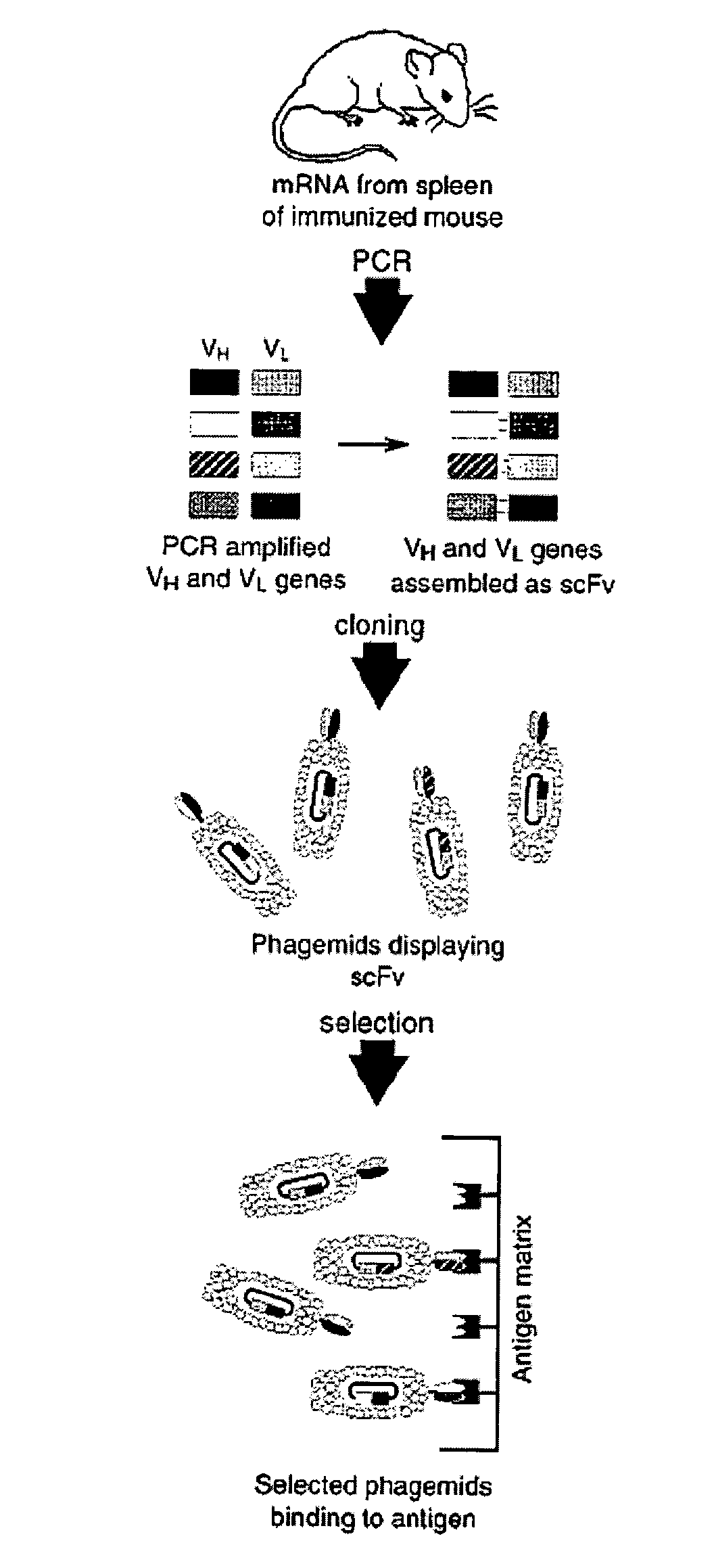
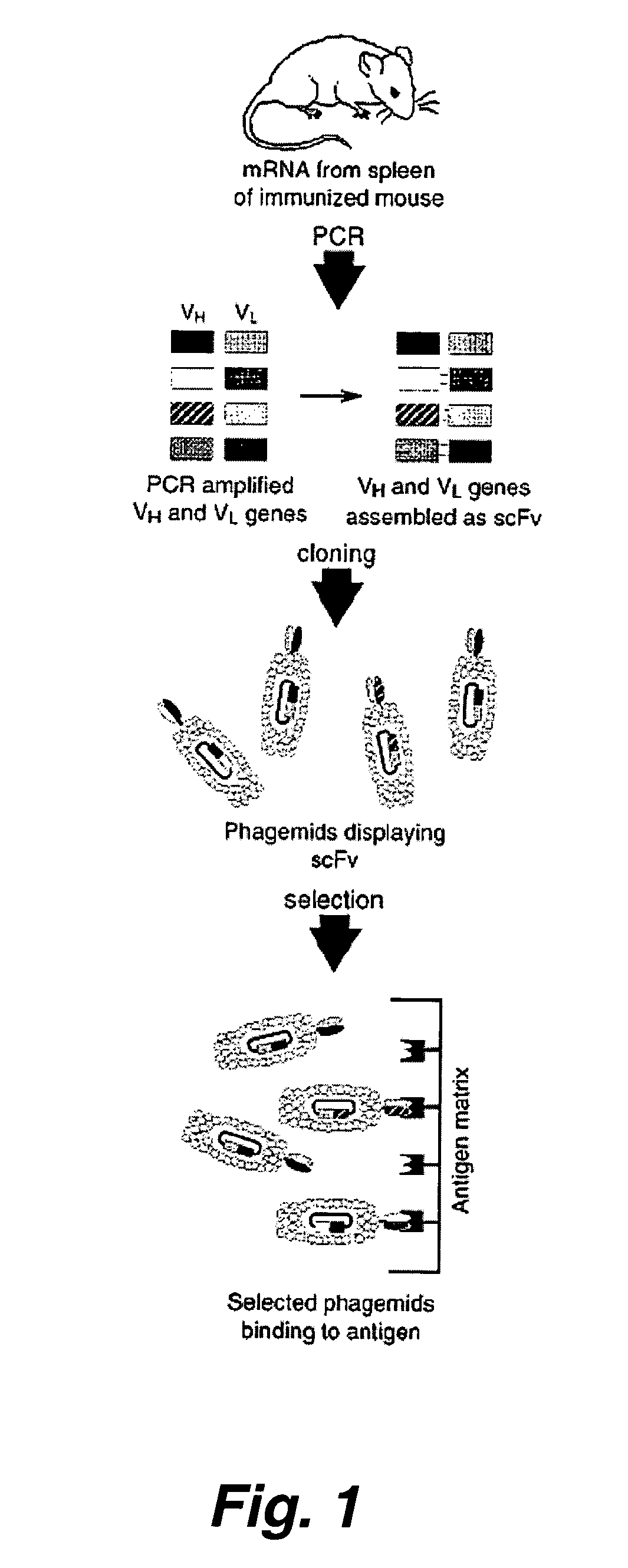
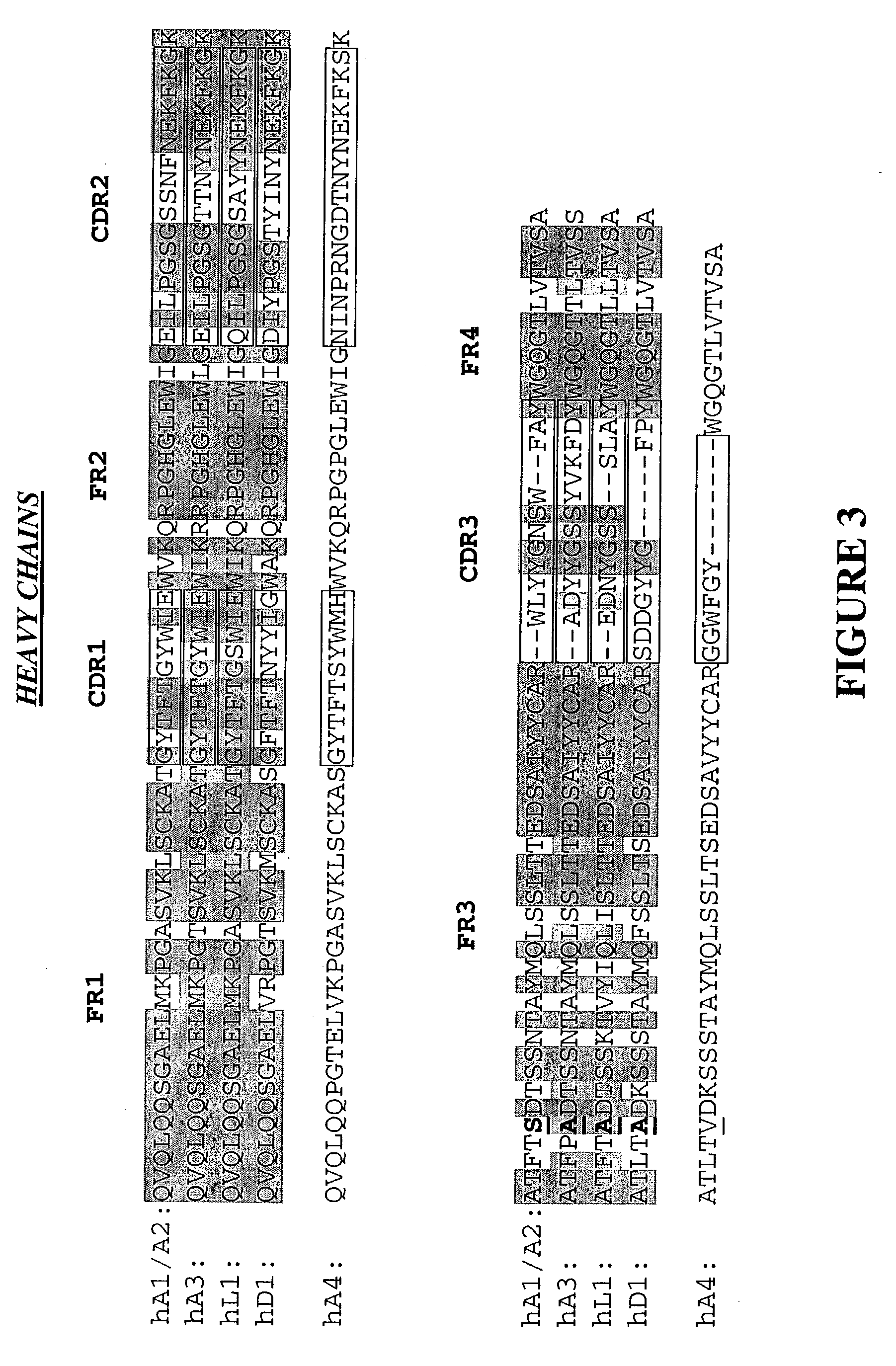
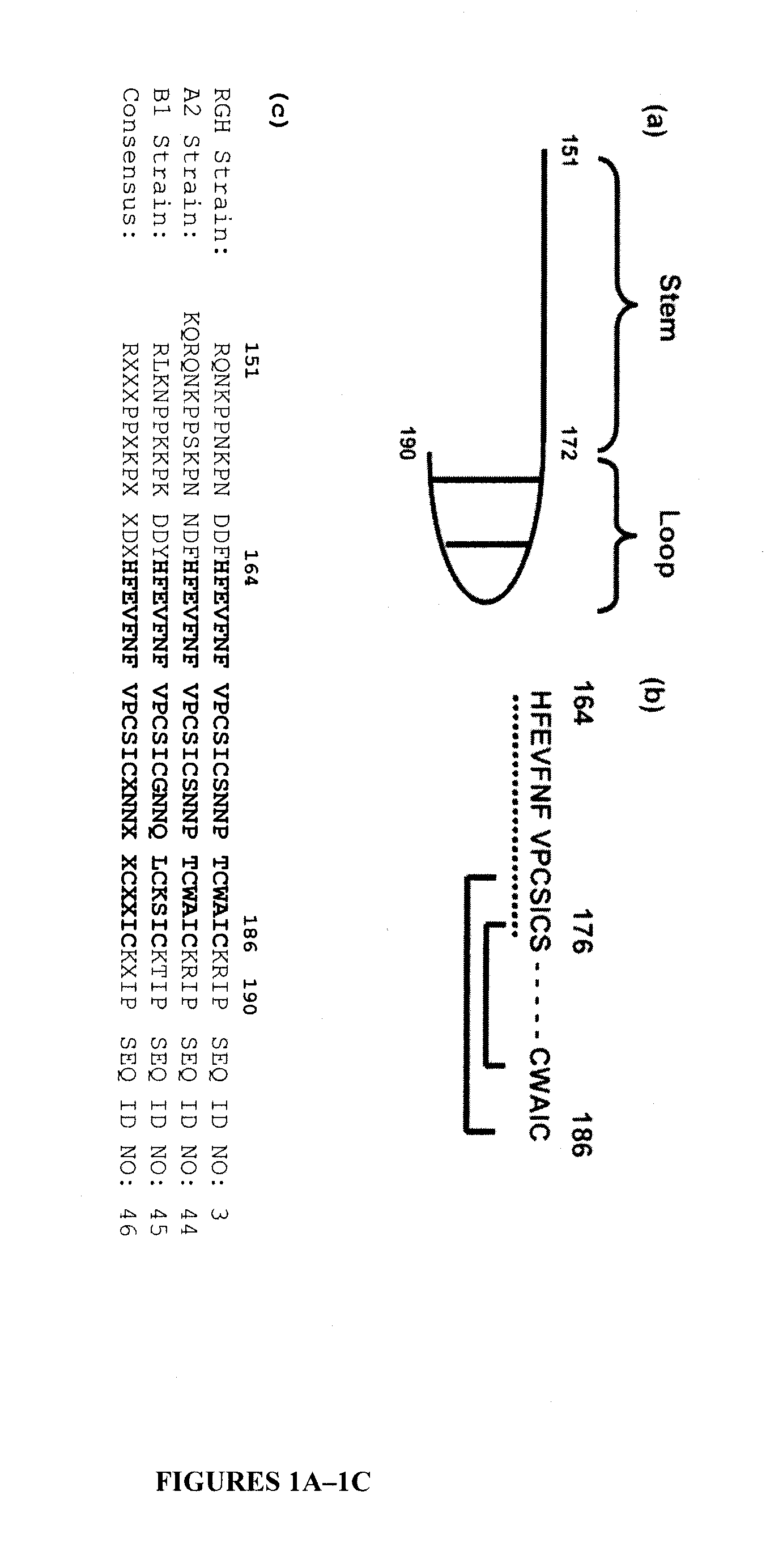
Patents
Literature
146 results about "Neutralizing epitope" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
HIV envelope polypeptides
InactiveUS6042836ANo accumulationReduce capacitySugar derivativesViral antigen ingredientsGeographic regionsHiv envelope
Owner:GENENTECH INC
Functional influenza virus-like particles (VLPs)
Recombinant influenza virus proteins, including influenza capsomers, subviral particles, virus-like particles (VLP), VLP complexes, and / or any portions of thereof, are provided as a vaccine for influenza viruses. The invention is based on the combination of two vaccine technologies: (1) intrinsically safe recombinant vaccine technology, and (2) highly immunogenic, self-assembled protein macromolecules embedded in plasma membranes and comprised of multiple copies of influenza virus structural proteins exhibiting neutralizing epitopes in native conformations. More specifically, this invention relates to the design and production of functional homotypic and heterotypic recombinant influenza virus-like particles (VLPs) comprised of recombinant structural proteins of human influenza virus type A / Sydney / 5 / 94 (H3N2) and / or avian influenza virus type A / Hong Kong / 1073 / 99 (H9N2) in baculovirus-infected insect cells and their application as a vaccine in the prevention of influenza infections and as a laboratory reagent for virus structural studies and clinical diagnostics.
Owner:NOVAVAX
