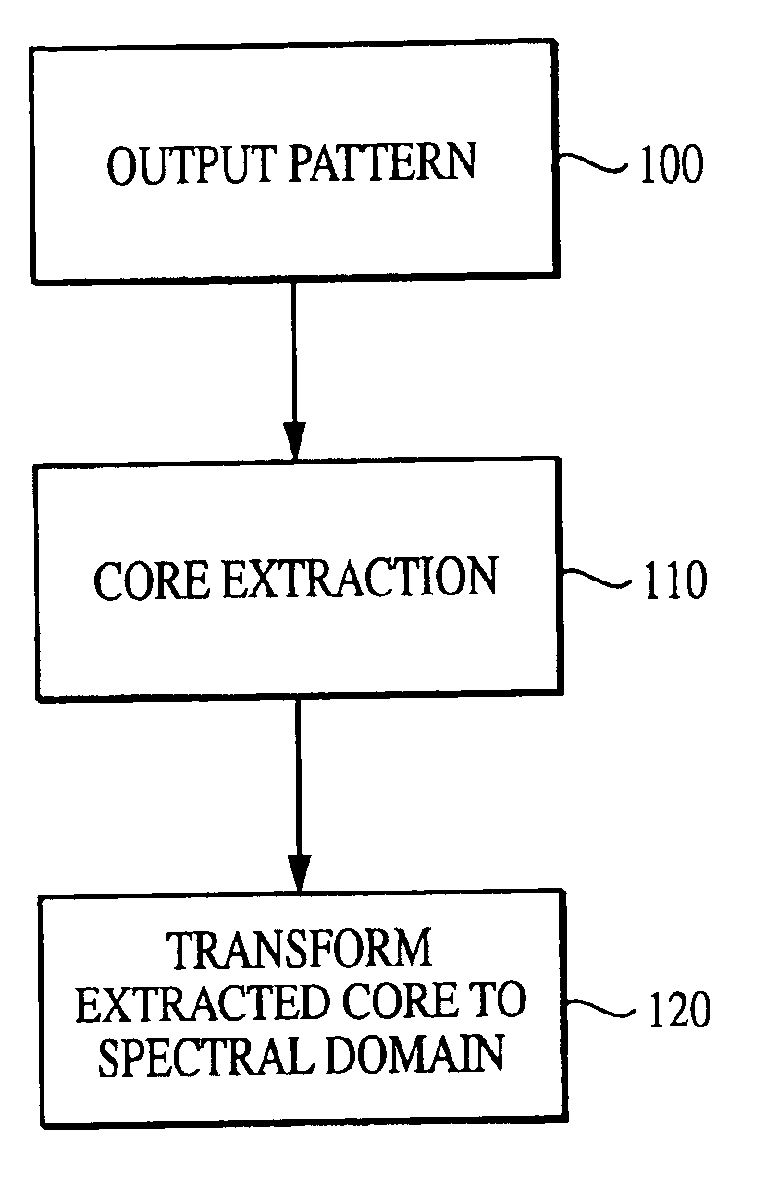
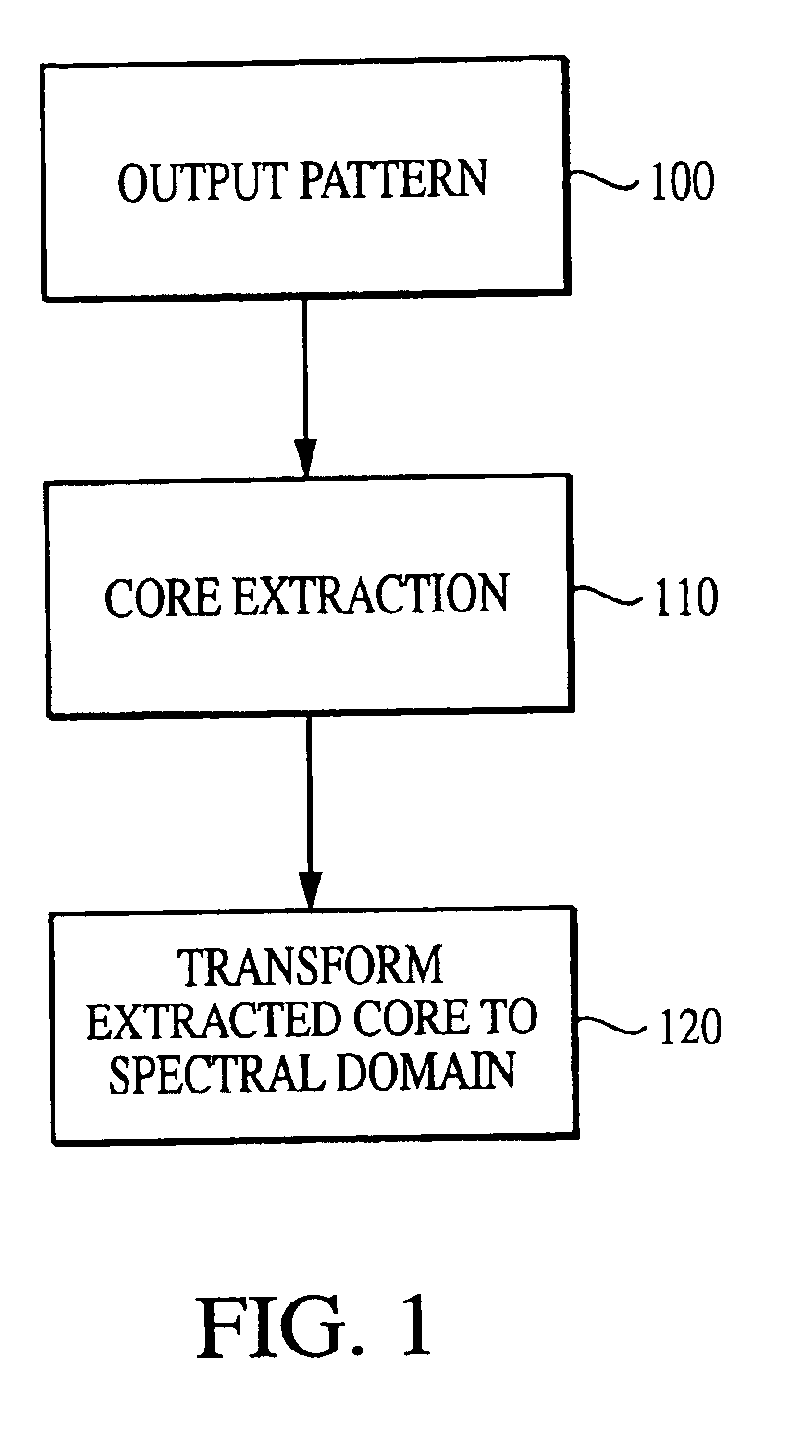
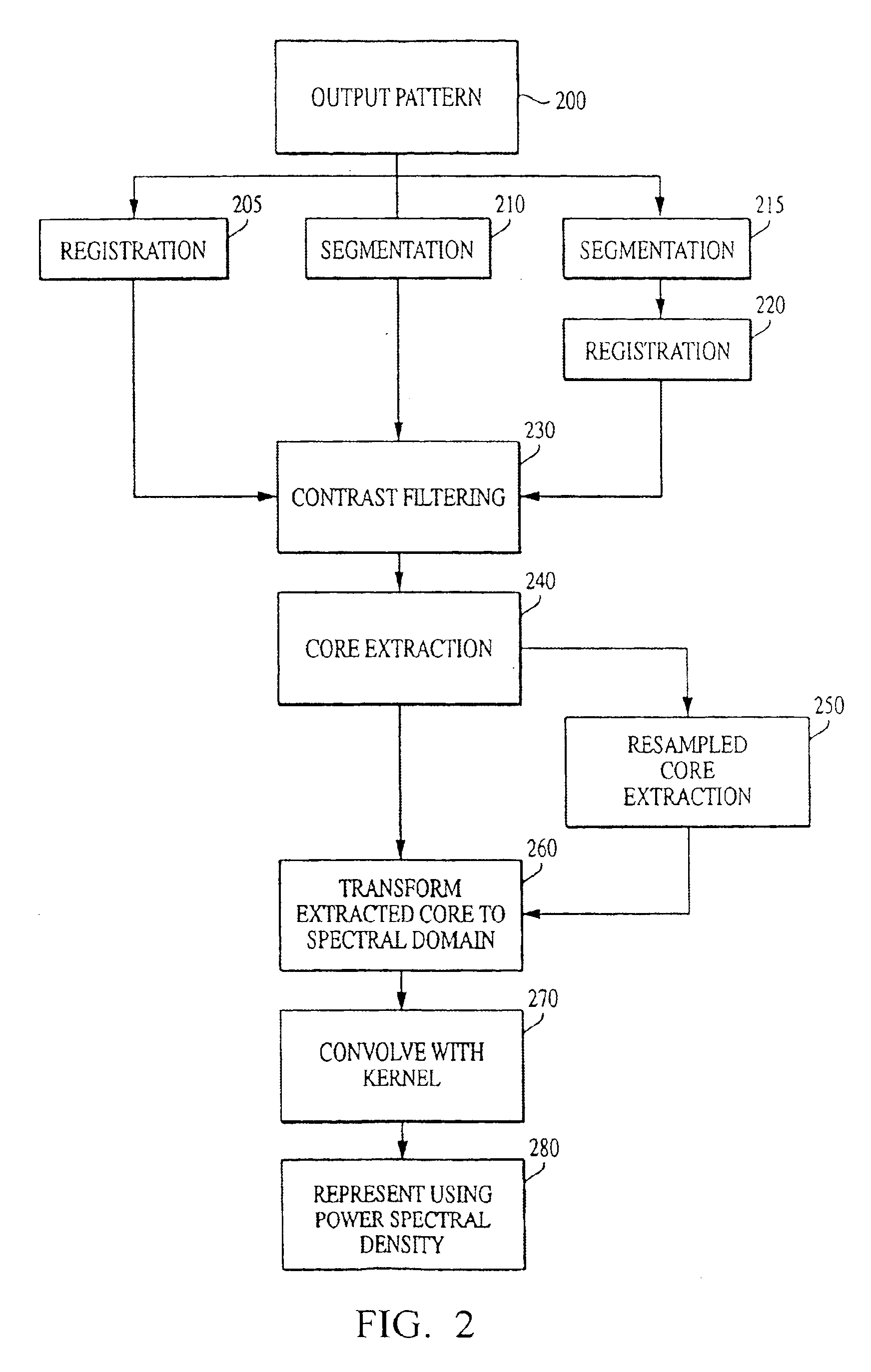
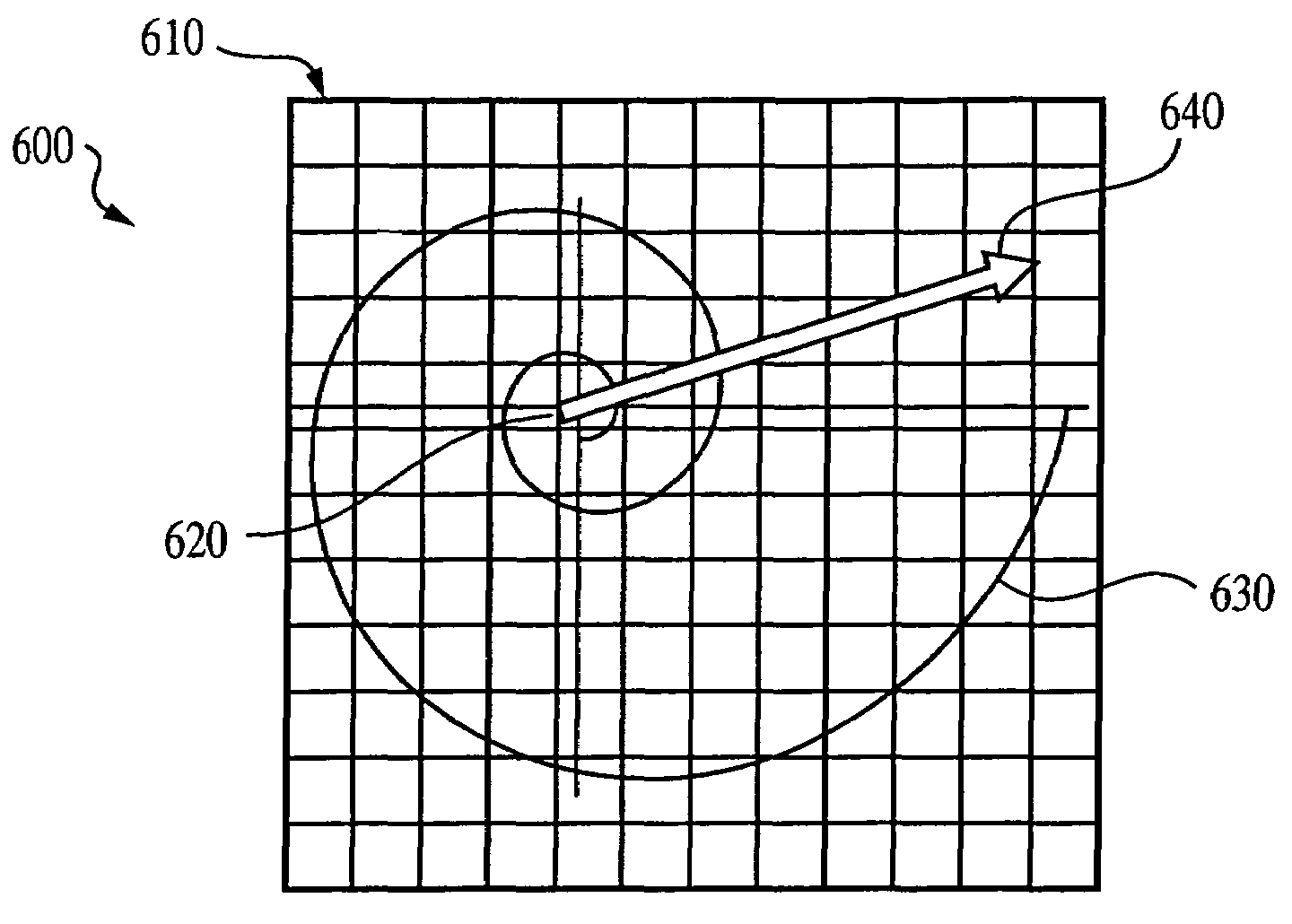
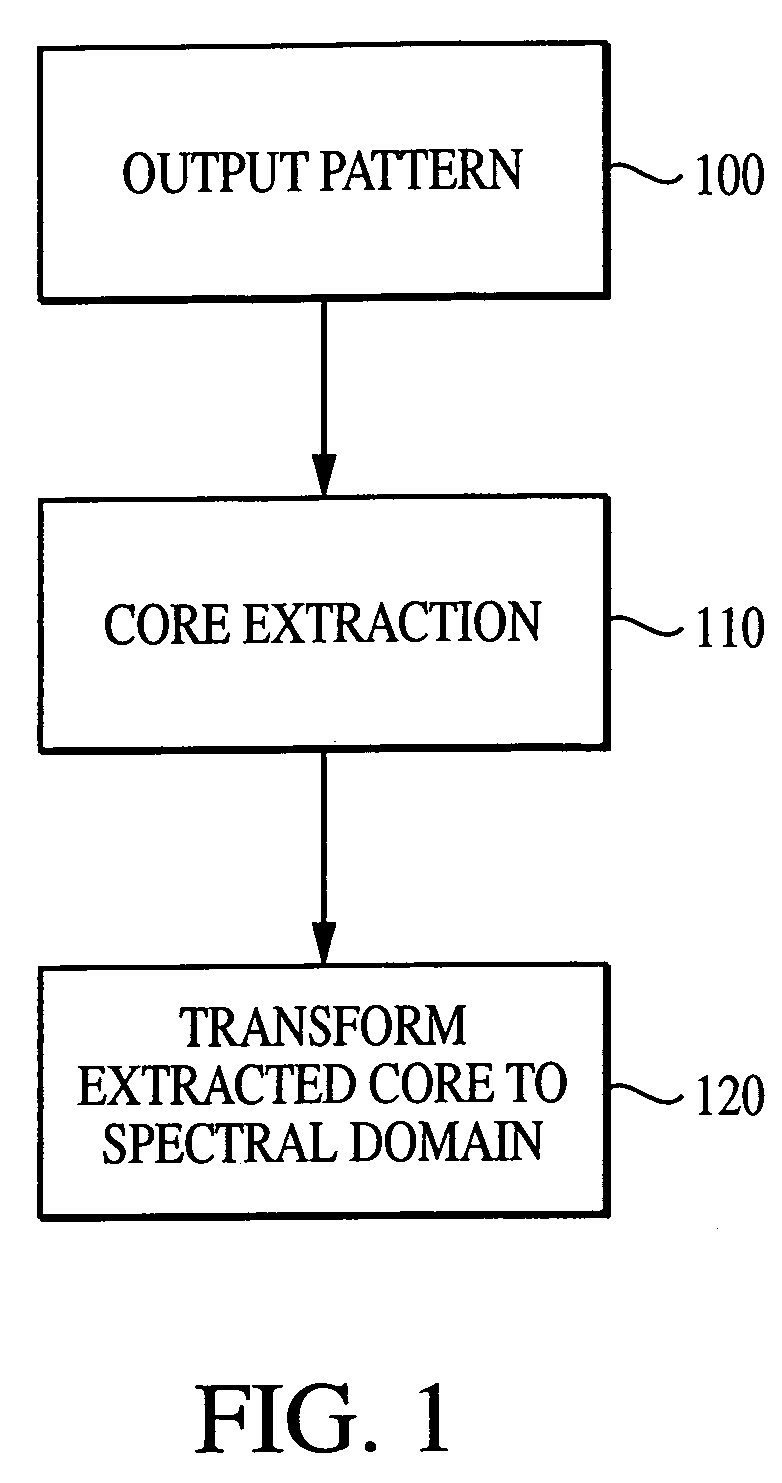
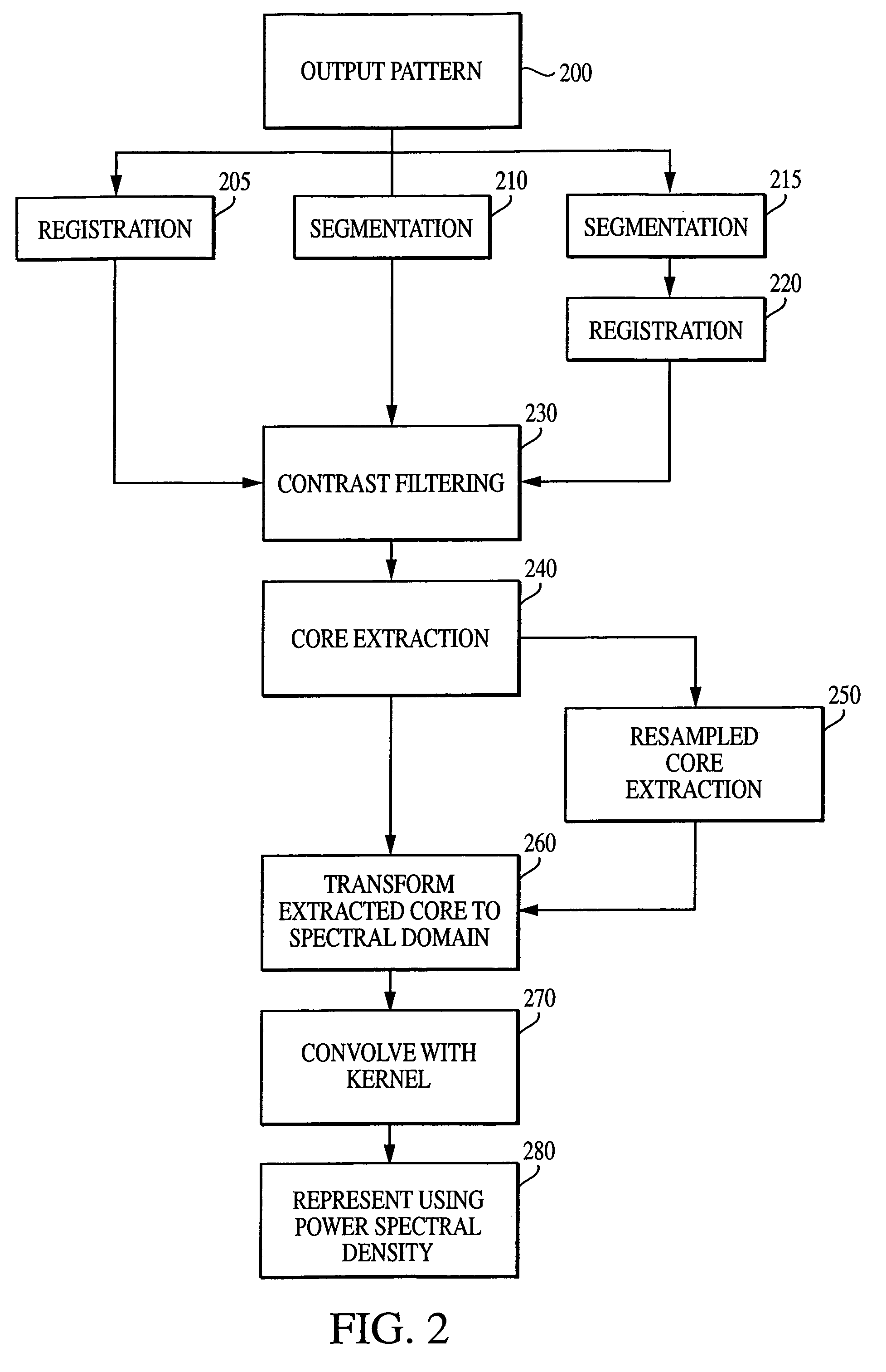
The current invention discloses a novel spectral transformation technique for characterizing digitized intensity output patterns from microarrays. This method yields improved sensitivity with reduced false positives and false negatives. Current microarray methods are overly sensitive to the detection of a visible distinction between pixels associated with probes and pixels associated with background. In one embodiment, a technique is disclosed that comprises the steps of: extracting pixels associated with an object of interest and transforming such pixels from an intensity representation to a spectral representation. In some embodiments, the extraction is based on a tessellated logarithmic spiral extraction that may yield a pixel core with a sampling of both foreground and background pixels. This core may then be computationally rescaled by 10×-10,000× to enhance spatial resolution. Once the extracted pixels are represented in the spectral regime, convolution with resolution-enhancement kernels may be used to accentuate morphological features capturing platform specific phenomenology.