HIV envelope polypeptides
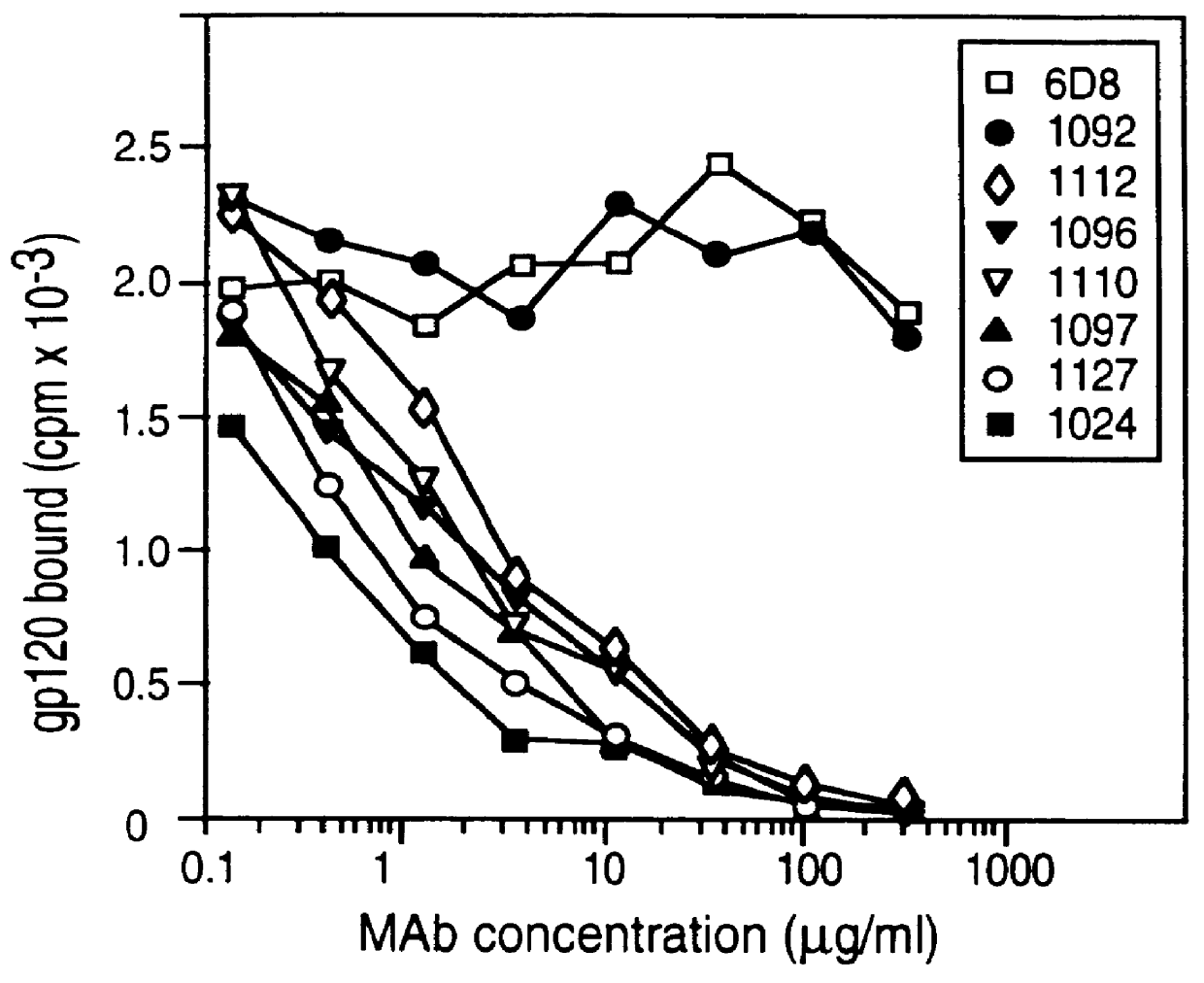
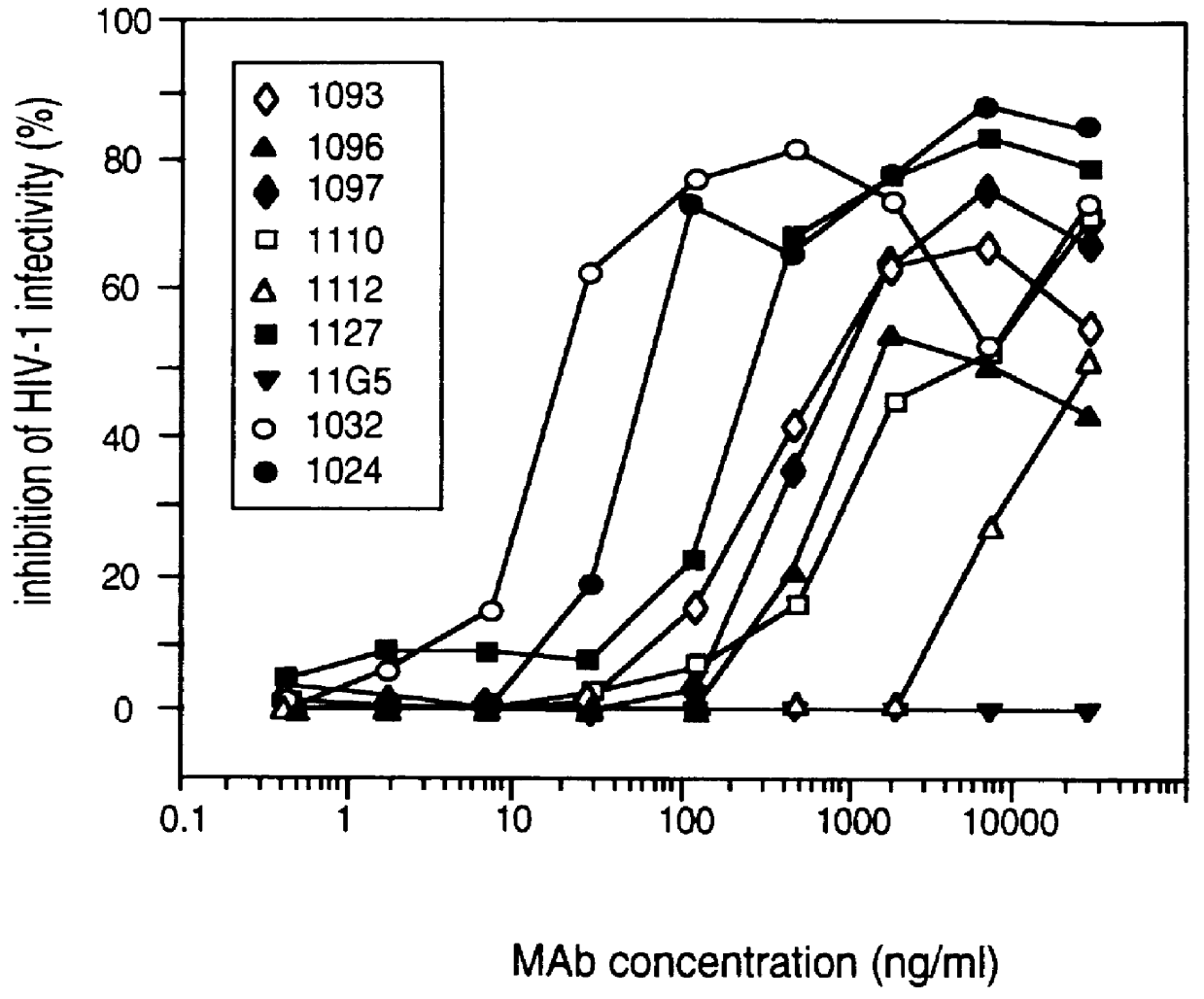
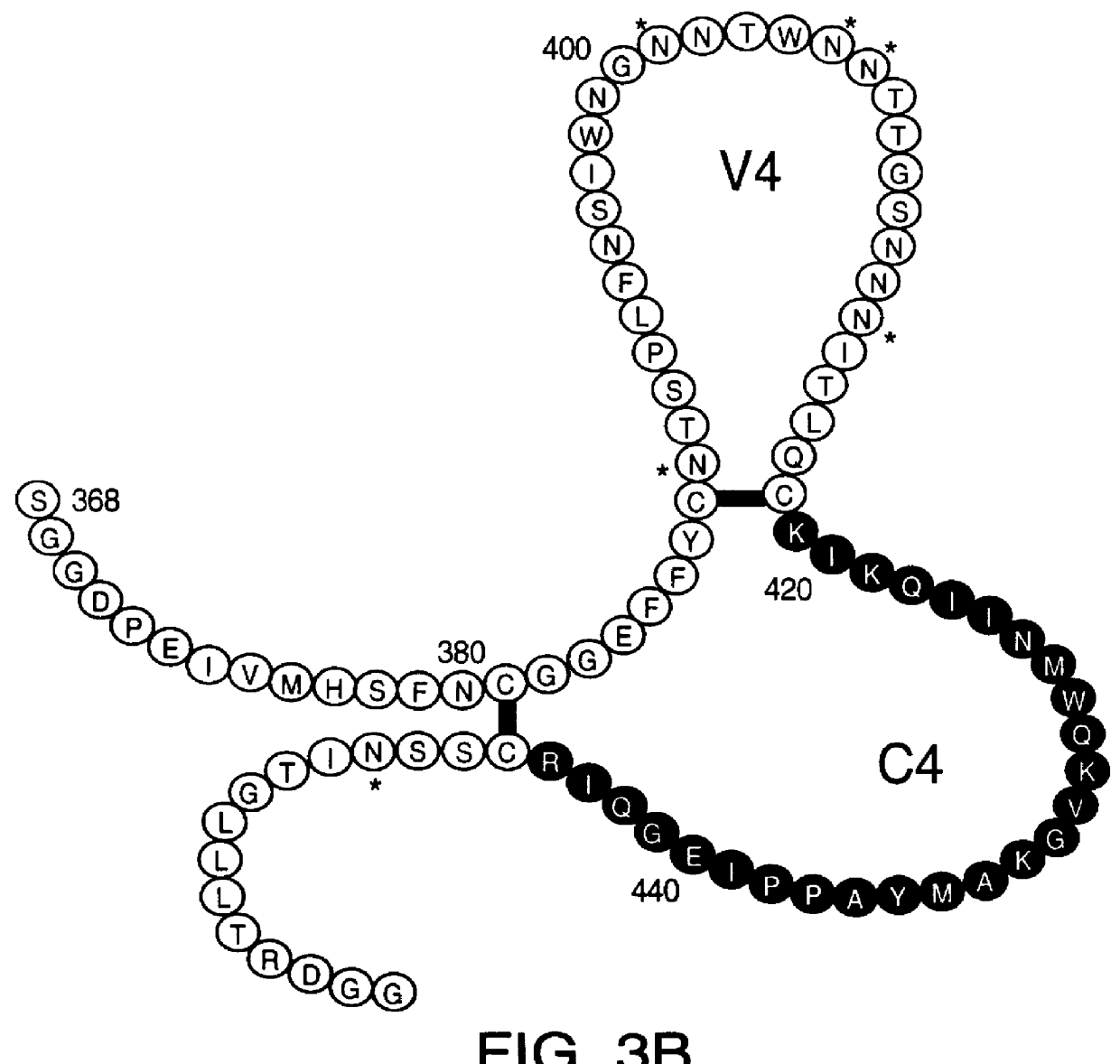
A method for the rational design and preparation of vaccines based on HIV envelope polypeptides is described. In one embodiment, the method for making an HIV gp120 subunit vaccine for a geographic region comprises determining neutralizing epitopes in the V2 and / or C4 domains of gp120 of HIV isolates from the geographic region and selecting an HIV strain having gp120 a neutralizing epitope in the V2 or C4-domain which is common among isolates in the geographic region. In a preferred embodiment of the method, neutralizing epitopes for the V2, V3, and C4 domains of gp120 are determined. At least two HIV isolates having different neutralizing epitopes in the V2, V3, or C4 domain are selected and used to make the vaccine. The invention also provides a multivalent HIV gp120 subunit vaccine. A DNA sequence encoding gp120 from preferred vaccine strains of HIV, GNE8 and GNE16, expression constructs comprising the GNE8-gp120 and GNE16-gp120 encoding DNA under the transcriptional and translational control of a heterologous promoter, and isolated GNE8-gp120 and GNE16-gp120 are also described.
Owner:GENENTECH INC
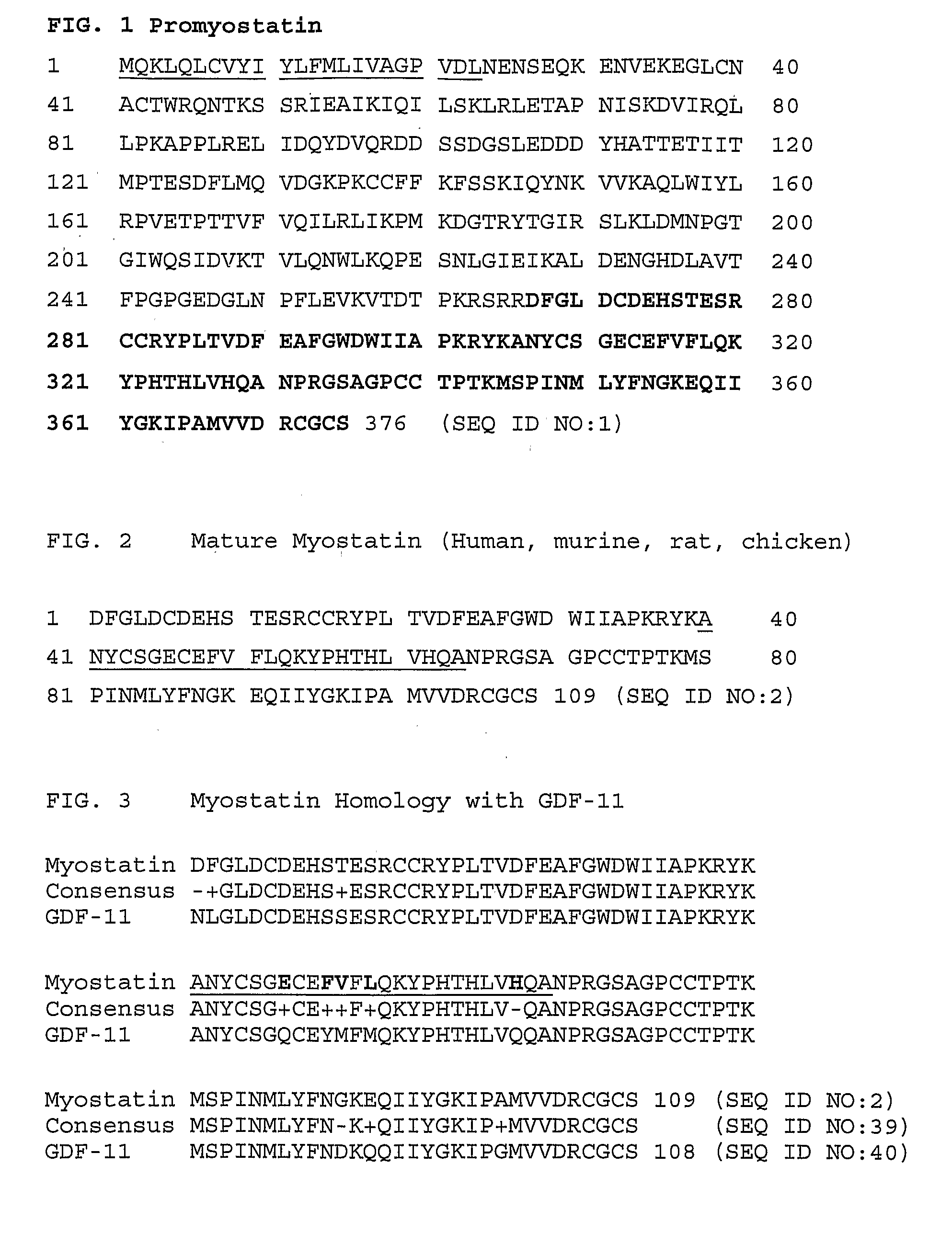
Anti-myostatin antibodies
InactiveUS20070178095A1Inhibitory activityQuality improvementAntibacterial agentsMuscular disorderMyostatinDisease