Owner:VIALOGY
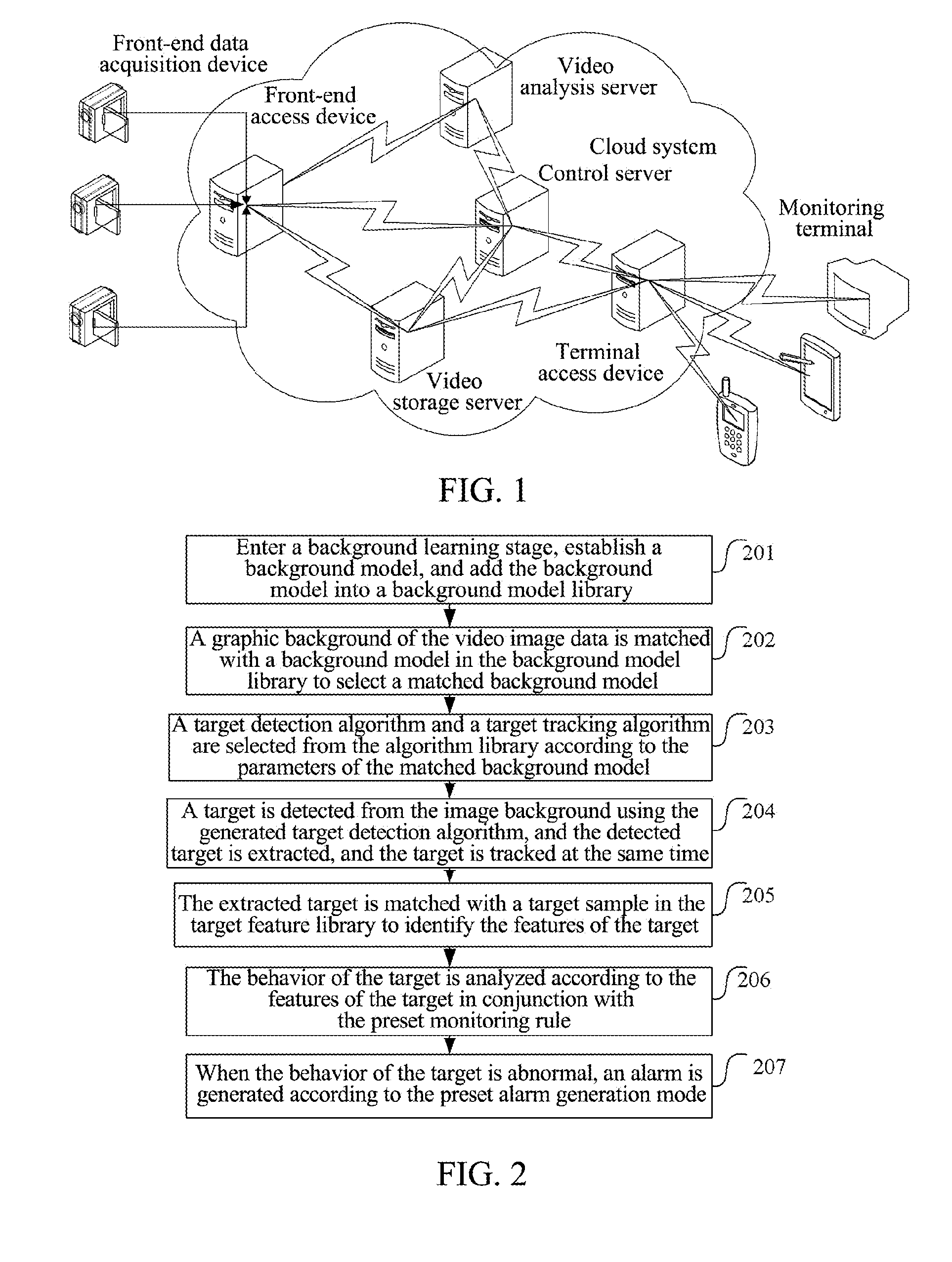
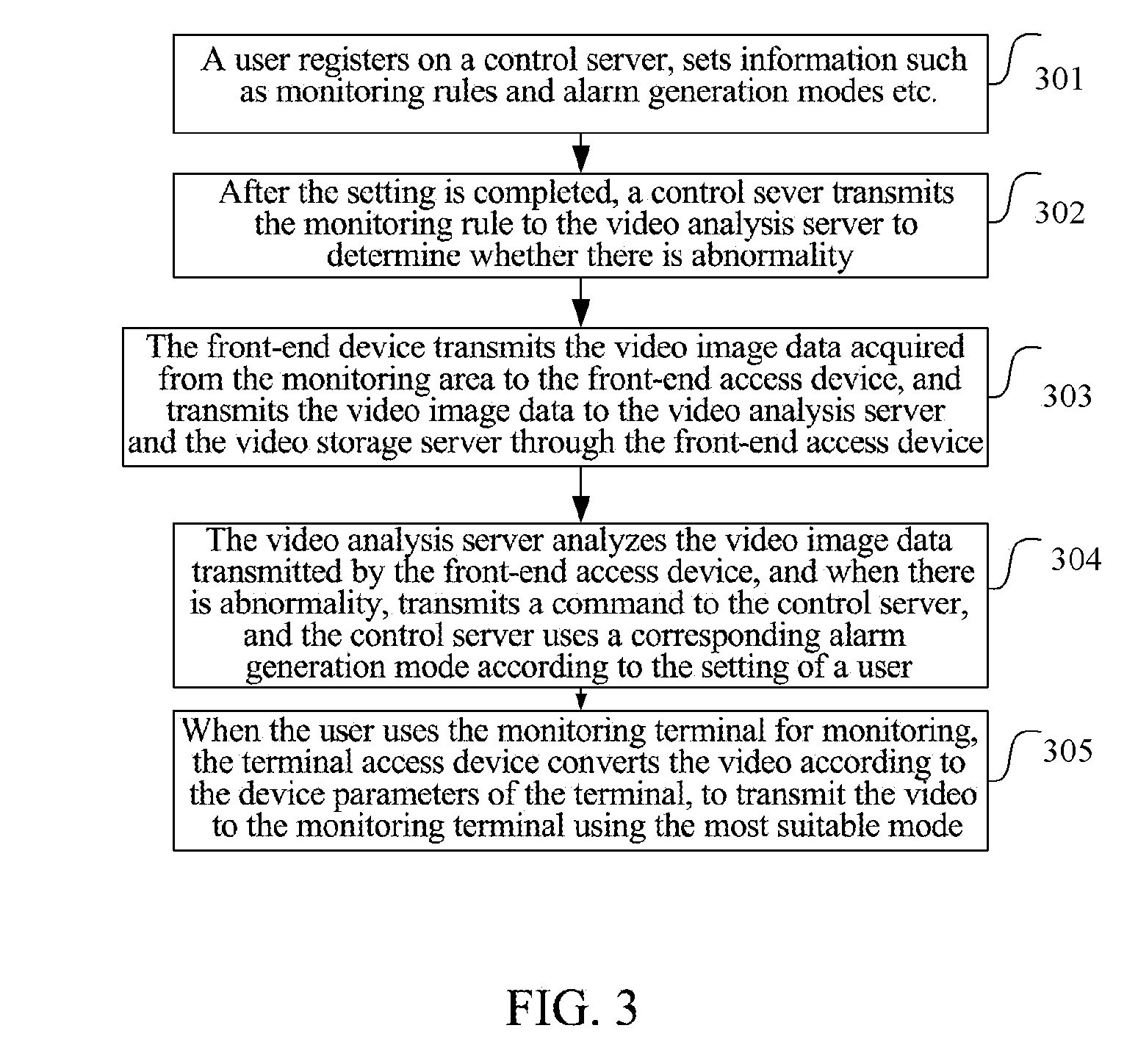
Video monitoring system and method
InactiveUS20140043480A1Save bandwidthEasy to deployColor television detailsClosed circuit television systemsVideo monitoringImaging analysis
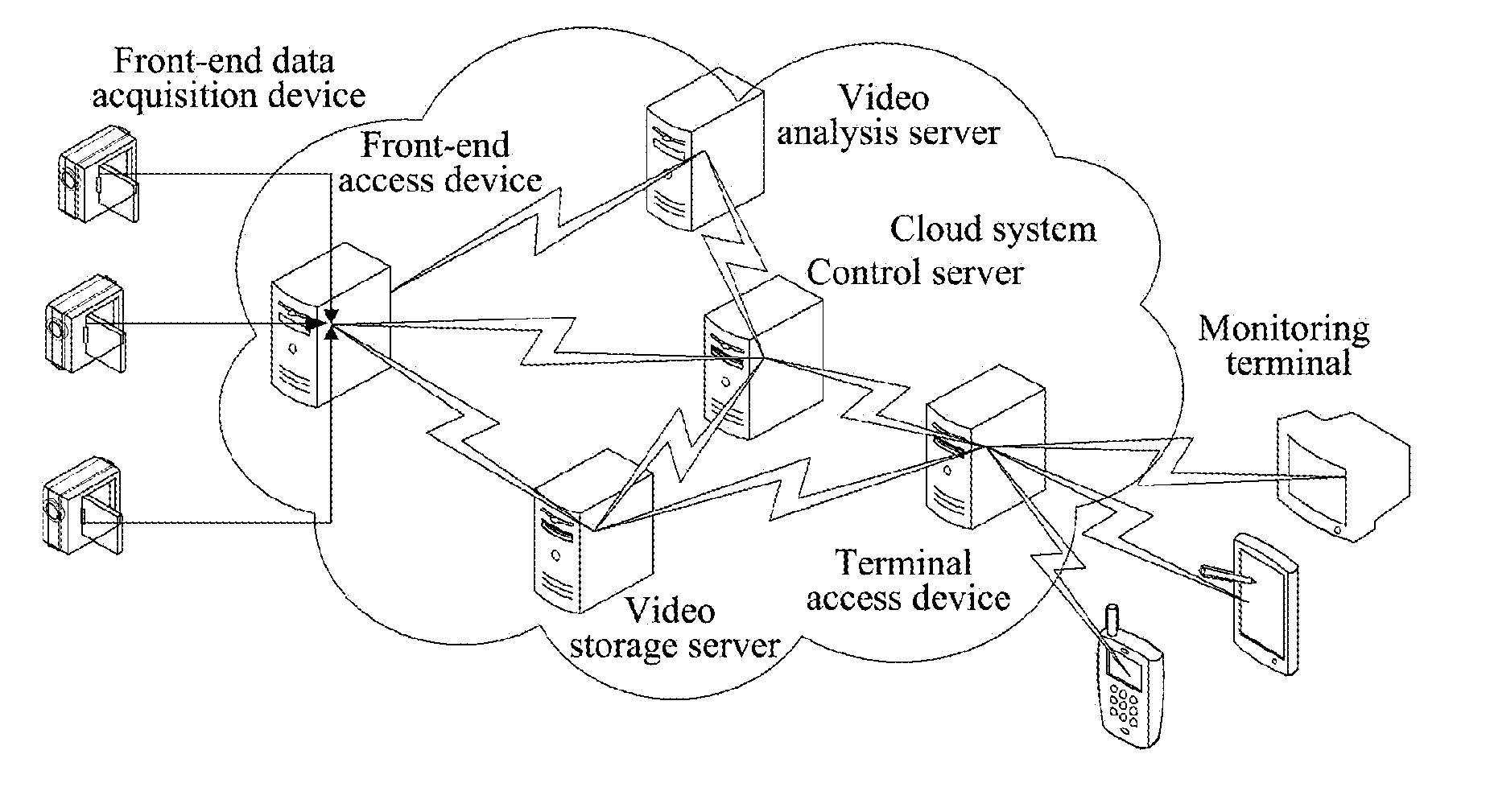
The present document discloses a video monitoring system and method, wherein, the system includes: a front-end data acquisition device, a front-end access device and a cloud system, wherein the front-end data acquisition device is configured to acquire a video image and transmit video image data to the front-end access device; the front-end access device is configured to transmit the video image data transmitted by the front-end data acquisition device to the cloud system; and the cloud system is configured to analyze the video image data and generate an alarm when a target in the video image acquired by the front-end data acquisition device behaves abnormally. The present document also discloses a cloud system. With the present document, full image analysis and processing can be performed on the monitored scenarios and false-positive and false-negative situations can be reduced.