A neutralizing epitope is identified within amino acids 40-64 of the mature form of human myostatin. Antibodies that bind this epitope fall within the scope of the invention and may be murine, chimeric, or humanized antibodies, immunoconjugates of the antibodies or antigen-binding fragments thereof. The antibodies of the invention are useful for increasing muscle mass, increasing bone density, or for the treatment of various disorders in mammals.
Owner:ELI LILLY & CO
INDUCTION OF BROADLY REACTIVE NEUTRALIZING ANTIBODIES BY FOCUSING THE IMMUNE RESPONSE ON V3 EPITOPES OF THE HIV-1 gp120 ENVELOPE
InactiveUS20080279879A1Vigorous Ab responseViral antigen ingredientsAntibody mimetics/scaffoldsHeterologousNeutralizing antibody
Compositions, kits and methods for boosting, or for priming and boosting, high titer broadly neutralizing cross-clade antibody responses focused on single HIV-1 neutralizing epitopes are disclosed. gp120 DNA plasmids comprising HIV env genes are used to prime the antibody response. Primed subjects are immunized with recombinant fusion proteins that comprise a “carrier” protein fusion partner, preferably a truncated form of the MuLV gp70 Env protein, and a desired HIV neutralizing epitopes. Preferred epitopes are epitopes of V3 from one or more HIV clades. Immune sera from such immunized subjects neutralized primary isolates from virus strains heterologous to those from which the immunogens were constructed. Neutralizing activity was primarily due to V3-specific antibodies and cross-clade neutralizing Abs were present. This approach results in more potent and broader neutralizing antibody levels, a result of “immunofocusing” the humoral immune response on neutralizing epitopes such as V3.