Owner:ZTE CORP
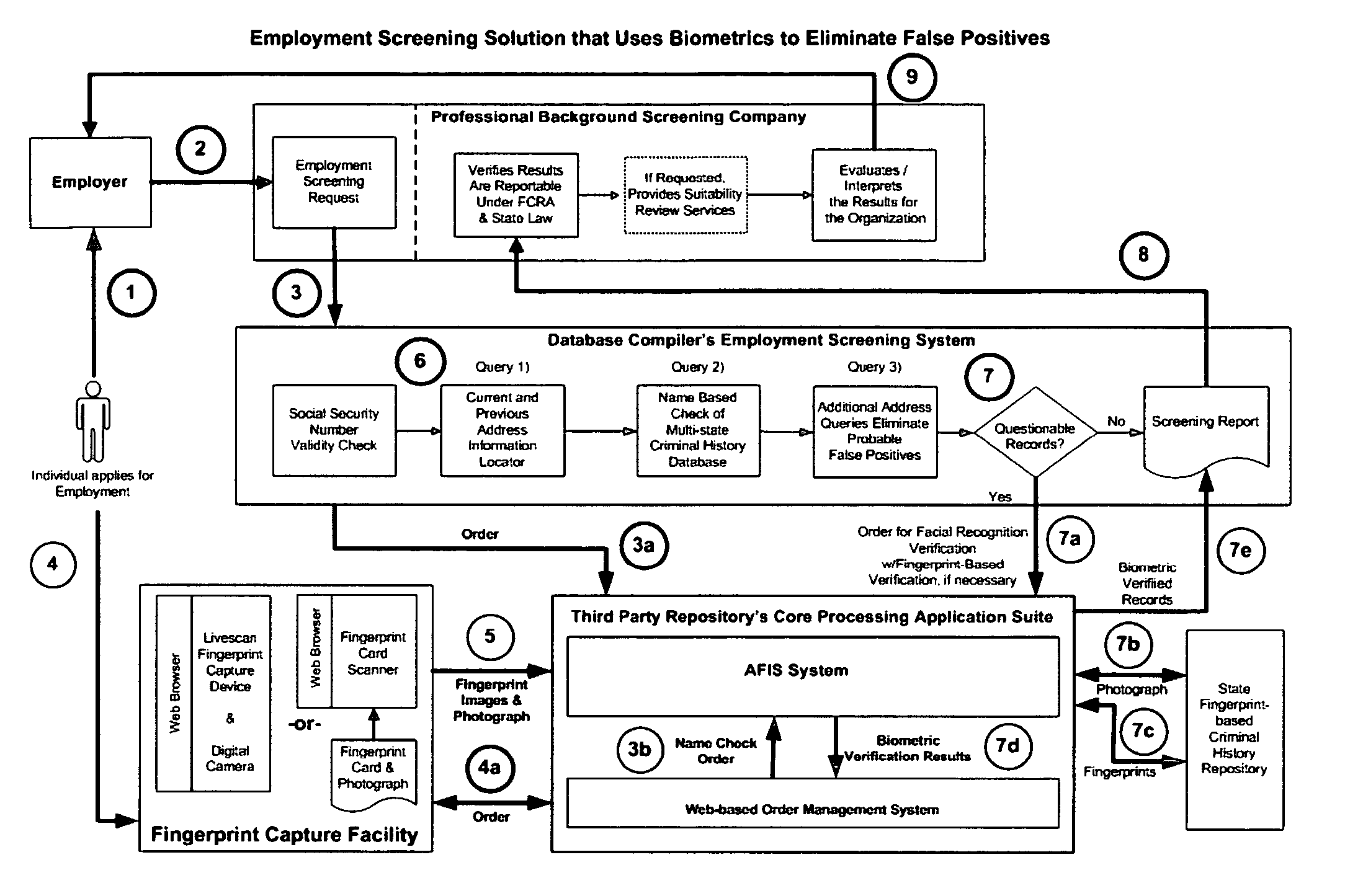
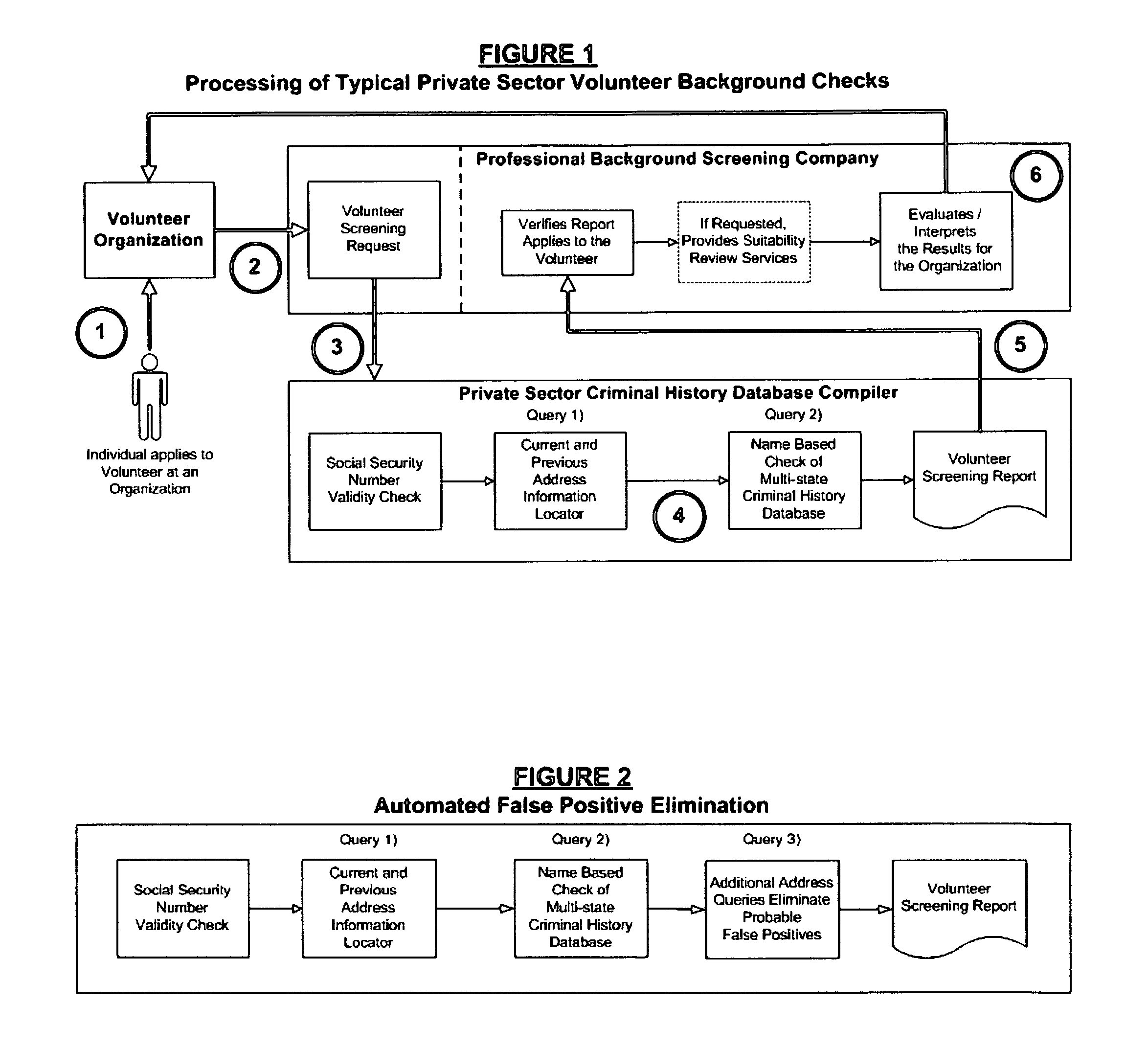
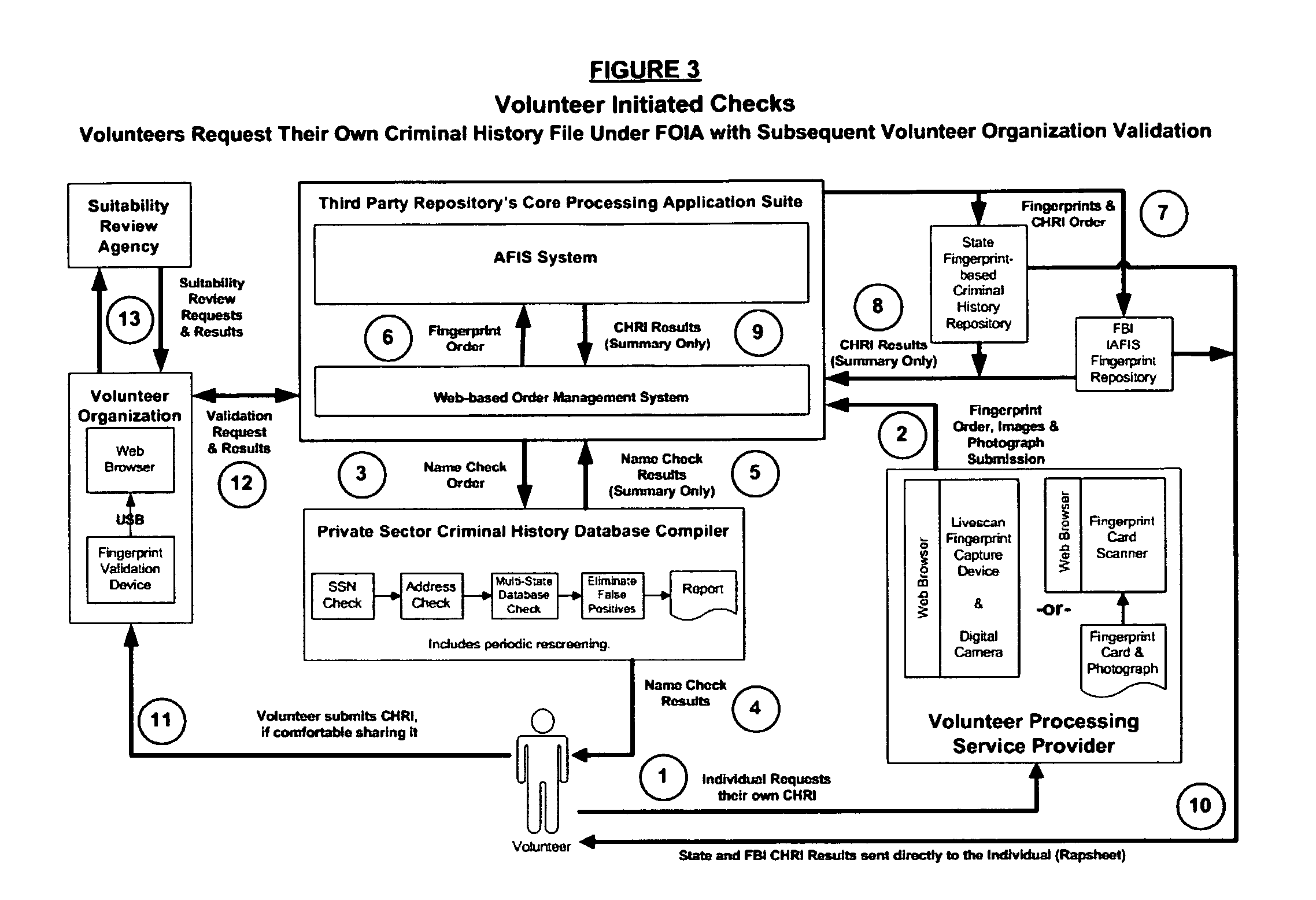
Biometric-supported name-based criminal history background checks
InactiveUS20060018520A1Improve recognition accuracyEasy to useCharacter and pattern recognitionOffice automationThird partyBiometric data
A method and apparatus for conducting biometric-supported name-based criminal history background investigations is disclosed as a means of eliminating both false-positive and false-negative results. Each of the embodiments disclosed employ the cooperative efforts of a professional background screening company, a database compiler, a trusted independent third-party evaluator of biometric data, and at least one government criminal history repository for providing more accurate screening reports for employers.
Owner:NAT BACKGROUND DATA
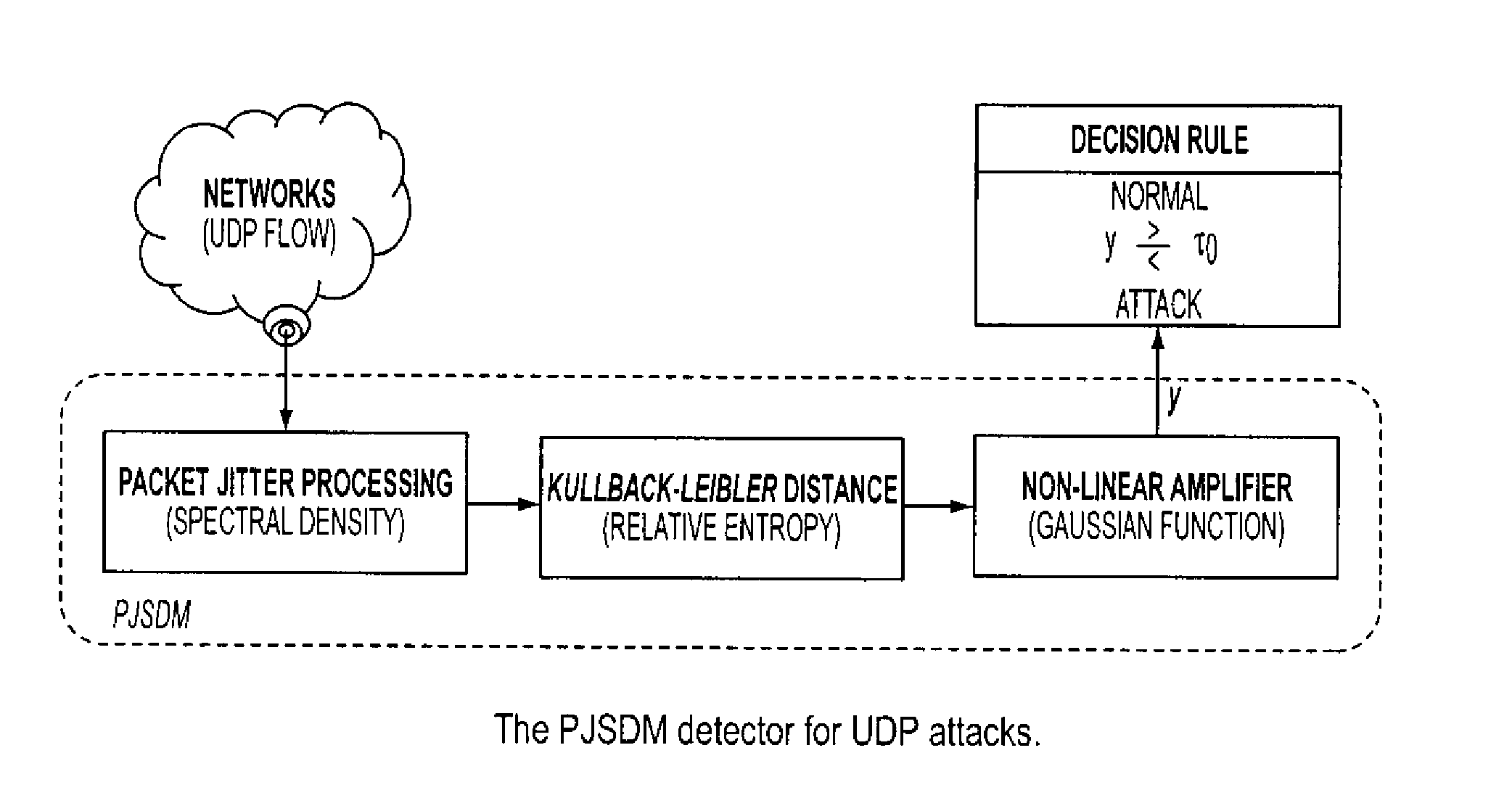
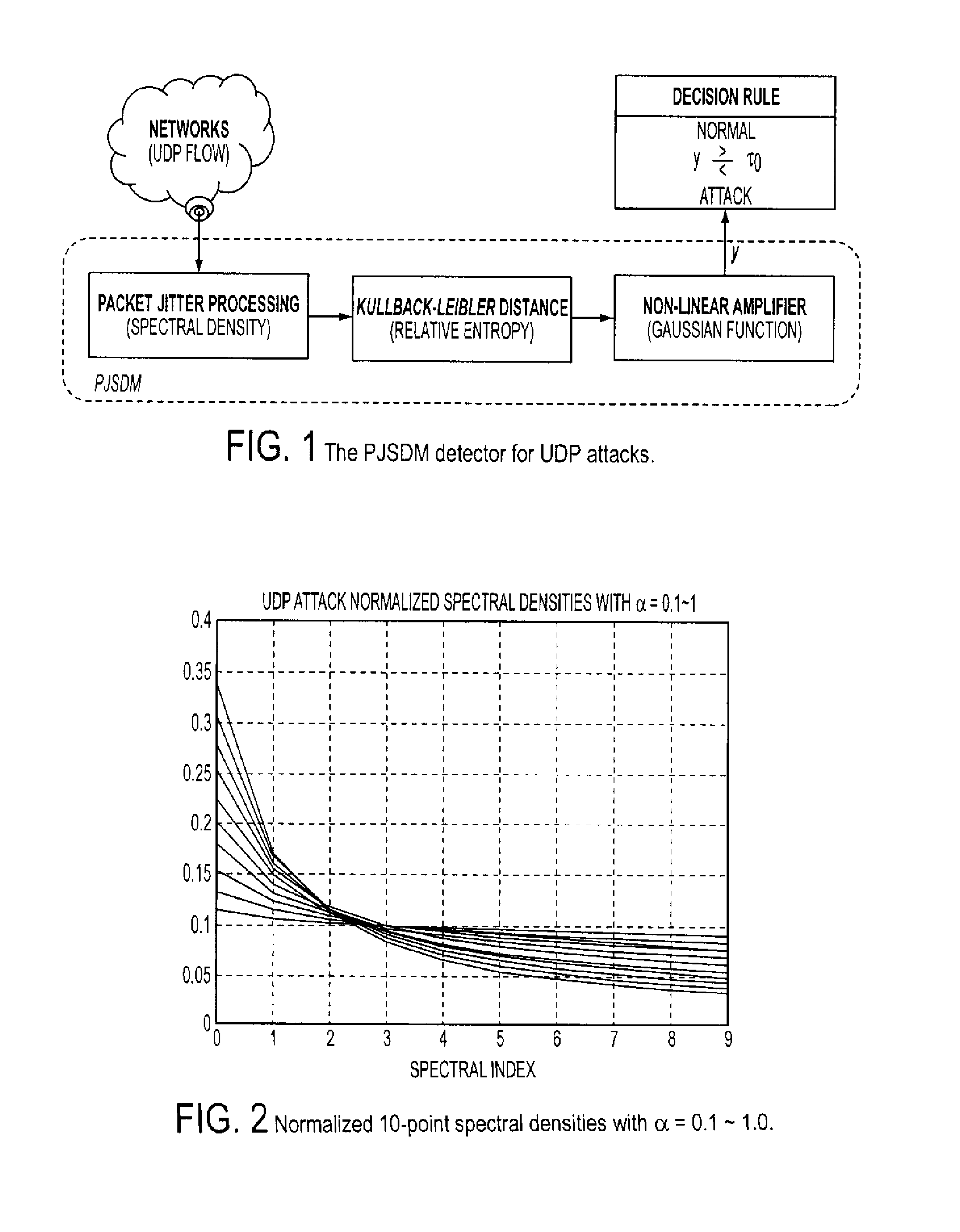
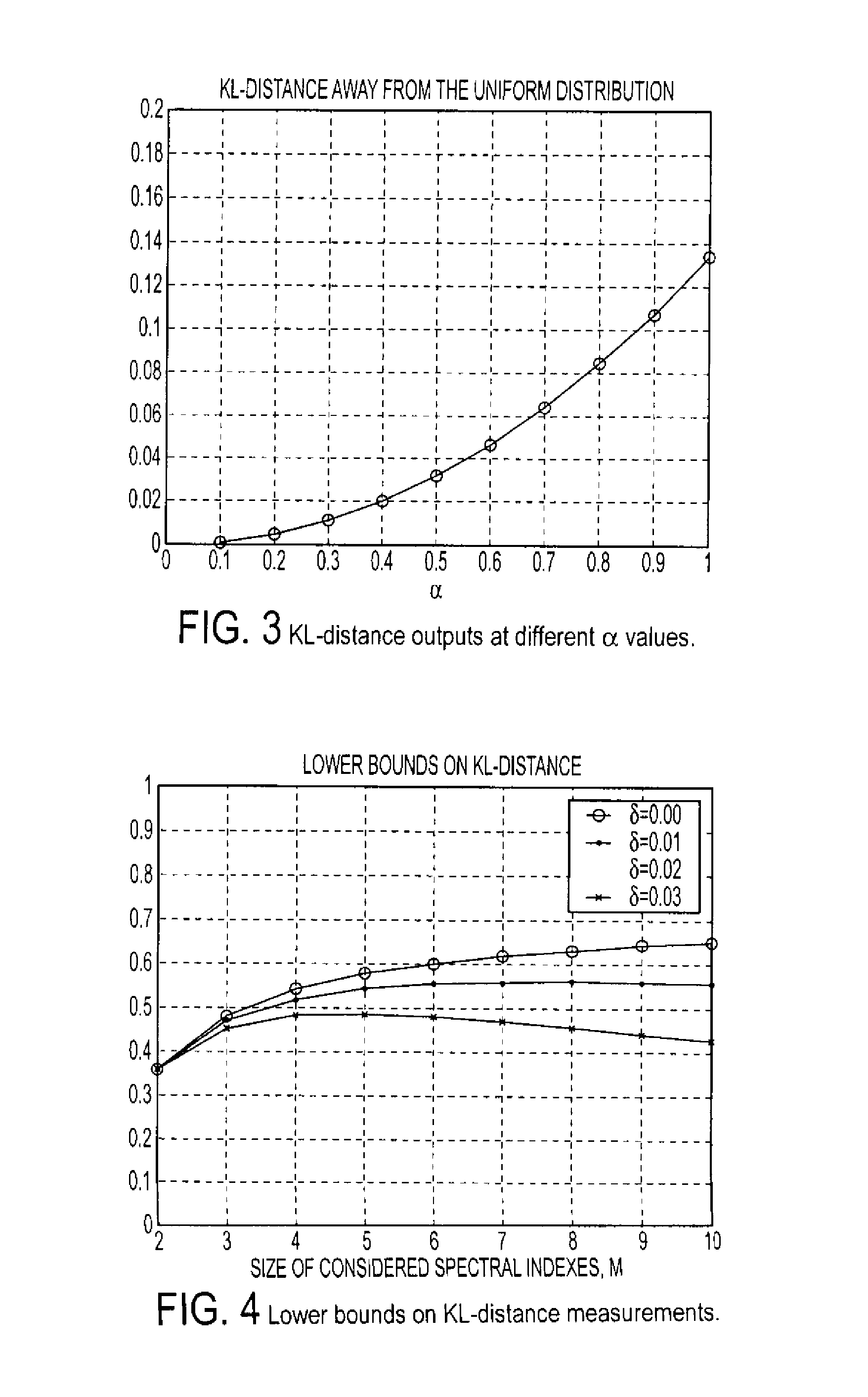
Method and system for UDP flood attack detection
ActiveUS8307430B1Easy to detectEasy to adjustMemory loss protectionError detection/correctionFrequency spectrumSystem requirements
A system and method is provided to identify UDP attacks. A processor determines a spectral density of packet timing intervals, a natural distance between the spectral density and a uniform distribution, and a non-linear amplifier applying a non-linear amplification to the natural distance to detect a denial-of-service attack. It uses the concept of traffic statistics analysis, i.e., spectral densities of arrived-packet timing intervals, calculates the KL-distance measurement and makes decision based on the output of a non-linear Gaussian amplifier, with which one can easily adjust the amplifier via selecting different parameters of mean and variance to satisfy system requirements of false-positive and false-negative UDP attack detections.