Owner:NEW YORK UNIV
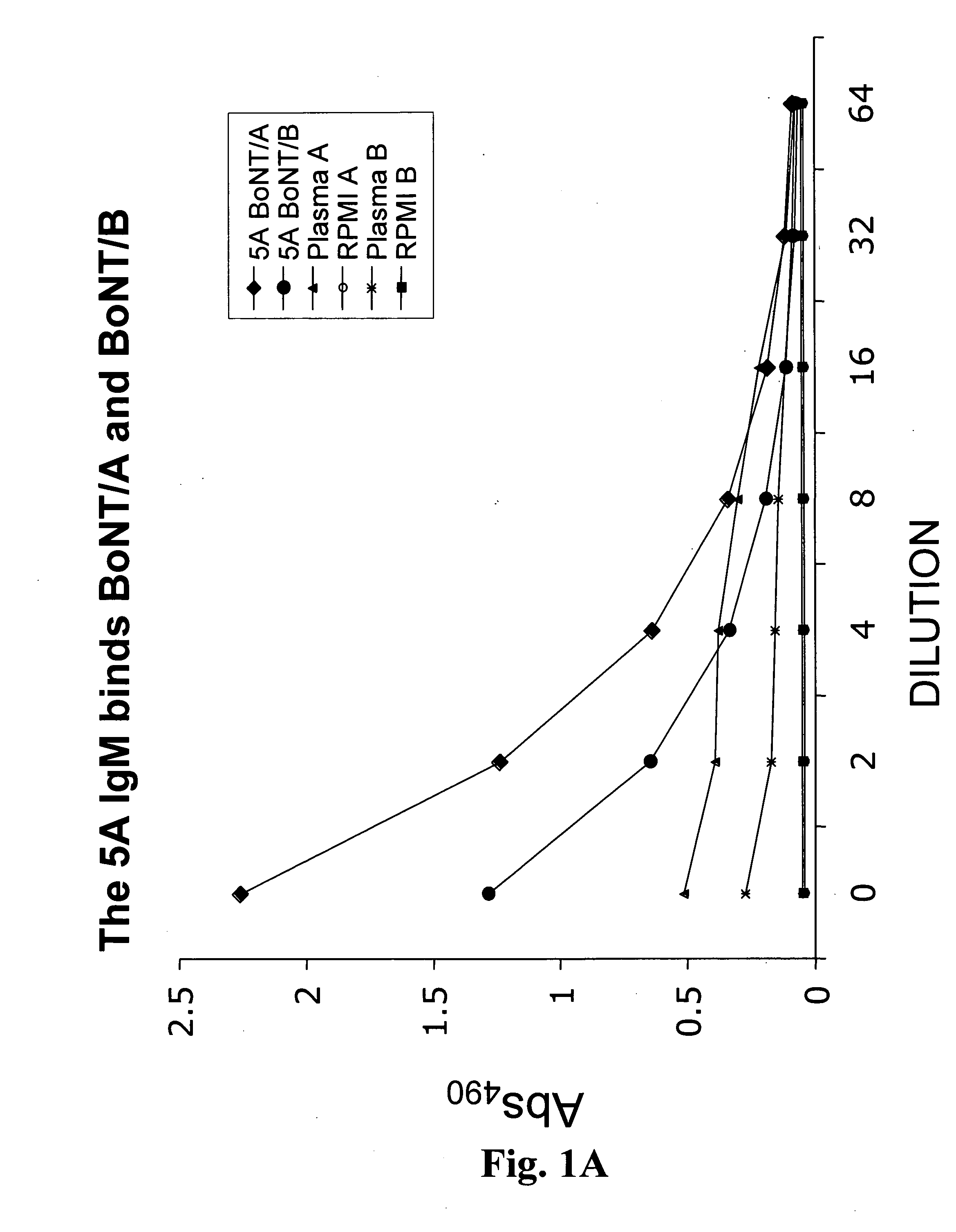
Antibodies for Botulinum Neurotoxins
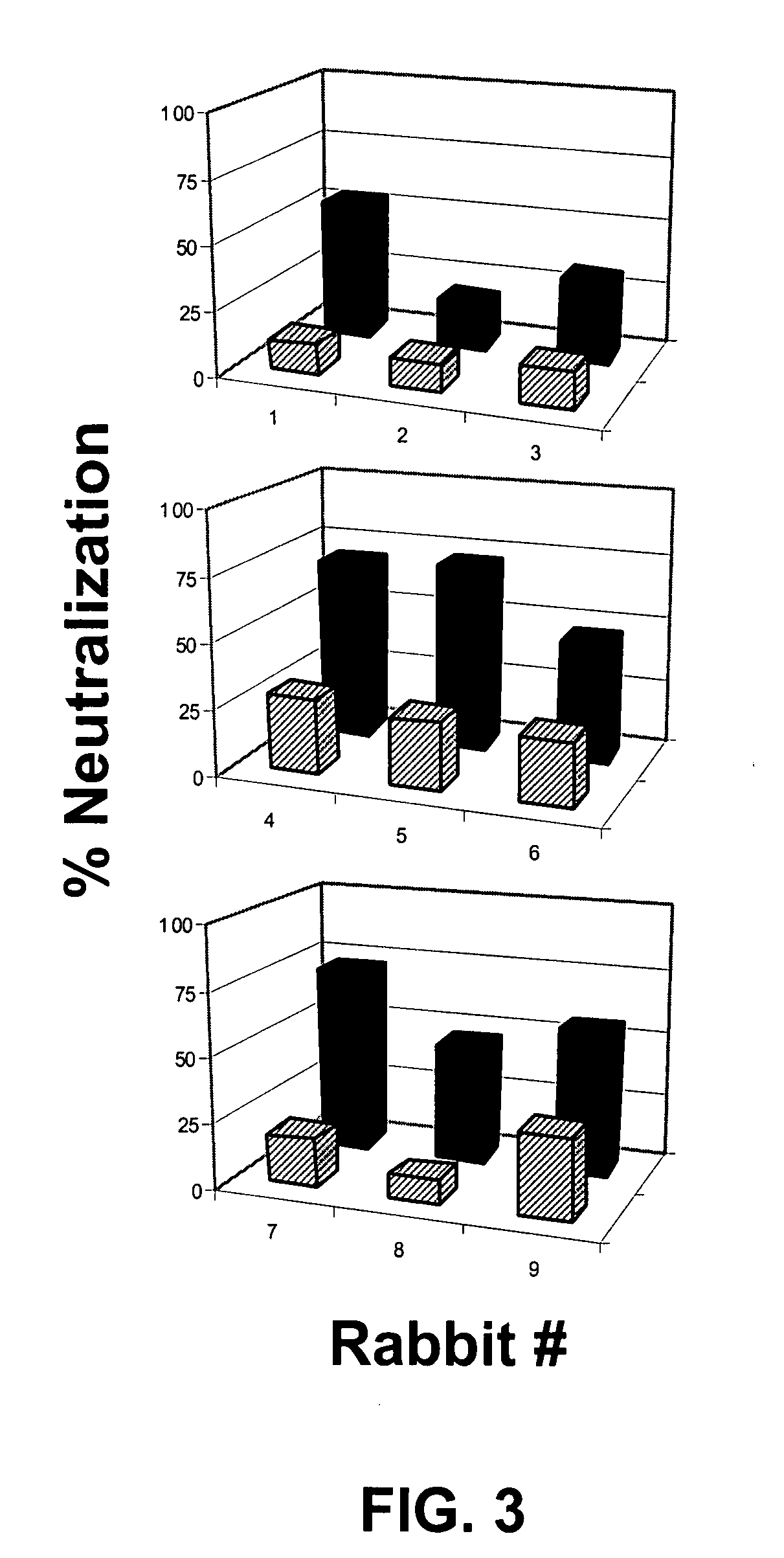
The present disclosure provides antibodies that specifically bind to botulinum neurotoxins (e.g., BoNT / A, BoNT / B, BoNT / C, BoNT / D, BoNT / E, BoNT / F, BoNT / G, etc.) and the epitopes bound by those antibodies. The antibodies and derivatives thereof that specifically bind to the neutralizing epitopes provided herein can be used to neutralize botulinum neurotoxin and are therefore also useful in the treatment of botulism.
Owner:RGT UNIV OF CALIFORNIA
Therapeutic monoclonal antibodies that neutralize botulinum neurotoxins
InactiveUS7563874B2Mitigate and eliminate symptomBiocidePeptide/protein ingredientsMedicineBotulinum Neurotoxin Type A
Owner:RGT UNIV OF CALIFORNIA
In vitro method for disassembly/reassembly of papillomavirus virus-like particles (VLPs)
InactiveUS6261765B1Stabilize VLPsHigh strength conditionNanotechMicroencapsulation basedEpitopeDiagnostic agent
A method of disassembly / reassembly of papillomavirus VLPs is provided. The resultant VLPs have enhanced homogeneity, present conformational, neutralizing PV epitopes, and therefore are useful prophylactic and diagnostic agents. Further, these VLPs can be used to encapsulate desired moieties, e.g., therapeutic or diagnostic agents, or "marker" DNAs, and the resultant VLPs used as in vivo delivery vehicles or as pseudovirions for evaluating vaccine efficacy.
Owner:MEDIMMUNE LLC
Amino acid sequences for therapeutic and prophylactic use against diseases due to clostridium difficile toxins
InactiveUS7151159B2Antibacterial agentsFungiClostridium difficile toxin AClostridium difficile (bacteria)
The invention relates to monoclonal antibodies capable of recognizing and neutralizing epitopes from the ligand domain, the translocation domain or the catalytic domain of the enterotoxin (toxin A) and cytotoxin (toxin B) from Clostridium difficile, as well as their production and therapeutic and prophylactic applications to diseases caused by said toxins.
Owner:VON EICHEL STREIBER CHRISTOPH
Antibodies that Neutralize Botulinum Neurotoxins
This disclosure provides antibodies that specifically bind to and typically neutralize botulinum neurotoxins (e.g., BoNT / A, BoNT / B, BoNT / E, etc.) and the epitopes bound by those antibodies. The antibodies and derivatives thereof and / or other antibodies that specifically bind to the neutralizing epitopes provided herein can be used to neutralize botulinum neurotoxin and are therefore also useful in the treatment of botulism.
Owner:RGT UNIV OF CALIFORNIA
Therapeutic monoclonal antibodies that neutralize botulinum neurotoxins
ActiveUS20100166773A1Low toxicityExtension of timeAntibacterial agentsImmunoglobulins against bacteriaMedicineNeutralizing epitope
This invention provides antibodies that specifically bind to and typically neutralize botulinum neurotoxins (e.g., BoNT / A, BoNT / B, BoNT / E, etc.) and the epitopes bound by those antibodies. The antibodies and derivatives thereof and / or other antibodies that specifically bind to the neutralizing epitopes provided herein can be used to neutralize botulinum neurotoxin and are therefore also useful in the treatment of botulism.