Owner:RIOREY LLC
Automatic teller machine (ATM) video surveillance method and apparatus
ActiveCN105100689ASolve the problem of low analysis accuracyStable and reliable outputComplete banking machinesClosed circuit television systemsVideo imageFalse positives and false negatives
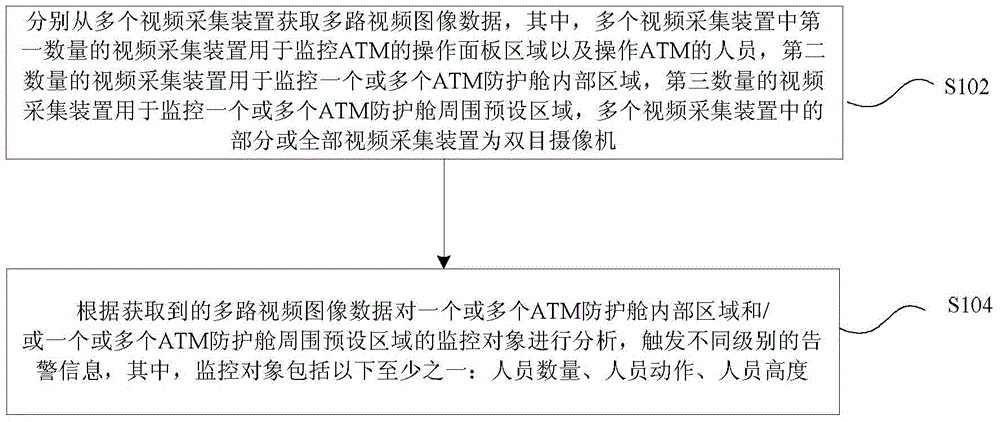
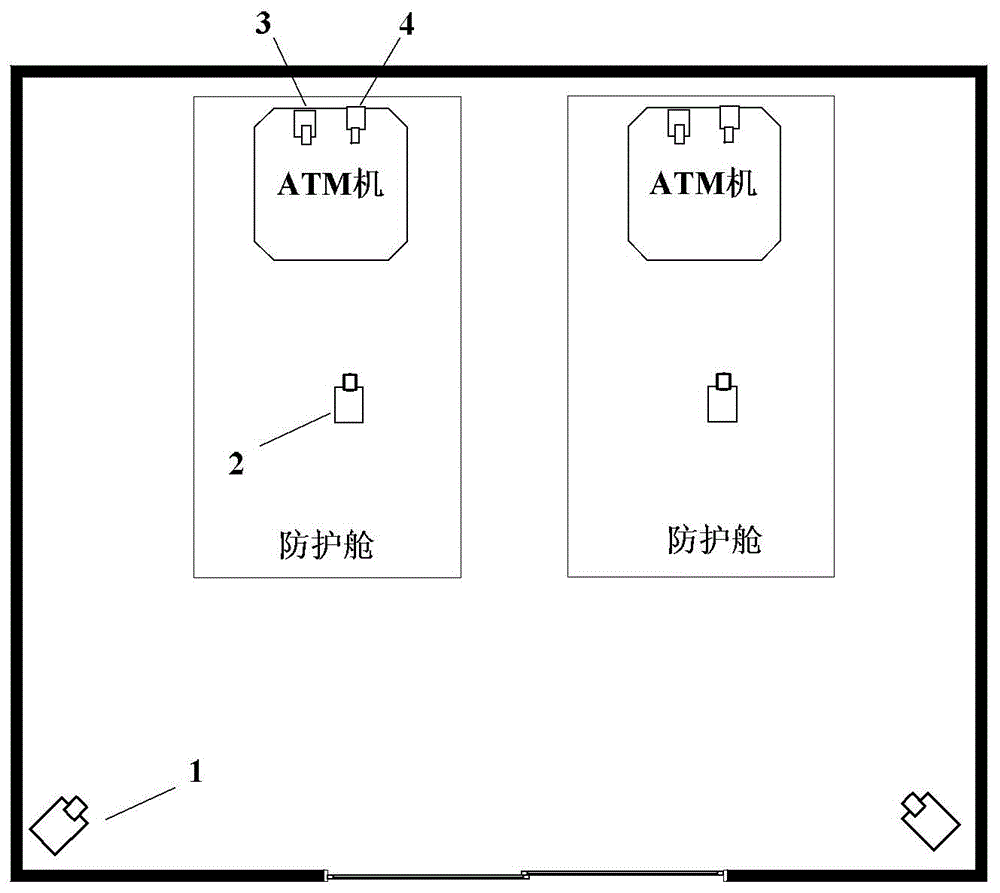
The invention discloses an automatic teller machine (ATM) video surveillance method. The method comprises the steps of acquiring multi-channel video image data from a plurality of video acquisition unit; and conducting analysis of surveillance objects in internal regions of one or more ATM protective cabins and / or preset regions surrounding the one or more ATM protective cabin according to the acquired multi-channel video image data, and triggering alarm information at different levels. The surveillance objects include at least one of the following: the number of persons, actions of a person and the height of a person. The technical scheme provided by the invention can be used for improving the accuracy of the video analysis, reducing false positives and false negatives and outputting reliable alarm information.
Owner:HANGZHOU HIKVISION DIGITAL TECH
Hybrid Hotspot Detection
ActiveUS20130031518A1Computer aided designSpecial data processing applicationsPattern recognitionPattern matching
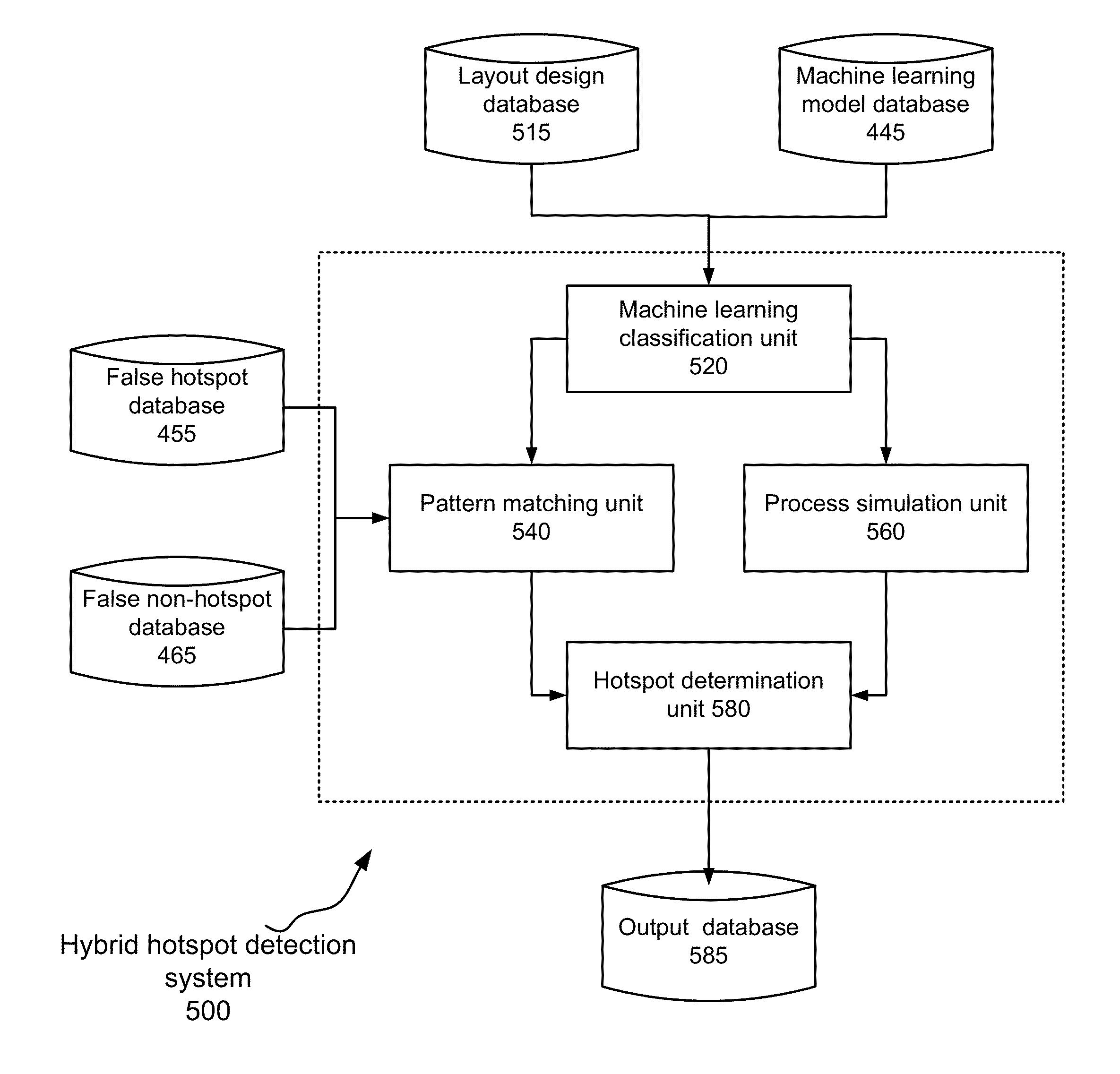
Aspects of the invention relate to hybrid hotspot detection techniques. The hybrid hotspot detection techniques combine machine learning classification, pattern matching and process simulation. A machine learning model, along with false hotspots and false non-hotspots for pattern matching, is determined based on training patterns. The determined machine learning model is then used to classify patterns in a layout design into three categories: preliminary hotspots, preliminary non-hotspots and potential hotspots. Pattern matching is then employed to identify false positives and false negatives in the first two categories. Process simulation is employed to identify boundary hotspots in the last category.
Owner:SIEMENS PROD LIFECYCLE MANAGEMENT SOFTWARE INC
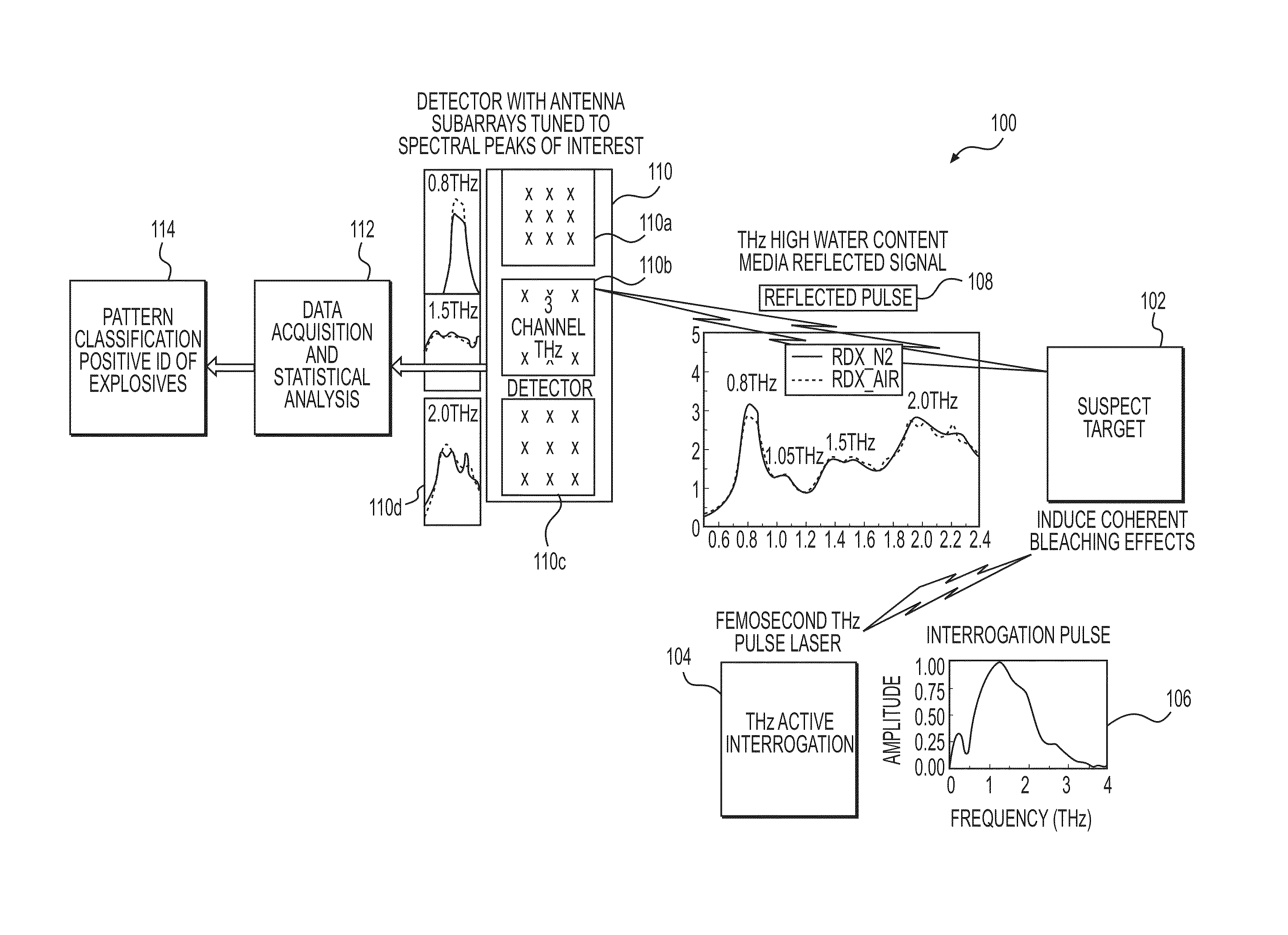
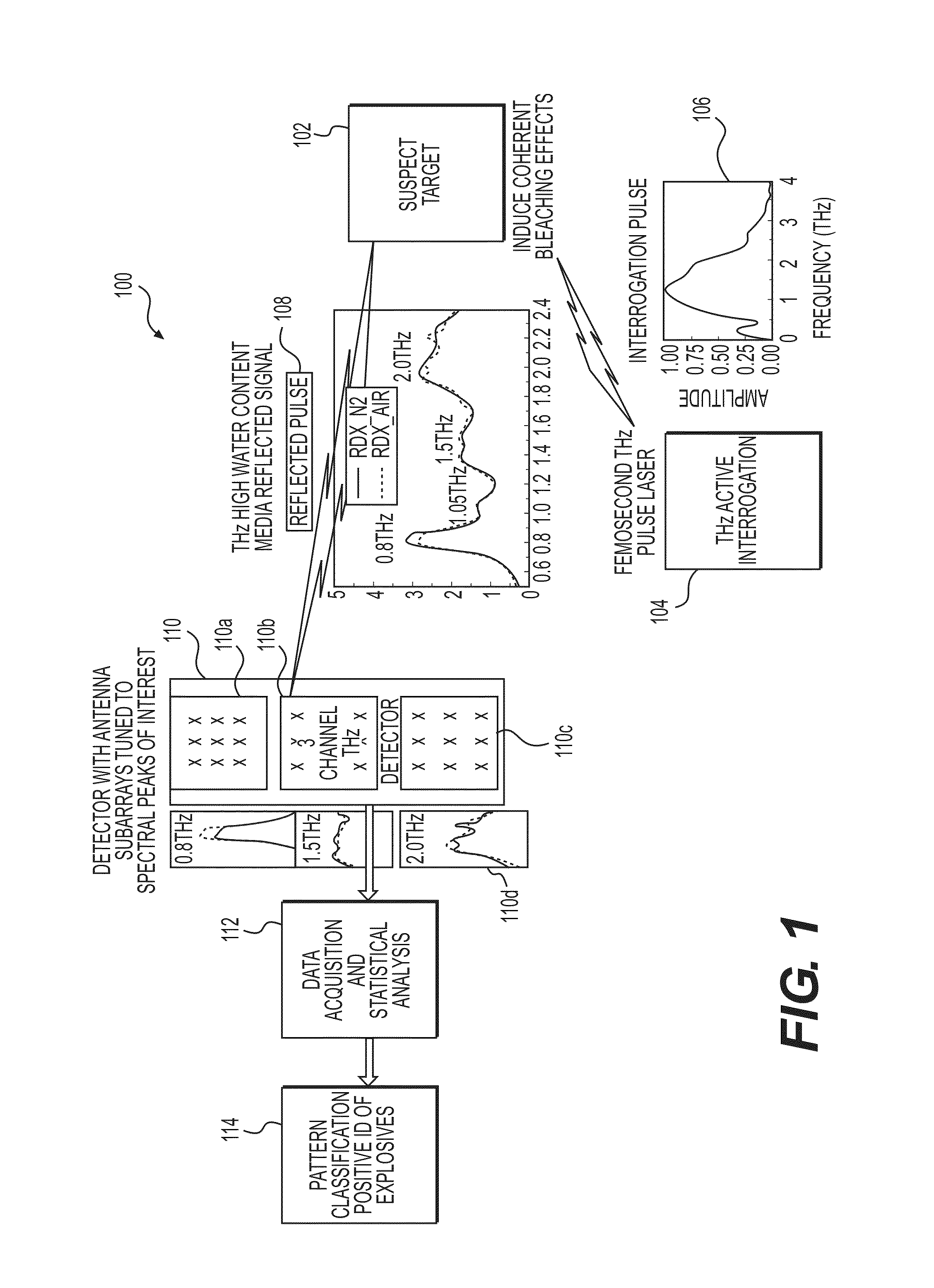
System and Method for Identifying Materials Using a THz Spectral Fingerprint in a Media with High Water Content
InactiveUS20140172374A1Reduce false positiveFalse negativeRadiation pyrometryAmplifier modifications to reduce noise influenceSpectral emissionStatistical analysis
A material detector includes a pulse generator to generate pulses to excite molecules in the material and a detector to detect a signal generated from excited molecules in the terahertz region. Spectral features in the material are analyzed to identify the material. Detection can be performed using a nanoantenna array structure having antennas tuned to detect the expected spectral emission. The nanoantenna array can include antennas having MIM or MIIM diodes. Signal processing and statistical analysis is use to reduce false positives and false negative in identifying the material.