Owner:RGT UNIV OF CALIFORNIA
African swine fever B and T cell tandem epitope fusion vaccine
InactiveCN111018995AGood immune effectAvoid the risk of accelerated viral infectionAntibody mimetics/scaffoldsViral antigen ingredientsAfrican swine feverTGE VACCINE
The invention, which belongs to the technical field of vaccines, particularly relates to an African swine fever B and T cell tandem epitope fusion vaccine. The main component of the African swine fever B and T cell tandem epitope fusion vaccine is African swine fever tandem epitope fusion protein. The African swine fever tandem epitope fusion protein comprises a B cell neutralizing epitope peptidefragment and a T cell epitope; and the B cell neutralizing epitope peptide comprises the following fragments: at least one neutralizing epitope peptide of each of p72, p54, p30 proteins. When the African swine fever tandem epitope fusion protein is used as a vaccine, the immune effect is good; and the antibody level significantly higher than that of a control group can be detected after one immunization. Since the non-neutralizing epitope is reduced as much as possible in the fusion protein, the risk of accelerating virus infection (ADE effect and antibody dependence enhancement effect) by anon-neutralizing antibody after immunization can be avoided.
Owner:河南省生物工程技术研究中心 +1
Neutralizing epitope from varicella-zoster virus (VZV) gE protein and antibody aiming the same
ActiveCN105669838AAvoid infectionInhibition of neutralizing antibodiesVirus peptidesInactivation/attenuationDiseaseChickenpox
The invention relates to a neutralizing epitope peptide (or a variant thereof) from varicella-zoster virus virus (VZV) gE protein, a recombinant protein containing the neutralizing epitope peptide (or the variant thereof) and a carrier protein, and applications of the neutralizing epitope peptide (or a variant thereof) and the recombinant protein, and also relates to an antibody aiming to the neutralizing epitope peptide, a cell strain producing the antibody, and the applications thereof, and also relates to a vaccine containing the neutralizing epitope peptide and the recombinant protein, a medicine composition including the antibody, and the applications thereof, e.g., the application for preventing and / or treating VZV infection or one or more diseases and symptoms related to the infection.
Owner:XIAMEN UNIV +1
Recombinant anti-interleukin-9 antibodies
The application describes neutralizing chimeric and humanized anti-human IL-9 antibodies, and the use thereof to identify neutralizing epitopes on human IL-9 and as medicaments to prevent and treat asthma, bronchial hyperresponsiveness, atopic allergy, and other related disorders. Particularly disclosed are recombinant antibodies derived from three murine anti-human IL-9 antibodies identified infra as MH9A3, MH9D1, and MH9L1.
Owner:LUDWIG INST FOR CANCER RES LTD +1
Neutralizing epitope-based growth enhancing vaccine
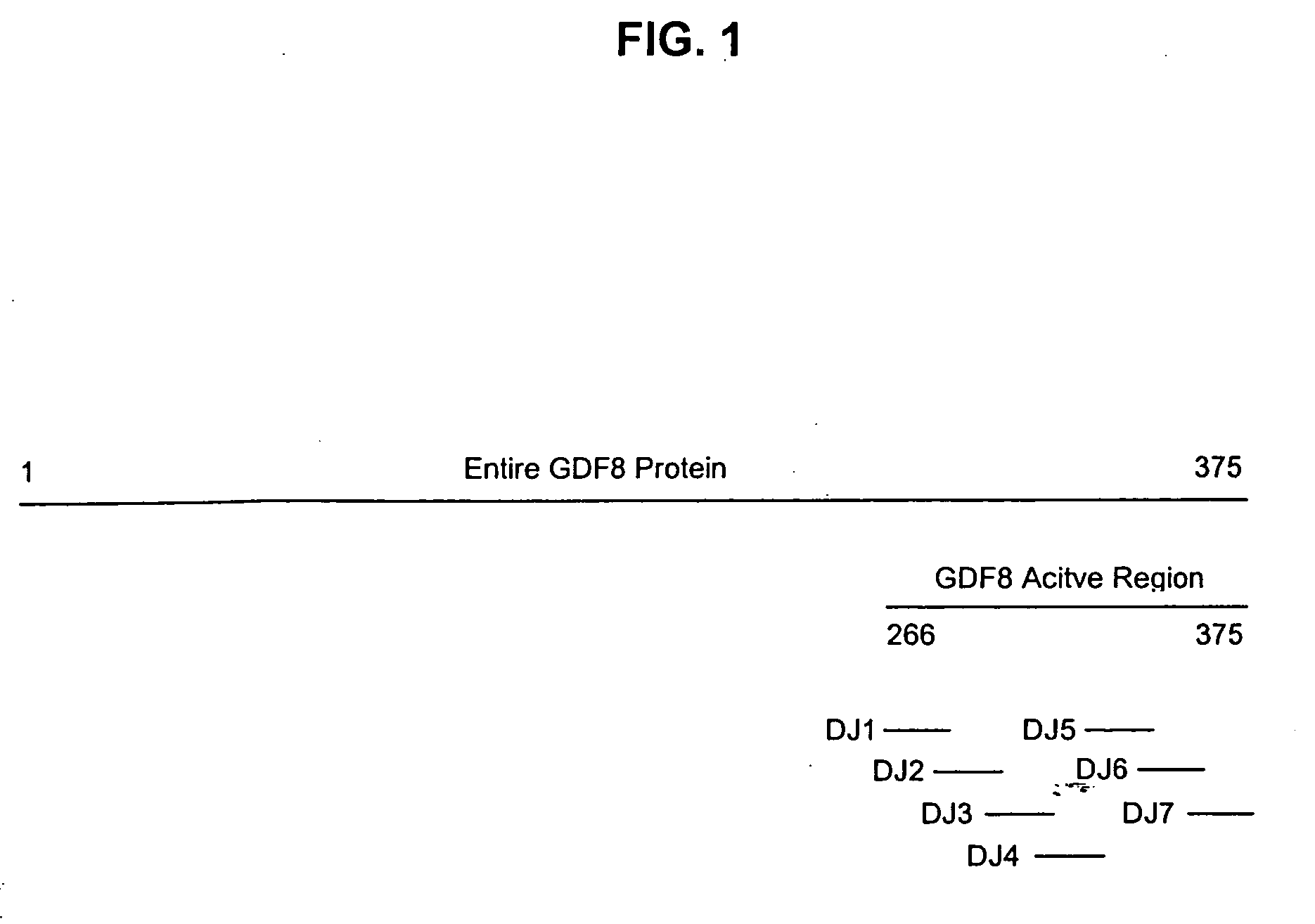
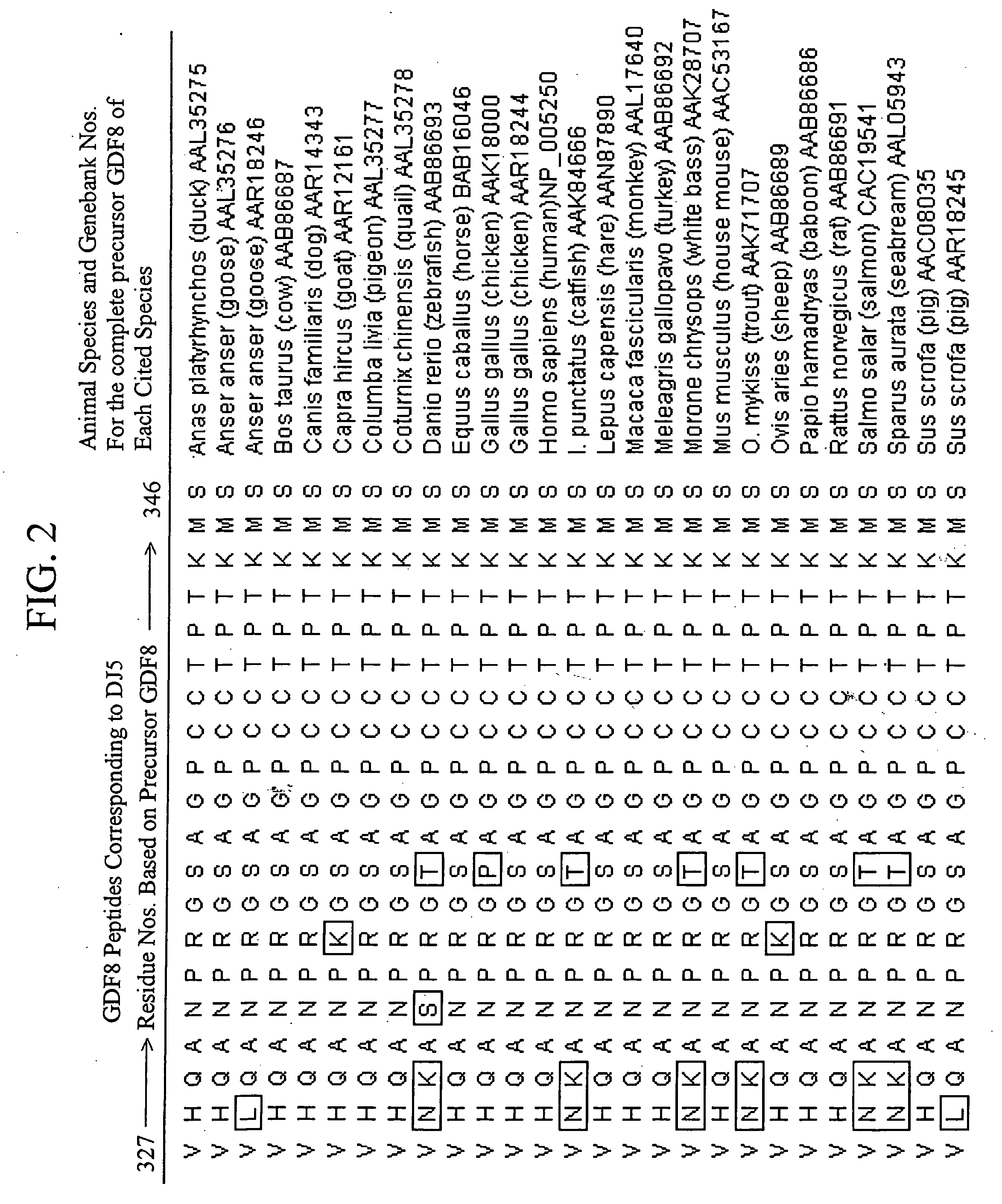
The invention provides new, specific antigenic peptides from the protein GDF8. The invention also provides fusion proteins comprising the new peptides, immunogens and vaccines based on the new peptides and / or fusion proteins, antibodies that specifically bind to the new peptides of GDF8, and methods of treating animals in order to modulate the activity of GDF8, employing vaccines or antibodies according to the invention.