Owner:REDWAVE ENERGY
Method for improving the accuracy of the semi-quantitative determination of analyte in fluid samples
InactiveUS6306660B1Microbiological testing/measurementBiological testingAnalyteQuantitative determination
Disclosed is an improved method for determining the concentration of a first analyte in a fluid test sample as a function of a second analyte also present in the sample whose concentration in the fluid sample is clinically related to that of the first analyte. The method involves determining the concentration of the first analyte, and, if this concentration is outside of its useful analytical range, dividing this concentration by the normal concentration of the second analyte. This method of ratioing the concentrations of the first and second analyte is advantageous because accuracy is increased with fewer false positive and false negative results being reported.
Owner:SIEMENS HEALTHCARE DIAGNOSTICS INC
Gastrointestinal insufflation device and method
Disclosed is an insufflation device and method operably configured to reduce the number of false positive and false negative tests associated with checking the integrity of an anastomotic connection. Versions include a valve assembly configured to facilitate the delivery of pressure via a catheter to an anastomotic site without having to remove an associated pressure source.
Owner:SLOAN DALE
Technique for extracting arrayed data
InactiveUS7466851B2Increase contrastImprove spatial resolutionImage enhancementImage analysisFrequency spectrumImage resolution
The current invention discloses a novel spectral transformation technique for characterizing digitized intensity output patterns from microarrays. This method yields improved sensitivity with reduced false positives and false negatives. Current microarray methods are overly sensitive to the detection of a visible distinction between pixels associated with probes and pixels associated with background. In one embodiment, a technique is disclosed that comprises the steps of: extracting pixels associated with an object of interest and transforming such pixels from an intensity representation to a spectral representation. In some embodiments, the extraction is based on a tessellated logarithmic spiral extraction that may yield a pixel core with a sampling of both foreground and background pixels. This core may then be computationally rescaled by 10×-10,000× to enhance spatial resolution. Once the extracted pixels are represented in the spectral regime, convolution with resolution-enhancement kernels may be used to accentuate morphological features capturing platform specific phenomenology.
Owner:VIALOGY
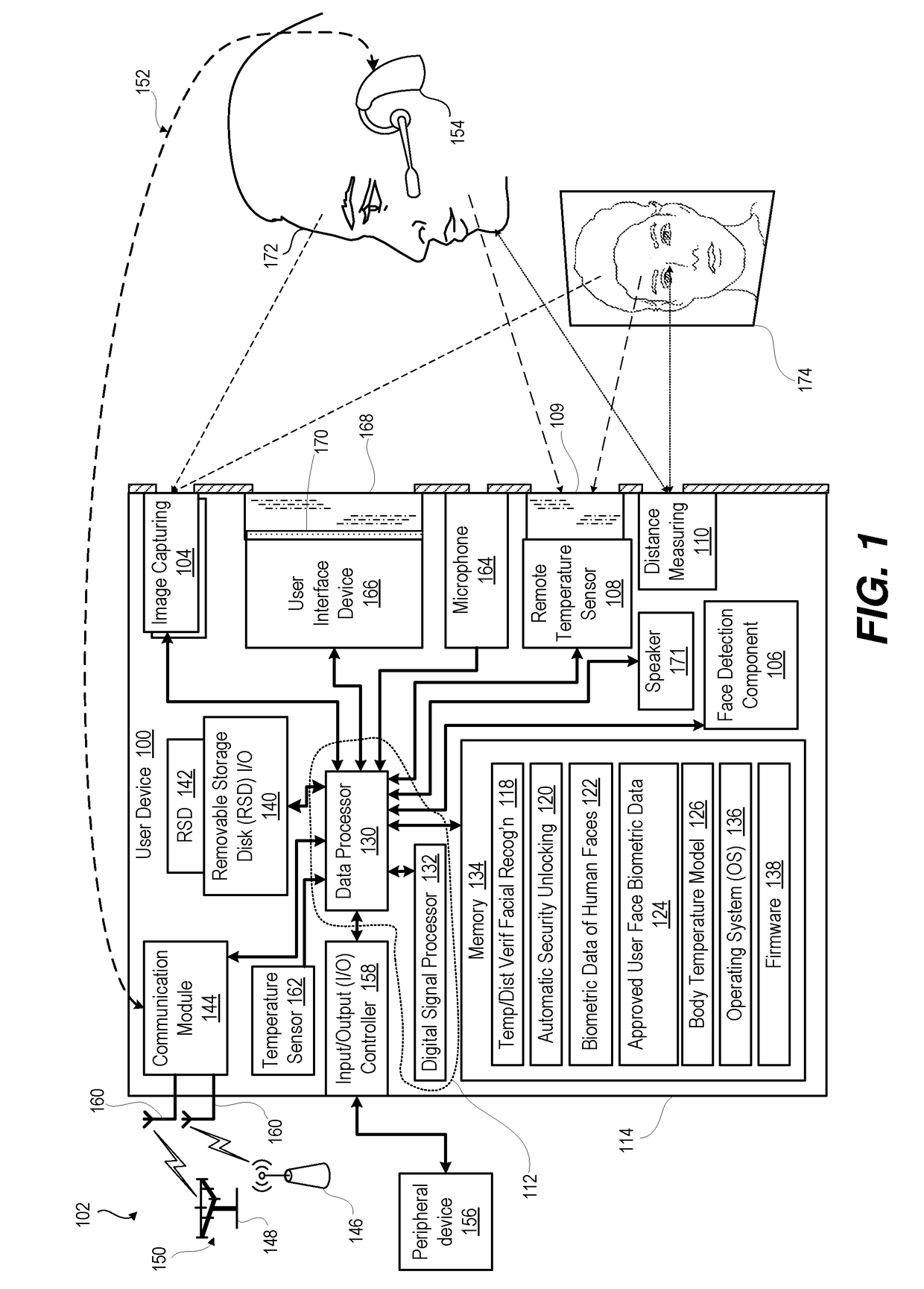
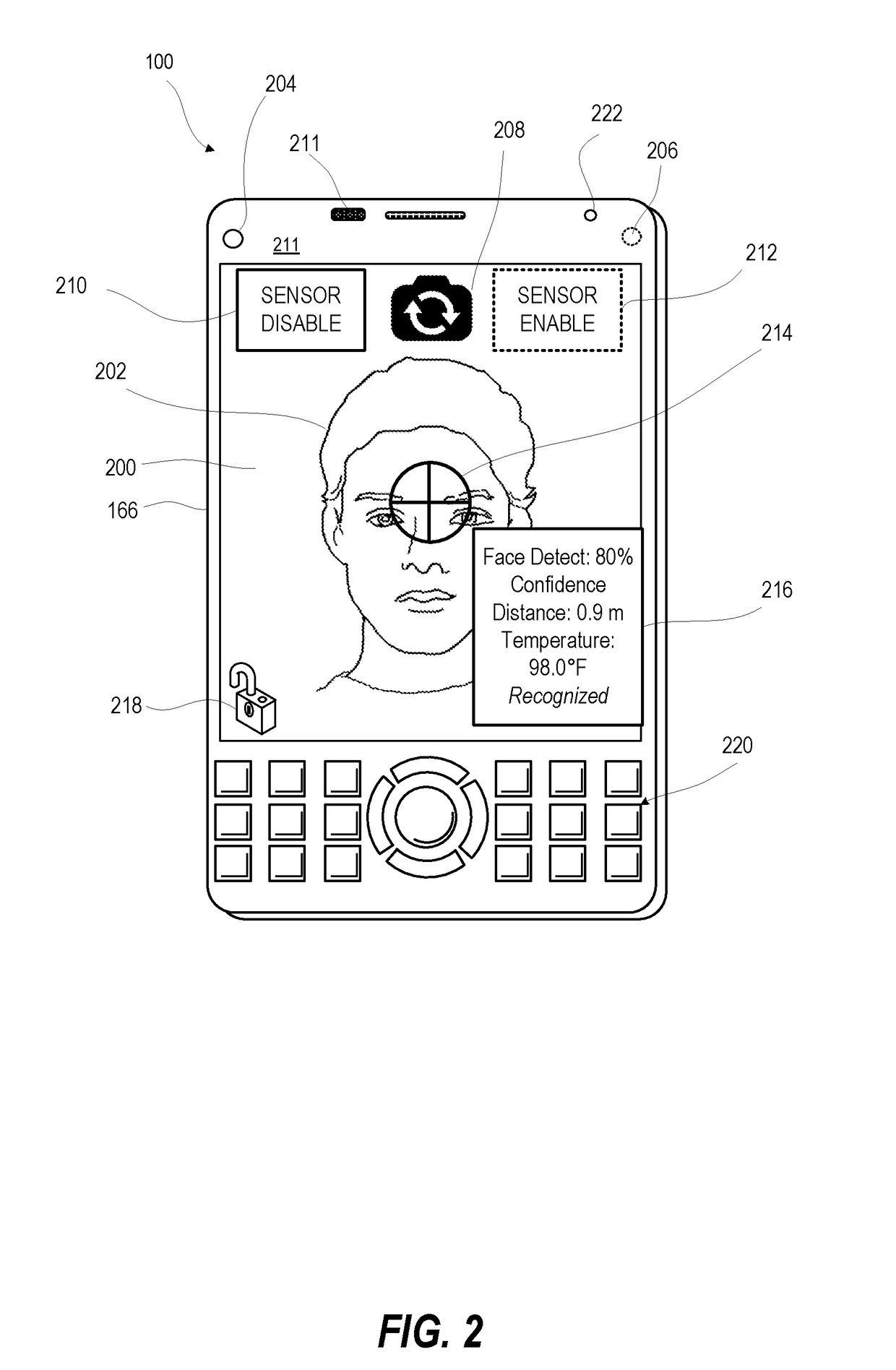
Face detection with temperature and distance validation
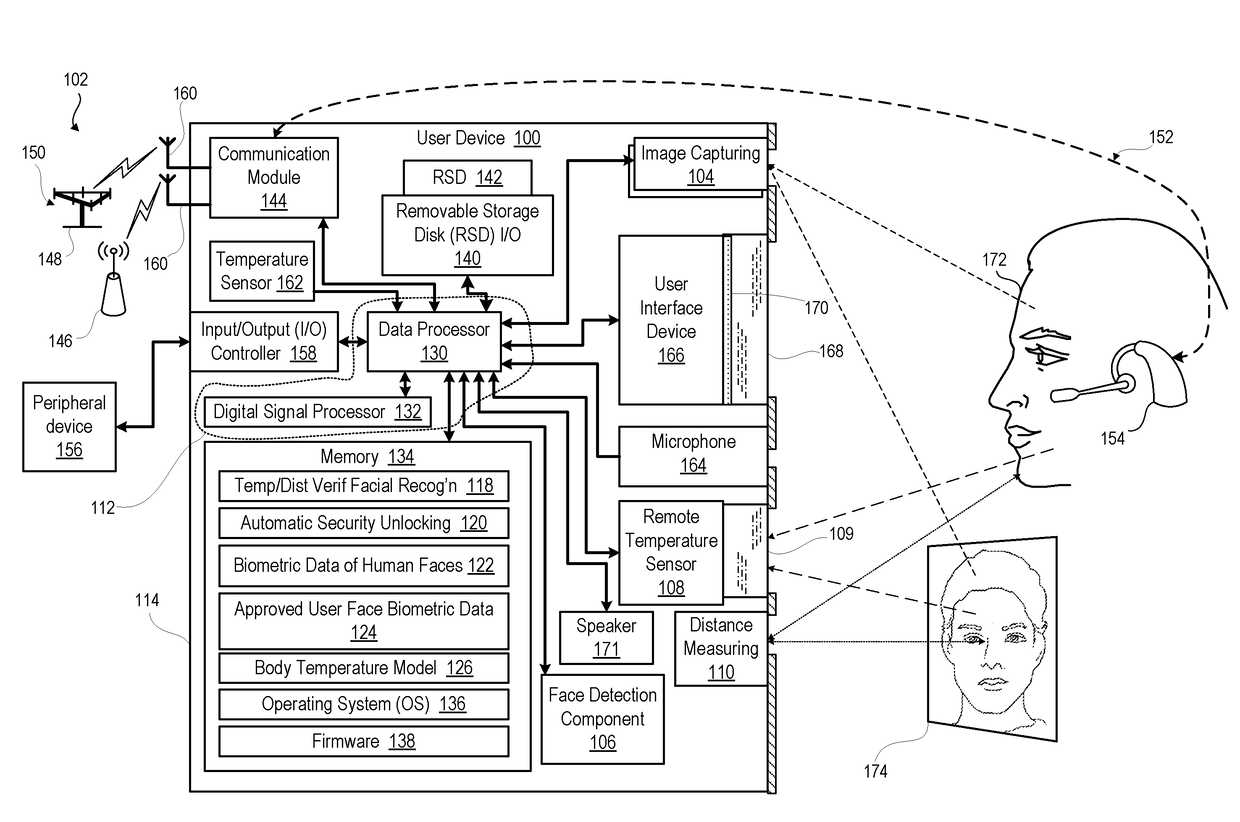
ActiveUS20180239977A1ConfidenceDecrease in confidenceTelevision system detailsColor television detailsCamera controlPattern recognition
An electronic device incorporates features that are dependent on finding a face by image processing of an image taken by an image capturing device. To avoid false positives and false negatives due to poor focus, exposure, or spoofing with a picture, the electronic device validates, by distance and / or temperature, a candidate face within a captured image. Distance information is used to scale the candidate face to an actual size for comparison against biometric data on a range of sizes of a human face. Detected temperature is compared against biometric data on the temperature of a human face sensed in the infrared spectrum. Confidence value for face detection is increased or decreased in relation to the validation by size / temperature. For a validated candidate having a confidence value above a threshold, the electronic device can adjust camera controls of an image capturing device or enable a face recognition security component.
Owner:MOTOROLA MOBILITY LLC
Technique for extracting arrayed data
InactiveUS20050105787A1Increase contrastImprove spatial resolutionImage enhancementImage analysisHigh spatial resolutionImage resolution
The current invention discloses a novel spectral transformation technique for characterizing digitized intensity output patterns from microarrays. This method yields improved sensitivity with reduced false positives and false negatives. Current microarray methods are overly sensitive to the detection of a visible distinction between pixels associated with probes and pixels associated with background. In one embodiment, a technique is disclosed that comprises the steps of: extracting pixels associated with an object of interest and transforming such pixels from an intensity representation to a spectral representation. In some embodiments, the extraction is based on a tessellated logarithmic spiral extraction that may yield a pixel core with a sampling of both foreground and background pixels. This core may then be computationally rescaled by 10×-10,000× to enhance spatial resolution. Once the extracted pixels are represented in the spectral regime, convolution with resolution-enhancement kernels may be used to accentuate morphological features capturing platform specific phenomenology.