Owner:SCHERING PLOUGH ANIMAL HEALTH +1
Therapeutic monoclonal antibodies that neutralize botulinum neurotoxins
ActiveUS7700738B2Low toxicityExtension of timeAntibacterial agentsAntinoxious agentsAntiendomysial antibodiesBotulinum Neurotoxin Type A
This invention provides antibodies that specifically bind to and neutralize botulinum neurotoxin type A (BoNT / A) and the epitopes bound by those antibodies. The antibodies and derivatives thereof and / or other antibodies that specifically bind to the neutralizing epitopes provided herein can be used to neutralize botulinum neurotoxin and are therefore also useful in the treatment of botulism.
Owner:RGT UNIV OF CALIFORNIA
Monoclonal antibodies that neutralize botulinum neurotoxin
InactiveUS20100222555A1Reduce development riskSugar derivativesImmunoglobulins against bacteriaMedicineBotulinum Neurotoxin Type B
This invention provides antibodies that specifically bind to botulinum neurotoxin type A (BoNT / A) and / or botulinum neurotoxin type B (BoNT / B) and the epitopes bound by those antibodies. The antibodies and derivatives thereof and / or other antibodies that specifically bind to the neutralizing epitopes provided herein can be used to neutralize botulinum neurotoxin and are therefore also useful in the treatment of botulism. Also included in the invention are diagnostic and therapeutic assays directed to botulinum neurotoxins.
Owner:THOMAS JEFFERSON UNIV
Recombinant light chains of botulinum neurotoxins and light chain fusion proteins for use in research and clinical therapy
Botulinum neurotoxins, the most potent of all toxins, induce lethal neuromuscular paralysis by inhibiting exocytosis at the neuromuscular junction. The light chains (LC) of these dichain neurotoxins are a new class of zinc-endopeptidases that specifically cleave the synaptosomal proteins, SNAP-25, VAMP, or syntaxin at discrete sites. The present invention relates to the construction, expression, purification, and use of synthetic or recombinant botulinum neutoroxin genes. For example, a synthetic gene for the LC of the botulinum neurotoxin serotype A (BoNT / A) was constructed and overexpressed in Escherichia coli. The gene product was purified from inclusion bodies. The methods of the invention can provide 1.1 g of the LC per liter of culture. The LC product was stable in solution at 4° C. for at least 6 months. This rBoNT / A LC was proteolytically active, specifically cleaving the Glu-Arg bond in a 17-residue synthetic peptide of SNAP-25, the reported cleavage site of BoNT / A. Its calculated catalytic efficiency kcat / Km was higher than that reported for the native BoNT / A dichain. Treating the rBoNT / A LC with mercuric compounds completely abolished its activity, most probably by modifying the cysteine-164 residue located in the vicinity of the active site. About 70% activity of the LC was restored by adding Zn2+ to a Zn2+-free, apo-LC preparation. The LC was nontoxic to mice and failed to elicit neutralizing epitope(s) when the animals were vaccinated with this protein. In addition, injecting rBoNT / A LC into sea urchin eggs inhibited exocytosis-dependent plasma membrane resealing.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
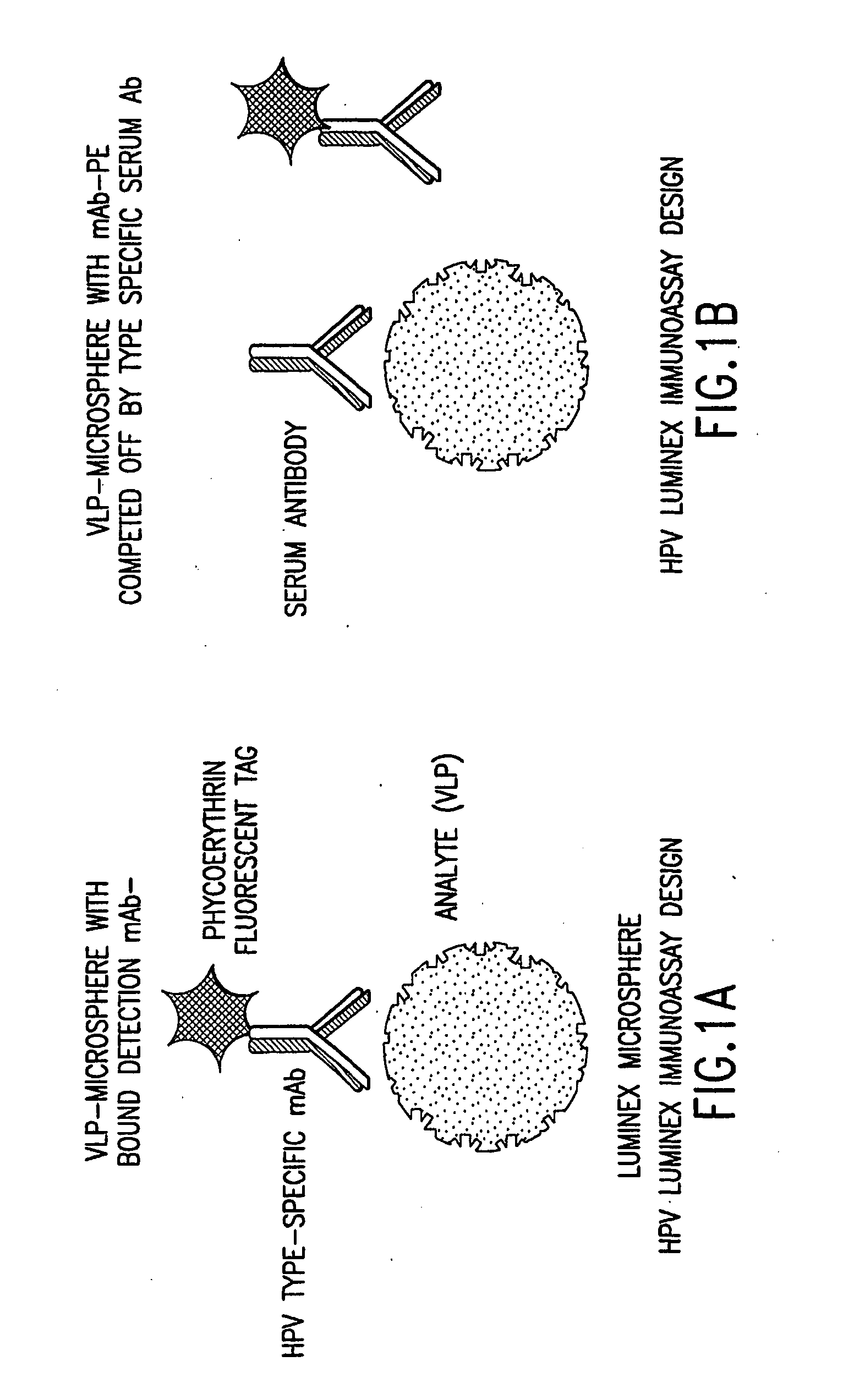
Human papillomavirus multiplexed assay
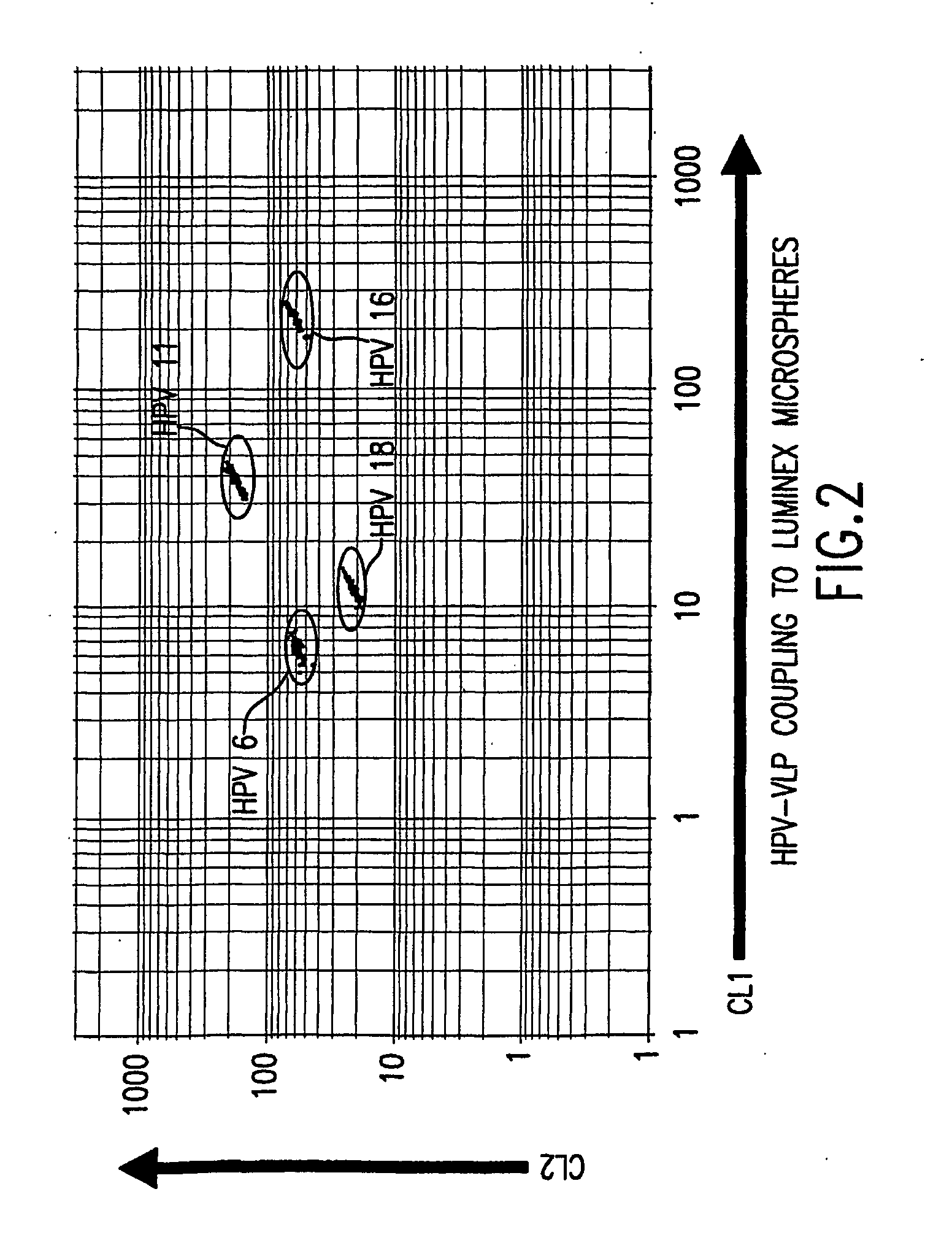
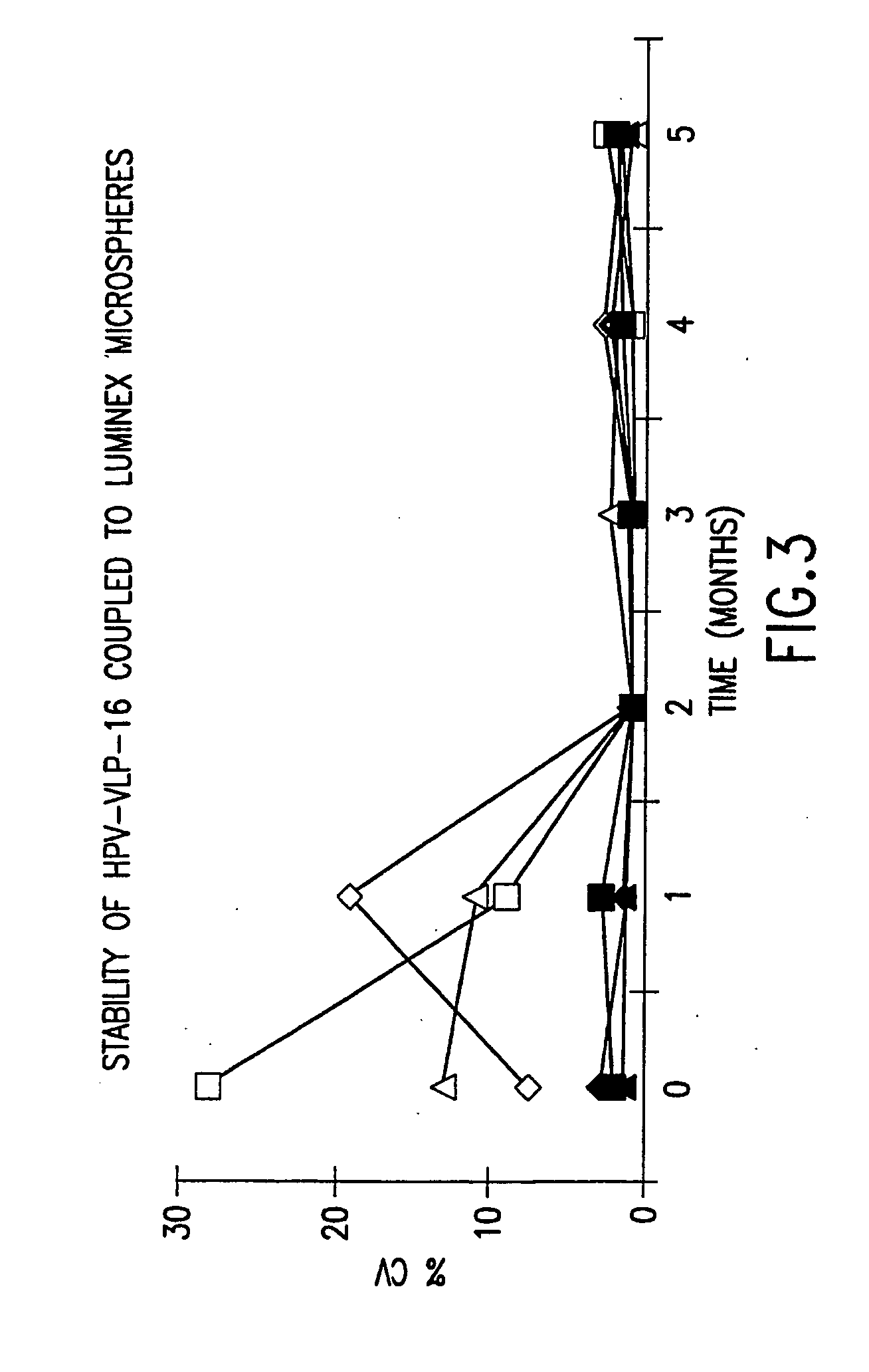
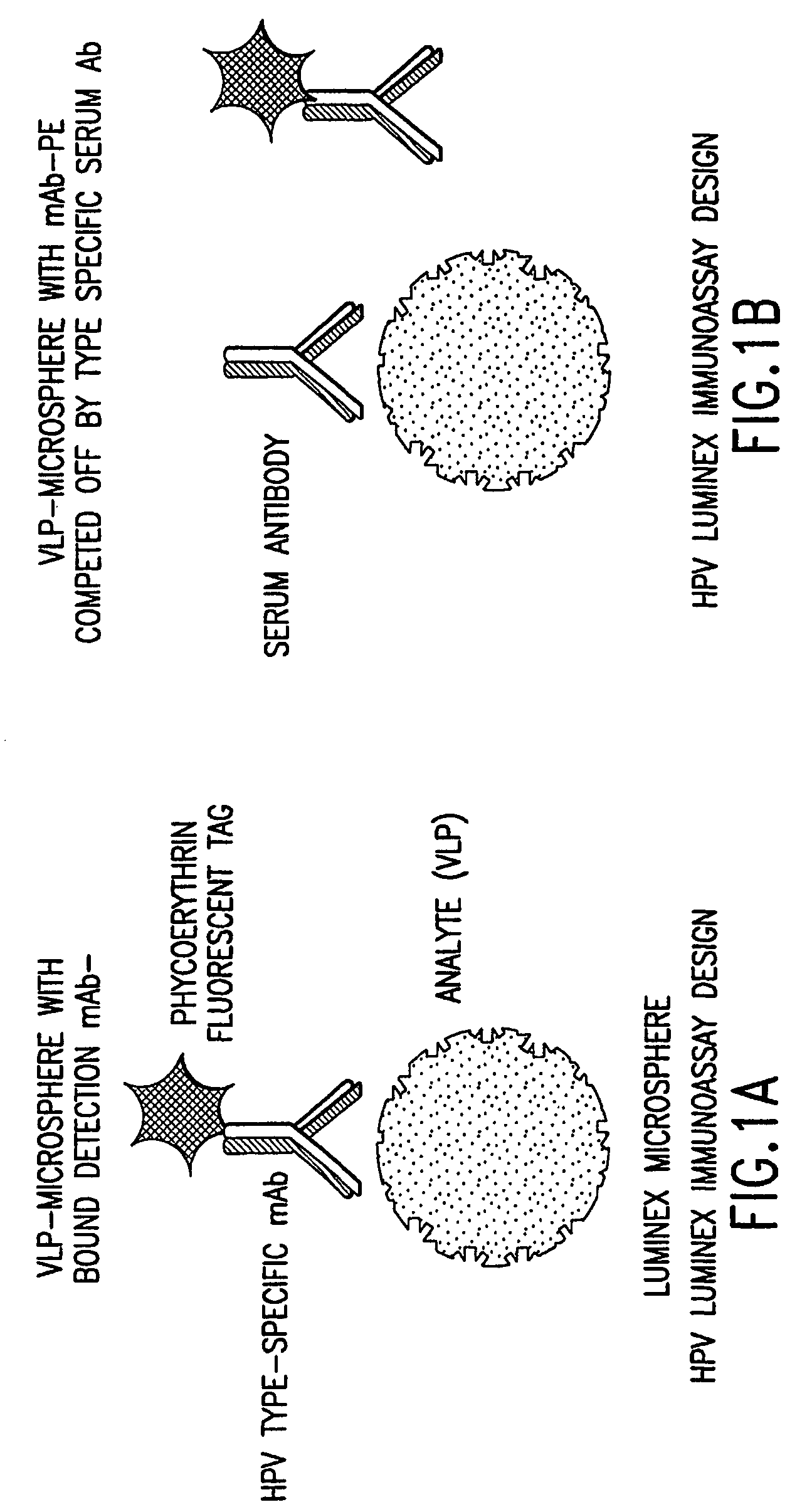
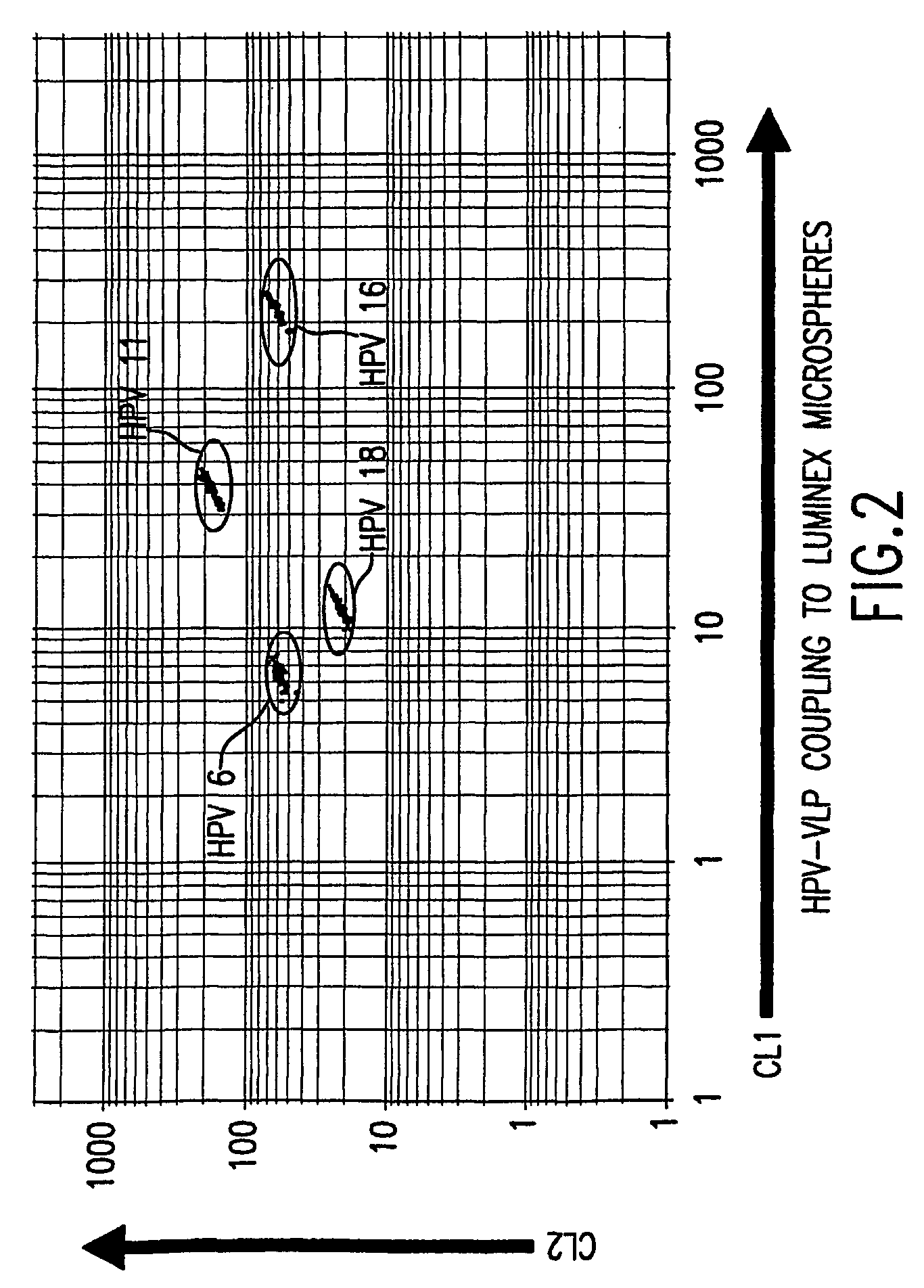
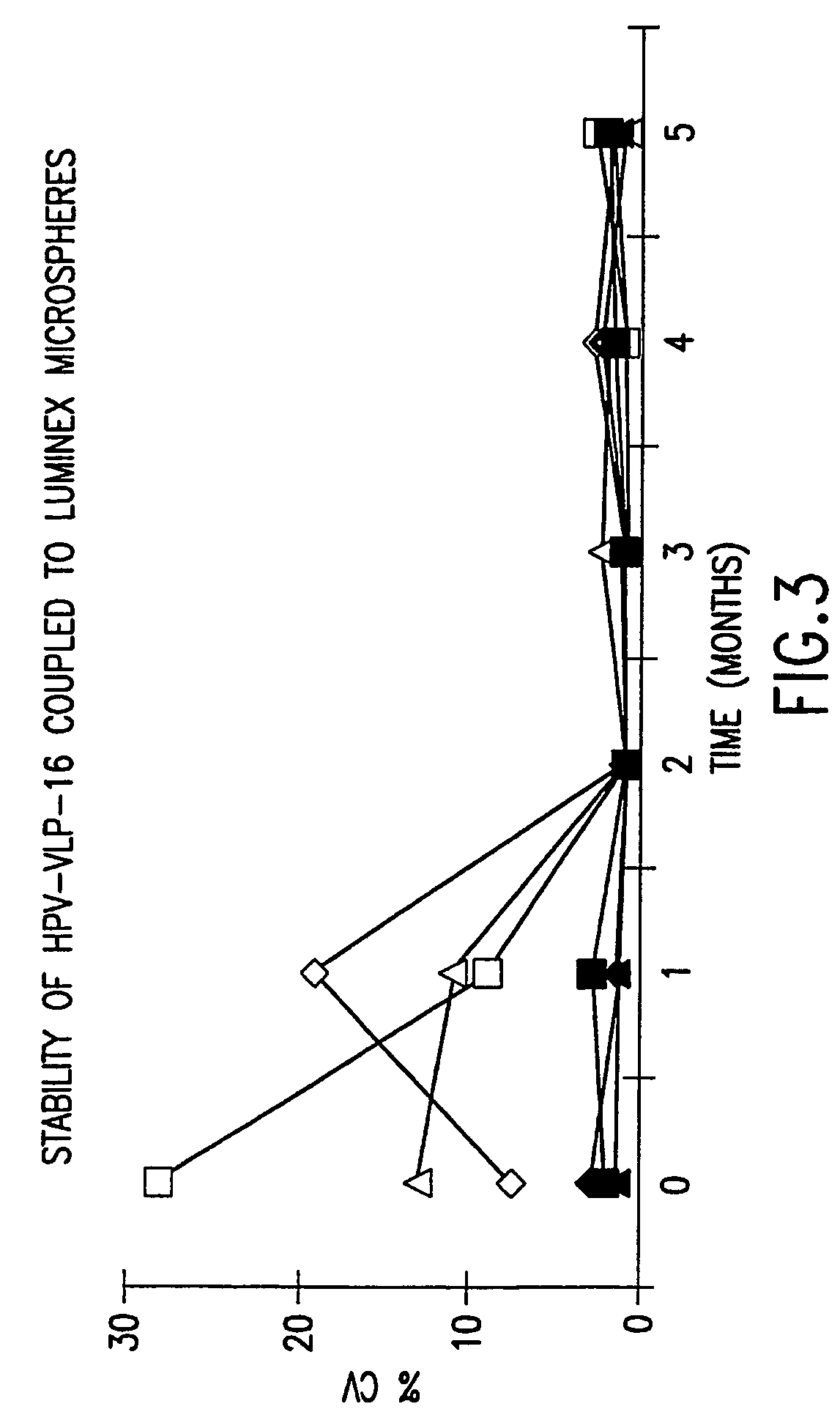
InactiveUS20050147961A1Inducing high titres of neutralizing antibodiesAnimal cellsHybrid immunoglobulinsMicrosphereFluorescence
The present invention relates to an immunoassay for simultaneously measuring the presence of antibodies to a plurlity of HPV types that utilizes particle-based flow cytometric analysis. The presence and / or titre of neutralizing antibodies in a test sample are determined in a competitive format, where known, type-specific, fluorescently labeled neutralizing monoclonal antibodies compete with antibodies within a test sample for binding to conformationally sensitive, neutralizing epitopes on specific HPV-VLPs. The invention also provides a microsphere complex comprising a microsphere coupled to an HPV VLP.
Owner:MERCK SHARP & DOHME CORP
Recombinant anti-interleukin-9 antibodies
The application describes neutralizing chimeric and humanized anti-human IL-9 antibodies, and the use thereof to identify neutralizing epitopes on human IL-9 and as medicaments to prevent and treat asthma, bronchial hyperresponsiveness, atopic allergy, and other related disorders. Particularly disclosed are recombinant antibodies derived from three murine anti-human IL-9 antibodies identified infra as MH9A3, MH9D1, and MH9L1.
Owner:LUDWIG INST FOR CANCER RES +1
Recombinant expression of self-folding neutralizing epitope-bearing subdomains of the respiratory syncytial virus attachment and fusion proteins
The present invention is directed to self-folding, soluble, stable RSV G and F polypeptides that contain a neutralizing epitope. Fusion proteins and immunogenic conjugates containing the RSV G and F polypeptides, along with recombinant transgenes and vectors, and host cells suitable for expression of such genetic constructs are also disclosed. Use of the RSV G and F polypeptides, fusion proteins, immunogenic conjugates, or a pharmaceutical composition containing the same, is contemplated for inducing a protective immune response against RSV.
Owner:UNIVERSITY OF ROCHESTER
Humanized broad-spectrum high-neutralizing-activity monoclonal antibody against novel coronavirus and application
PendingCN113512113AHigh sourceImprove stabilityGenetically modified cellsImmunoglobulins against virusesAntigenTGE VACCINE
The invention discloses a group of humanized broad-spectrum monoclonal neutralizing antibodies for resisting SARS-COV-2 virus, and the neutralizing antibodies are obtained by screening through a single B cell flow sorting-antibody gene amplification pairing expression technology and have a unique CDR region; theneutralizing antibodies can be specifically combined with SARS-COV-2 and can effectively neutralize a plurality of international epidemic virus strains (a novel coronavirus mutant strain A, a novel coronavirus mutant strain B.1. 1.7, a novel coronavirus mutant strain B.1.351, a novel coronavirus mutant strain P.1, a novel coronavirus mutant strain B.1.617.1 and a novel coronavirus mutant strain B.1.617.2) at present, wherein the IC50 is about 0.1 [mu]g / mL. The present invention also relates to methods of preparation and uses of the set of neutralizing antibodies. The three antibodies have the effect of synergistically neutralizing viruses when being used in a pairwise combined manner, so that the combination of the three antibodies can be used for emergency prevention and / or treatment of COVID-19, has the characteristics of full humanization, high expression and good stability, and is suitable for industrialization. In addition, the antibody can also be used for preparing an SARS-COV-2 virus detection reagent, finding effective neutralizing epitopes and developing SARS-COV-2 recombinant protein and subunit vaccines.
Owner:THE FIRST AFFILIATED HOSPITAL ZHEJIANG UNIV COLLEGE OF MEDICINE
Modified pig propagation and respiratory syndrome virus ORF5 gene and use thereof
The invention belongs to the animal gene engineering technology field. It is about a gene and its application, the gene is modificatory porcine reproductive and respiratory syndrome virus ORF5. Its attribute is to insert nucleotide sequence between the neutralization and cover list of GP5, the nucleotide sequence is artificial synthesis coding accessorial T-lymph cell list. The sequence of this nucleotide is just as the sequence list of SEQ ID NO:1 and attached figure 1. This modificatory gene is included in eukaryotic expression plasmid, and the Escherichia coliDH5 / pCI-52M, which includes the plasmid, is conserved in CCTCC, and the number is CCTCC NO: M204080. This invention also presents the use of this gene in the preparation of pig bread and respiratory syndrome DNA vaccineíú