Owner:VIALOGY
Parity vector method-based double-satellite failure recognition method
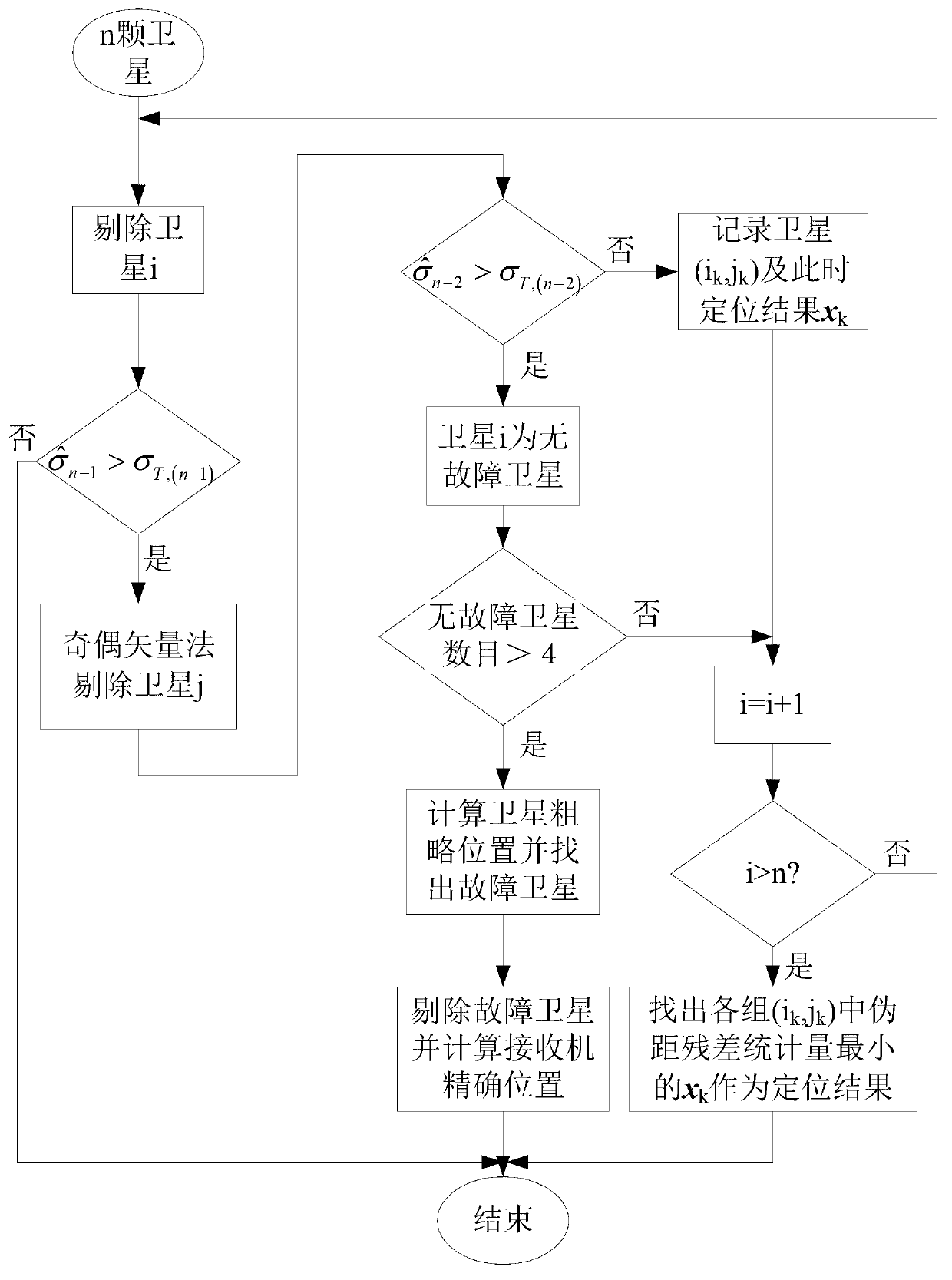
InactiveCN102901971ASolve the problem of fault offset offsetRealize detection and identificationSatellite radio beaconingRemote sensingReceiver autonomous integrity monitoring
The invention relates to global satellite navigation system receiver autonomous integrity monitoring technology and discloses a parity vector method-based double-satellite failure recognition method, aiming at the problems of false positives and false negatives caused by fault deviation offsetting when the parity vector method is used for recognizing two fault satellites. According to the technical scheme, the parity vector method is used for recognizing one fault satellite; with the fault satellite as the basis, four fault-free satellites are found out, and the information of the fault-free satellites is used for roughly locating, so that the fault satellites can be recognized; the recognized fault satellites are removed, and then the position resolution is carried out again, so that the locating accuracy is improved; therefore, the problems of false positives or false negatives caused by fault deviation offsetting can be avoided. The method solves the problem of the fault deviation offsetting caused by parity vector residual error and realizes the detection and the recognition for a plurality of fault satellites. After the method is used for detecting and recognizing satellite failure, the locating accuracy is improved. The method is mainly used for monitoring the autonomous integrity of a global satellite navigation system receiver.
Owner:UNIV OF ELECTRONICS SCI & TECH OF CHINA
Systems and Methods for Monitoring Health in a Shared Living Environment
InactiveUS20160196735A1Rapid responseDiagnostic recording/measuringSensorsSexual coercionLiving environment
The systems and method for monitoring health in a shared living environment may provide a collection of components and / or accessories that may, in various aspects, assist in deterring sexual coercion, sexual assault, and rape, provide a rapid response pathway in the event of sexual coercion or assault, stimulate and support event reporting by complainants, reduce false positive and false negative rates (for both accused and accusers), and / or enable later differentiation between meritorious and meretricious complainants and counter complainants (defendants / respondents) in future disciplinary or legal proceedings. In addition, other aspects of the systems and method described herein may assist in monitoring promoting other healthy lifestyle choices, such as sleep and study choices.
Owner:CLAYMAN ADAM
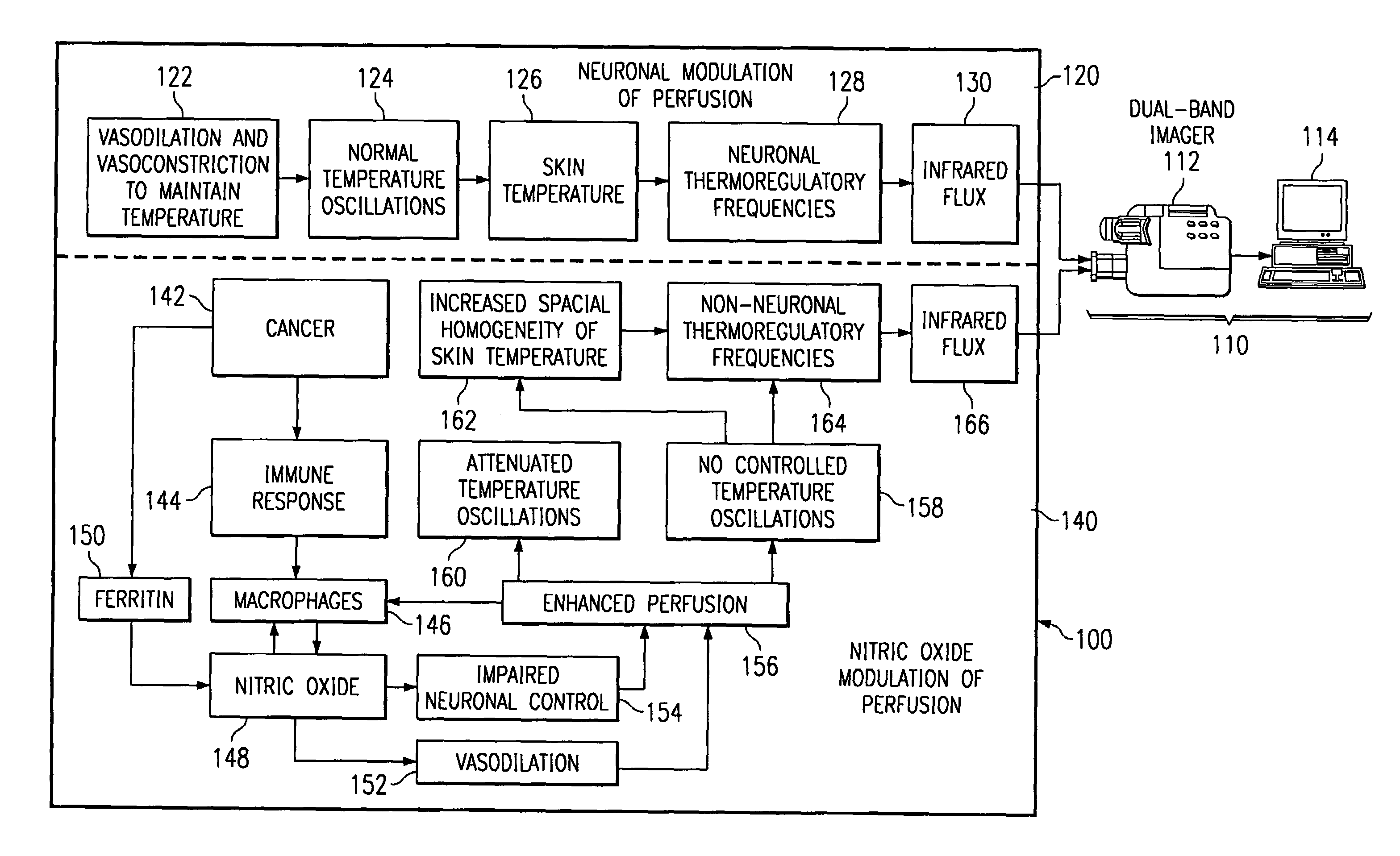
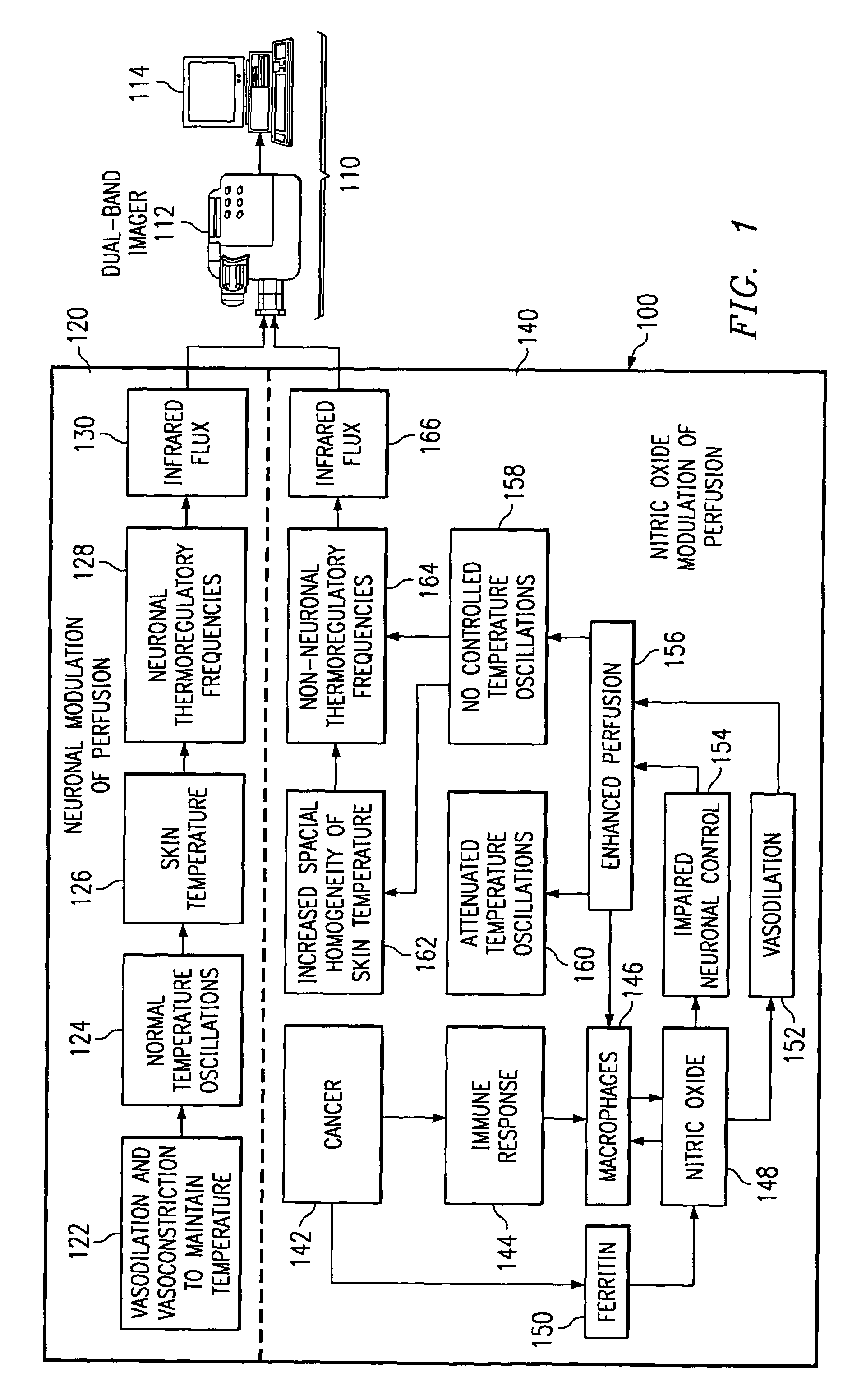
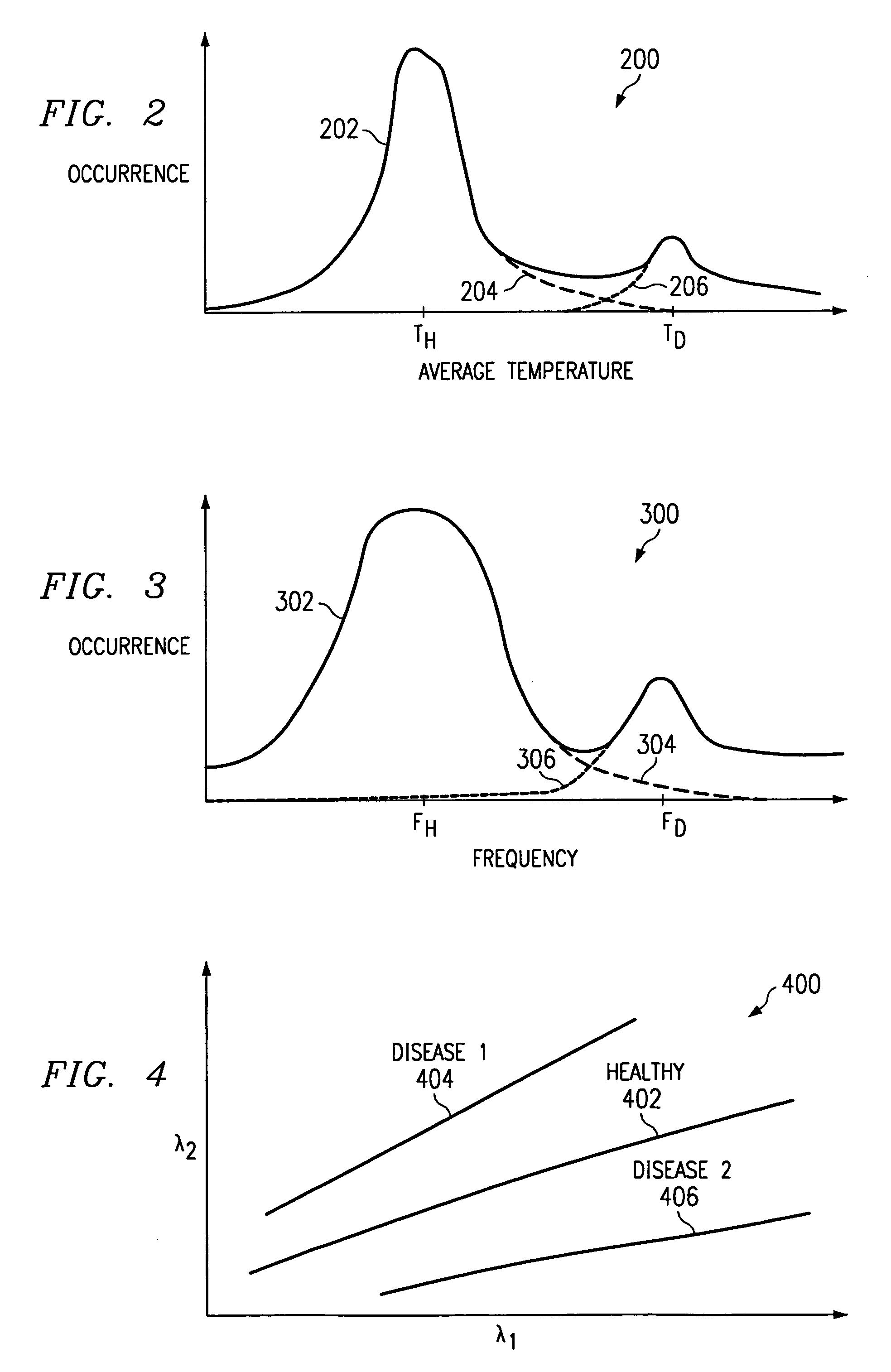
Method of and apparatus for detecting diseased tissue by sensing two bands of infrared radiation
A method of and apparatus for detecting diseased tissue based upon infrared imaging in two different bands of infrared wavelengths is described. The use of two series of infrared images taken in two different bands of infrared wavelengths increases sensitivity to the subtle temperature changes caused by diseased skin and tissue, especially in the case of cancerous tissue. By sensing skin temperature, the homogeneity thereof, the time variations thereof and the correlation between the two series of infrared images, the present invention decreases the rate of false positives and false negatives. The increased discrimination due to two series of infrared images allows for reliable detection of diseased or cancerous tissue even in the presence of skin tone variations such as birthmarks, tattoos and freckles. The present invention finds special application in the field of breast cancer detection where subtle skin temperature variations may readily be sensed using two series of infrared images.
Owner:LOCKHEED MARTIN CORP
Method and device for detecting instability of microsatellite locus
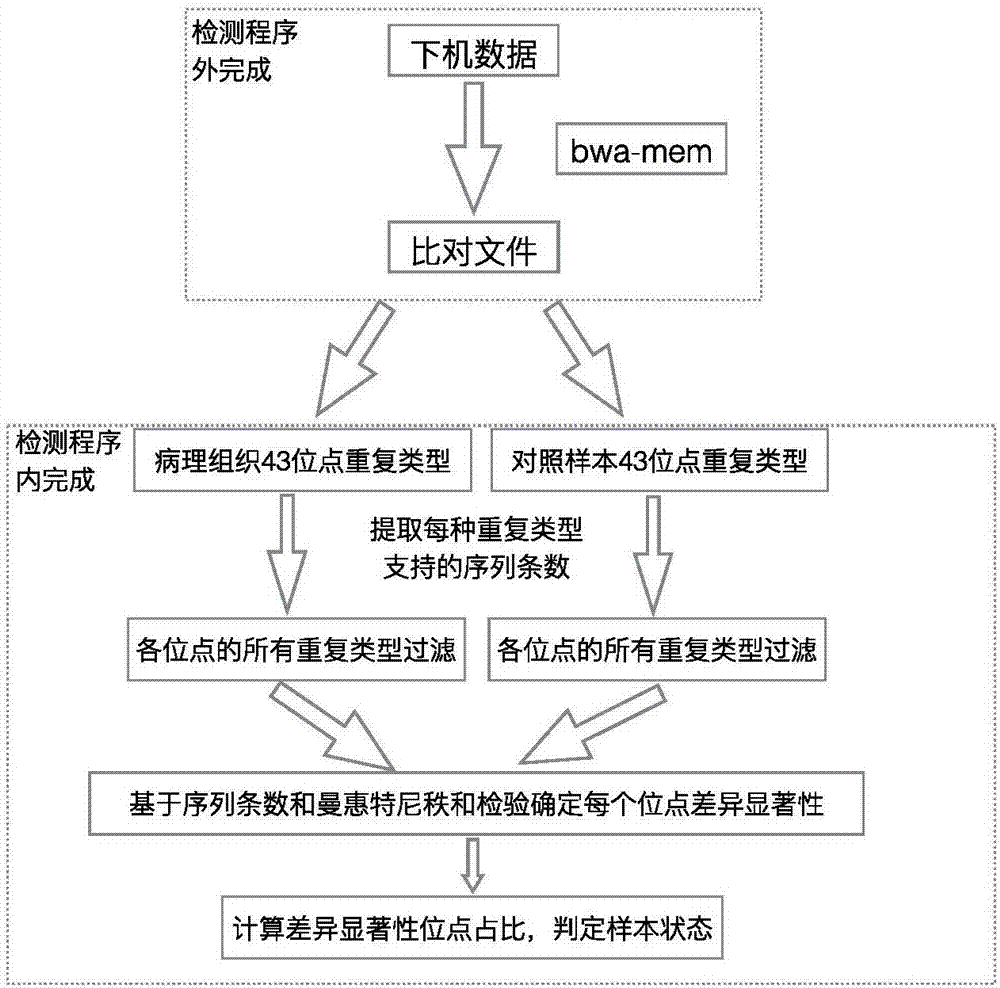
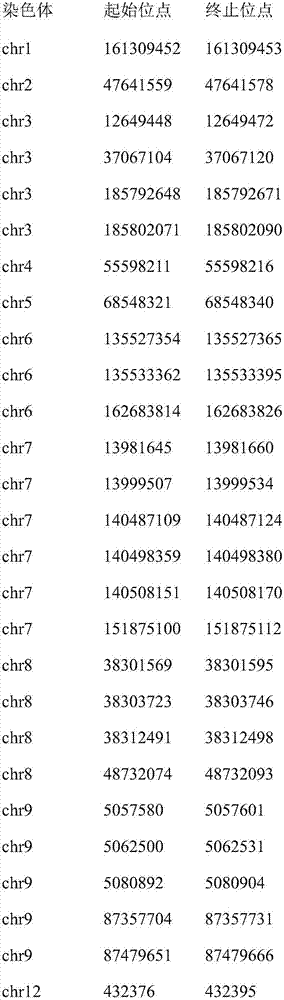
ActiveCN107058551AIncrease the number of sitesReduce false positivesMicrobiological testing/measurementSequence analysisInstabilityData treatment
The invention discloses a method and device for detecting instability of a microsatellite locus, wherein the method comprises the steps of S1, treating a sample; S2, processing data, to be specific, using comparison software to compare determined sequences to a reference genome to obtain a comparison file, allowing non-matched sequences to form soft cut-off, ranking according to comparison positions, and using the software samtools to establish an index; S3, detecting the instability of the microsatellite locus, to be specific, using locus coverage rates having significant differences to determine the microsatellite instability of the sample. By using the method and device in connection with high-throughput sequencing techniques for capturing a target area, the quantity of loci for detection can be increased greatly; differences between a pathological sample and a control sample are judged, the microsatellite stability of the sample is determined through the differences in connection with statistical testing, and chances for false positives and false negatives to occur in the detection of microsatellite locus stability in the prior art are slimmed.
Owner:天津诺禾致源生物信息科技有限公司
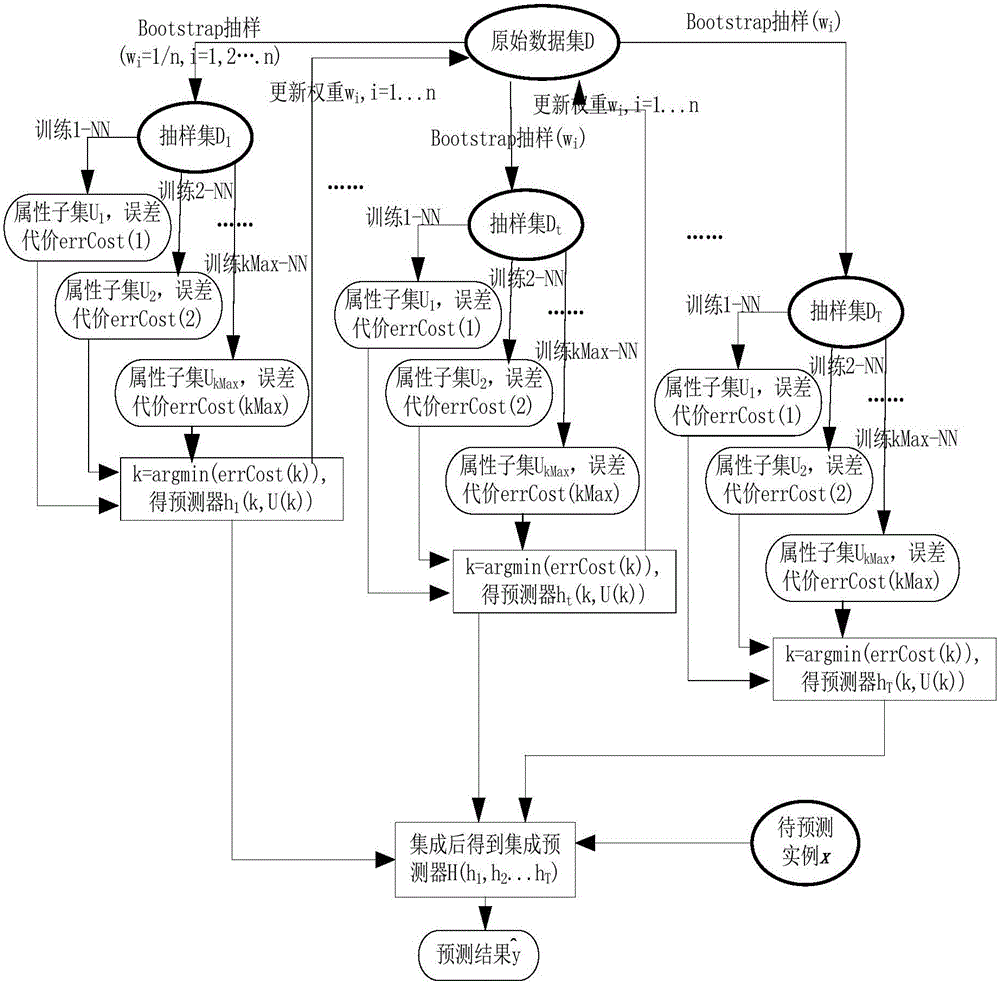
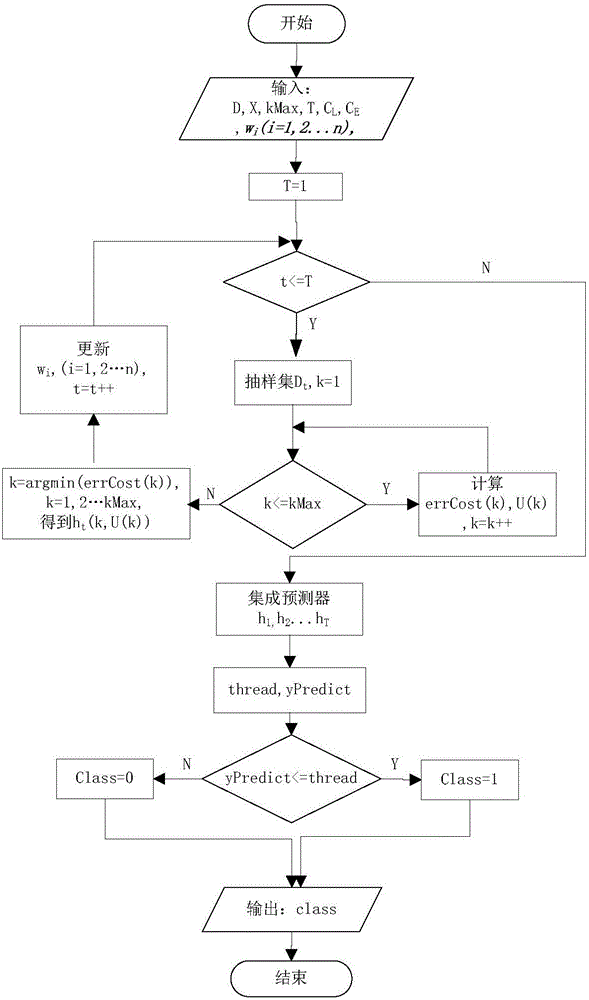
Boosting based cost sensitive software defect prediction method
InactiveCN106203534AMake sure to learnAvoid accidental deletionCharacter and pattern recognitionCost sensitiveSmall sample
The invention discloses a boosting based cost sensitive software defect prediction method, belonging to the technical field of software engineering application. According to the invention, re-sampling is carried out by means of Bootstrap, the erroneous deletion of valuable attributes can be prevented by using a cost-sensitive subclass selection manner of randomly deleting an attribute during attribute selection, and meanwhile, the selected attribute sub-class facilitates the reduction of prediction error costs, when the weight is updated, a cost-sensitive weight updating mechanism is adopted, large weight is endowed to a data set with high cost, so that the data can be guaranteed to be studied for many times to obtain a reasonable integrated prediction model, and the prediction model is applied to small sample data to accurately predict software defects, and therefore, the technical problems that the prediction effects are not satisfactory due to the shortage of training data under the small sample data and the unequal cost of false positives and false negatives in the prediction process are solved.
Owner:NANJING UNIV OF AERONAUTICS & ASTRONAUTICS
Pcr-free sample preparation and detection systems for high speed biologic analysis and identification
InactiveUS20100216657A1Great reagent stabilityPrevent degradationNucleotide librariesLibrary screeningPersonal protective equipmentBiology
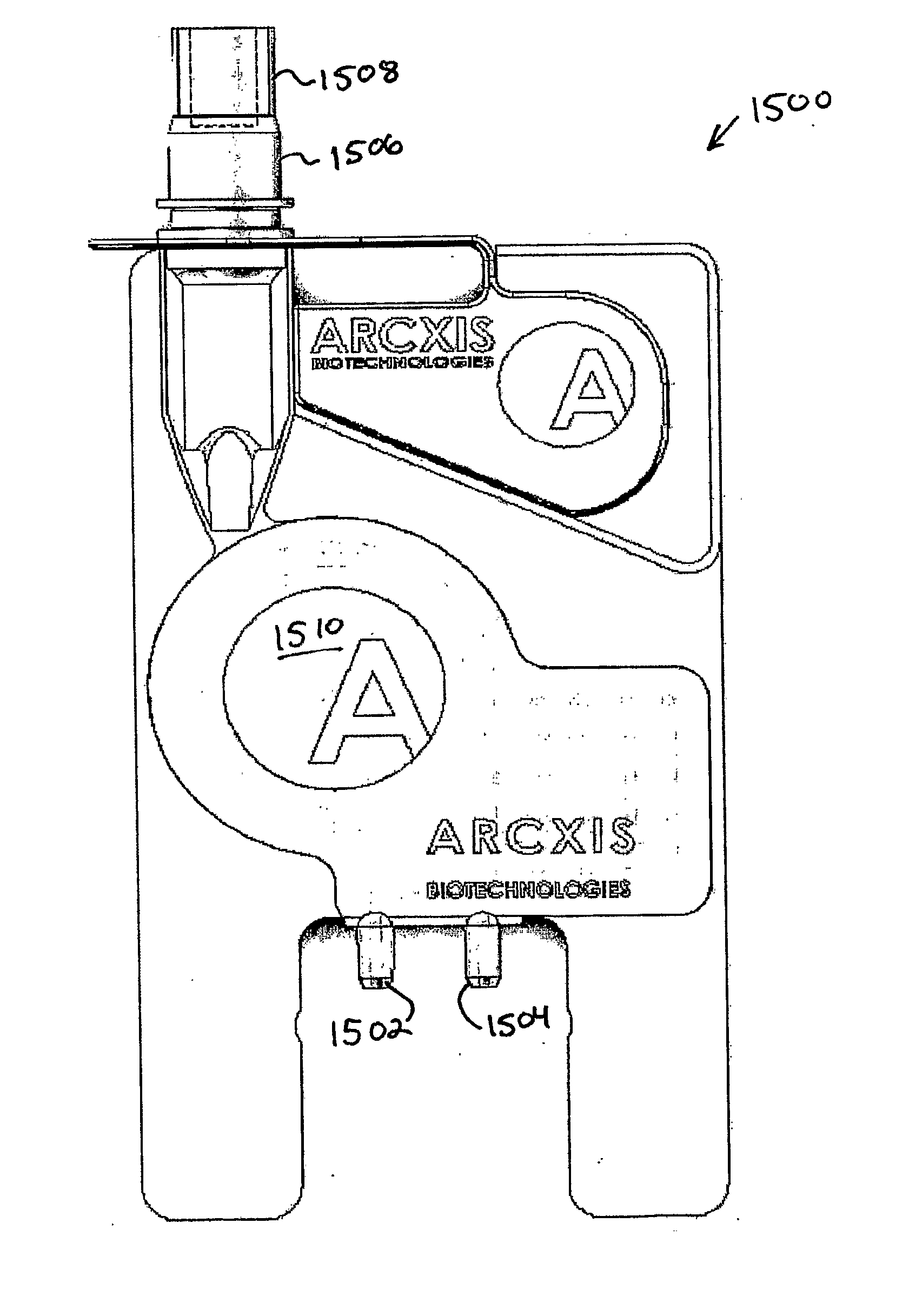
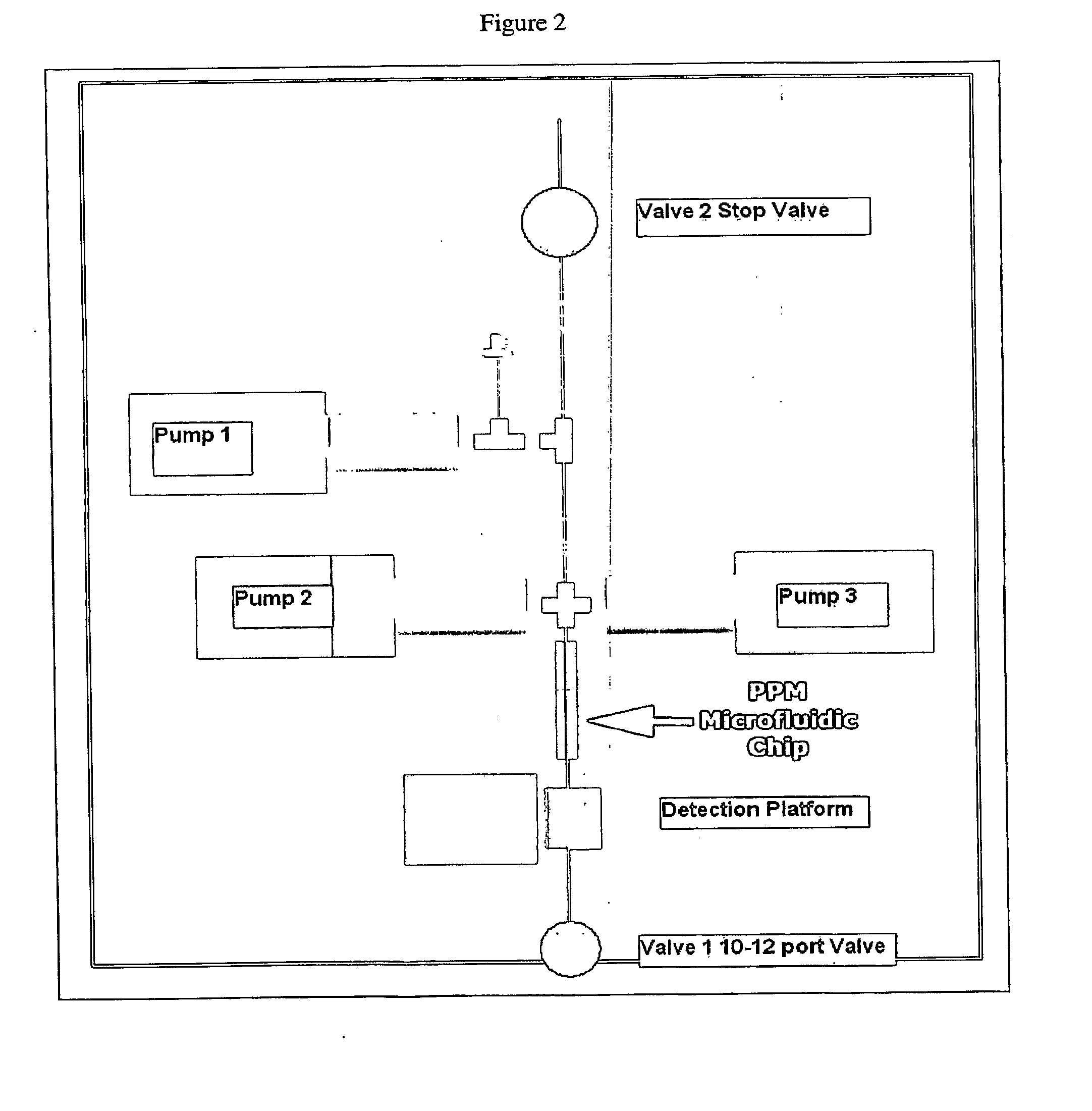
Provided herein are biologic sample preparation and analysis systems that are rapid, portable, robust detection system for multiplexed detection of bio-threats, and which can be ruggedized to operate in harsh environments. A new method of detection called Combinatorial Probe Analysis (CPA), which provides an exponential increase in detection reliability, has been incorporated into these systems. This type of analysis greatly reduces false positives and false negatives; in addition it is reusable and eliminates special storage requirements for reagents. Specific technical advancements in the optimization of hybridization assays for nucleic acid detection on porous polymer monoliths (PPM) are also disclosed. Performing rapid and complete solubilization of viruses, vegetative bacteria and bacterial spores with an ultra high temperature solubilization protocol is also described. The systems provided herein provides the ability to perform rapid highly multiplexed analysis of a variety of bioagents, including bacteria viruses, and protein biotoxins. The systems and assays described herein are perform completely automated sample preparation and analysis, in a time frame of five minutes or less. The assay is simple in design allowing users in personal protective equipment to easily operate the system. The disclosed systems are robust, simple to use, and address the goals of the first responder community.
Owner:FLUIDIGM CORP
Method for screening xanthine oxidase inhibitor by ultra performance liquid chromatography and mass spectrometry
ActiveCN102095825AQuick checkAccurate detectionComponent separationMass spectrometry imagingGenistein
The invention provides a method for screening a xanthine oxidase inhibitor by ultra performance liquid chromatography and mass spectrometry. The method is used for analyzing the in-vitro inhibition rate of the extract or monomer of a natural product on the xanthine oxidase and the catalytic activity of the xanthine oxidase. By using the ultra performance liquid chromatography-mass spectrometry in the xanthine oxidase inhibitor screening, the method is rapid and accurate in sample detection, and the correlation coefficients of the linear equation reaches 0.998. The mass spectrometry has high accuracy and good specificity in detecting the mass electron ratio of a compound, and can be used for screening the xanthine oxidase inhibitor and for the kinetic study of the xanthine oxidase inhibitor without false positive and false negative results which occur in the spectrometry. The results showed that the inhibition rate I of 20 mumol / L allopurinol is 80%; the inhibition rate of 20 mumol / L isorhamnetin is 73%; the inhibition rate of 20 mumol / L genistein is 50%; and the inhibition rate of 0.1 mg / mL aqueous extract of Ginkgo biloba is 27%.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
Colloidal gold chromatographic test strip for rapidly testing lead ion
InactiveCN102426239AStrong specificityIncreased sensitivityMaterial analysisSocial benefitsGlass fiber
A colloidal gold chromatographic test strip for rapidly testing lead ion belongs to the field of immunological technique. The test strip provided by the invention is composed of a liner plate and a sample pad, a colloidal-gold combination pad, a coating membrane and a water absorption pad which are successively connected on the liner plate. The colloidal-gold combination pad is glass fiber cotton for adsorbing lead ion colloidal-gold antibody. A linear invisible detection line T printed by a lead ion and carrier protein coupled solution and a linear contrast line C printed by a goat-anti-mouse IgG solution are successively arranged on the coating membrane, wherein the two lines are parallelly arranged. The colloidal gold chromatographic test strip for rapidly testing lead ion in the invention has strong specificity and high sensitivity, and can be used to more rapidly, sensitively and simply test lead ion residues. By the adoption of the test strip, the determination result is vivid, visual, accurate, simple and clear, and false positive and false negative human misjudgments are not easy to occur. The test strip provided by the invention can be used to save cost, has a wide application range, is convenient for popularization, and has an extensive market prospect as well as obvious economic and social benefits.
Owner:王利兵 +2
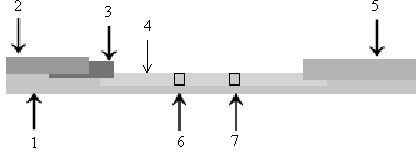
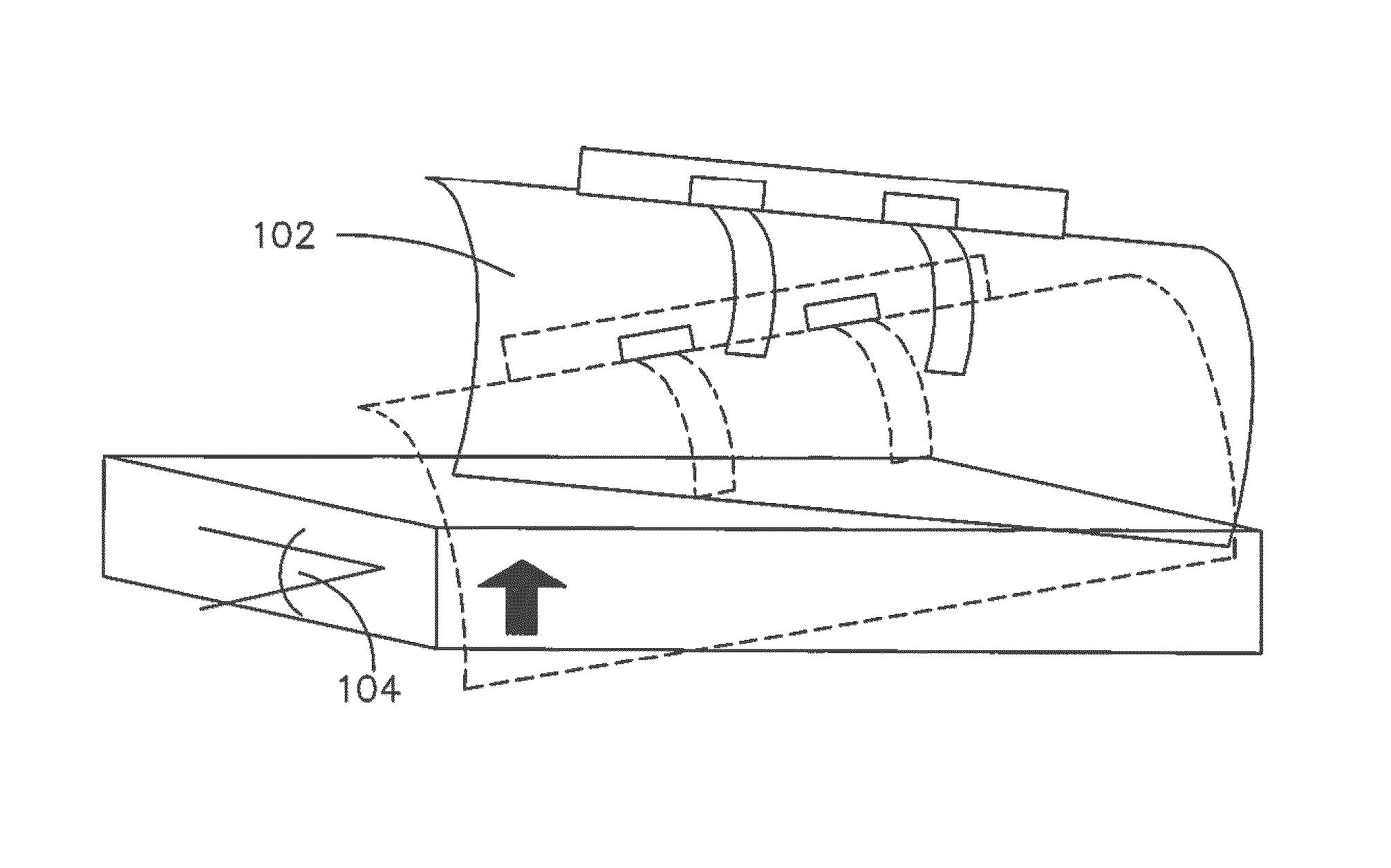
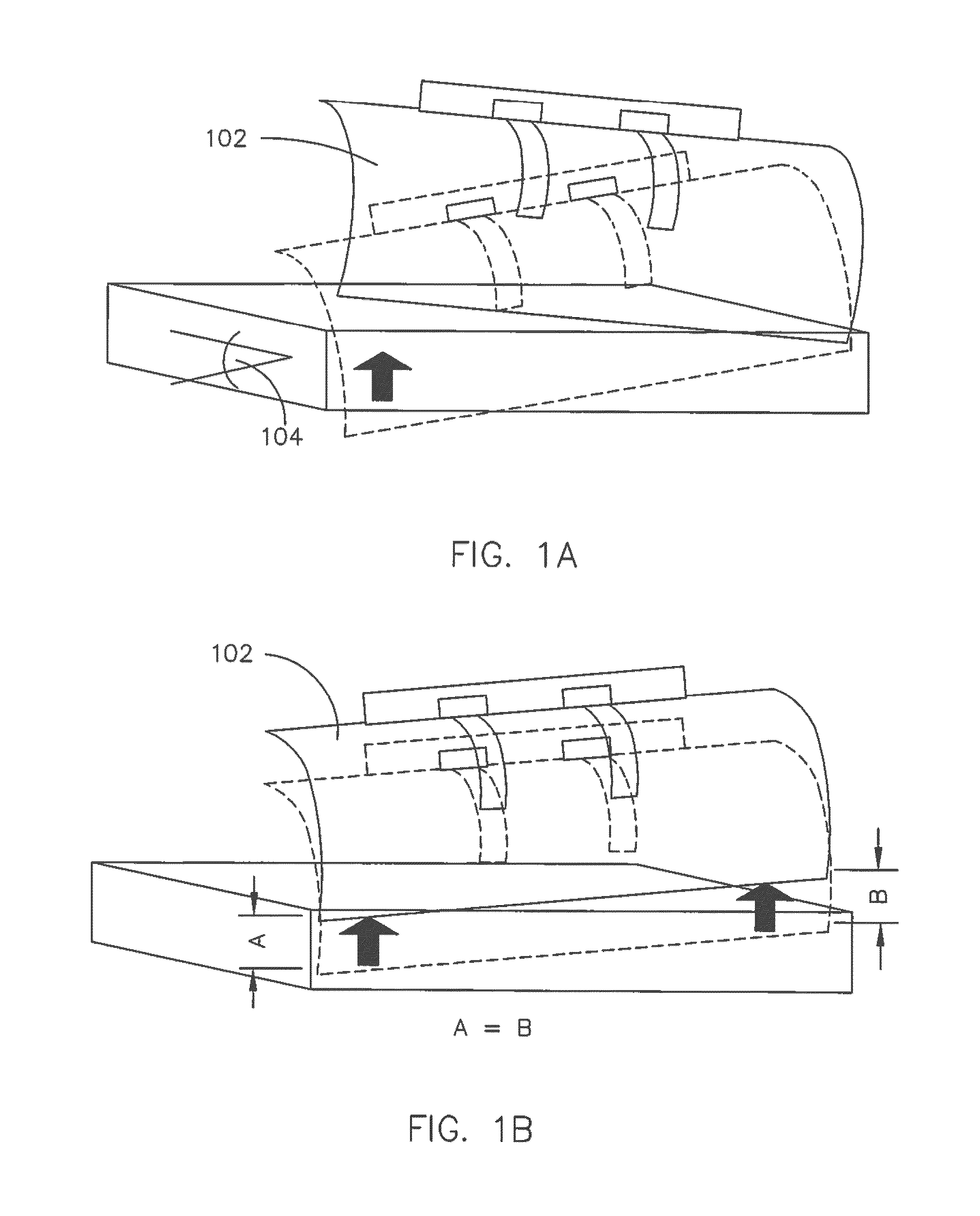
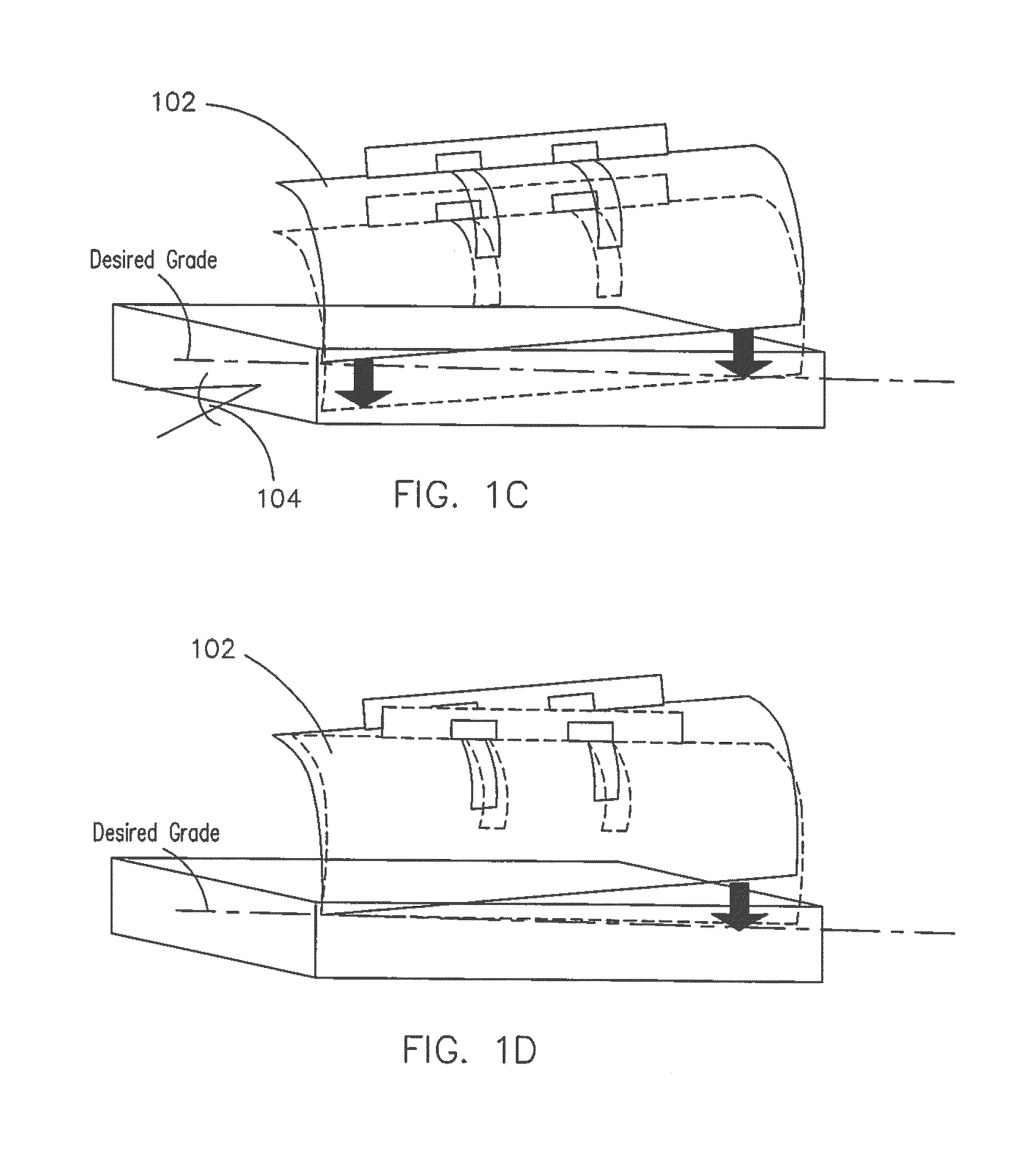
Coordinated and proportional grade and slope control using gain matrixes
ActiveUS9458581B1Error can be accountedSpatial transmit diversitySoil-shifting machines/dredgersComputer control systemMultiple sensor
A multiple-input multiple-output (MIMO) computer control system in a heavy equipment machine is in communication with multiple sensors in order to measure deviations from a path to be followed. Sensor corrections are applied to return the heavy equipment machine to a path to be followed or to restrain the machine from deviating from the path to be followed. Sensor corrections affect a controlled variable, such as cross-slope. Sensor corrections may account for false positives and false negatives. Sensor corrections are applied to the heavy equipment machine using a gain matrix (G). The multiple vectors of gain values comprising the gain matrix (G) are utilized by the MIMO computer control system to simultaneously and proportionally actuate each drive leg of the machine to obtain a desired grade including a compensated slope and / or elevation.
Owner:GOMACO
Magnetic fluorescent bifunctional nano biological probe and preparation method thereof
InactiveCN102127586AWide variety of sourcesEasy to synthesizeMicrobiological testing/measurementMaterial analysis by electric/magnetic meansCancer cellBifunctional
The invention discloses a magnetic fluorescent bifunctional nano biological probe and a preparation method thereof. The invention is characterized by preparing magnetic fluorescent bifunctional nanoparticles which can be used a nano biological probe, wherein each nanoparticle is in a core-shell spherical structure, and the nanoparticles are prepared from hydrophobic superparamagnetic nanoparticles as the core layer and a fluorescent amphipathic polymer as the shell layer by a nano emulsion process. After entering cells, the composite magnetic fluorescent nanoparticles can be used for imaging analysis on the cells respectively by a fluorescence process and a magnetic resonance imaging process. The magnetic fluorescent nano probe disclosed by the invention has high sensitivity and stability, greatly enhances the sensitivity and accuracy of cell imaging, reduces the emergence of false positive and false negative in cell diagnosis and analysis, and can be well used for diagnosing various diseases, such as tumor cells, cancer cells and the like.
Owner:苏州同科生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com