Owner:HUAZHONG AGRI UNIV
African swine fever neutralizing epitope subunit vaccine
PendingCN111018996AImprove securityAvoid the risk of infectionViral antigen ingredientsVirus peptidesAfrican swine feverNeutralising antibody
The invention, which belongs to the technical field of vaccines, particularly relates to an African swine fever neutralizing epitope subunit vaccine that is mainly composed of African swine fever neutralizing epitope fusion protein. The African swine fever neutralizing epitope fusion protein mainly comprises a B cell neutralizing epitope peptide fragment including at least one neutralizing epitopepeptide of p72 protein, at least one neutralizing epitope peptide of p54 protein and at least one neutralizing epitope peptide of p30 protein. With the African swine fever neutralizing epitope fusionprotein, the risk of accelerating virus infection possibly caused by non-neutralizing antibodies can be effectively eliminated and the immune safety of the fusion protein can be effectively improved.Besides, when the African swine fever neutralizing epitope fusion protein is used for immunizing pigs, the antibody level obviously higher than that of a control group can be generated after one immunization.
Owner:河南省生物工程技术研究中心 +1
Foot-and-mouth disease virus (FMDV) resistant monoclonal antibody and identified epitope and application thereof
ActiveCN101724605AGood passive immunityImprove immunityVirus peptidesImmunoglobulins against virusesIn vivoAmino acid
The invention discloses a foot-and-mouth disease virus (FMDV) resistant monoclonal antibody and an identified epitope and application thereof, and belongs to the field of prevention and control of the FMDV. The microbial collection number of a hybridoma cell line, which can secrete the neutralizing monoclonal antibody resisting to Asia-1 FMDV, is CGMCC No.2692; and the microbial collection number of the hybridoma cell line, which can secrete the neutralizing monoclonal antibody resisting to O-type FMDV, is CGMCC No.2691. The invention also discloses amino acid sequences of a conformational neutralizing epitope of the Asia-1 FMDV VP1 protein and a linear neutralizing epitope of the O-type FMDV VP1 protein which are identified by the two monoclonal antibodies respectively. In-vitro neutralization tests and in-vivo animal protection tests show that both the two monoclonal antibodies have excellent passive immunity effect, can be applied to emergency prevention of the FMDV and have excellent immunity effect on the passive immunity of the FMDV.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Antibodies for botulinum neurotoxins
The present disclosure provides antibodies that specifically bind to botulinum neurotoxins (e.g., BoNT / A, BoNT / B, BoNT / C, BoNT / D, BoNT / E, BoNT / F, BoNT / G, etc.) and the epitopes bound by those antibodies. The antibodies and derivatives thereof that specifically bind to the neutralizing epitopes provided herein can be used to neutralize botulinum neurotoxin and are therefore also useful in the treatment of botulism.
Owner:RGT UNIV OF CALIFORNIA
EV71 virus neutralization epitope detection kit or reagent and preparation method thereof
The invention relates to an EV71 virus neutralization epitope detection kit or a reagent and a preparation method thereof. Particularly, the invention performs quantitative detection on EV71 virus antigen by adopting an HD6 monoclonal antibody with spectral activity. The kit or the reagent has the advantages of easy operation, high sensitivity and the like.
Owner:SINOVAC BIOTECH
Therapeutic monoclonal antibodies that neutralize botulinum neurotoxins
This invention provides antibodies that specifically bind to and typically neutralize botulinum neurotoxins (e.g., BoNT / A, BoNT / B, BoNT / E, etc.) and the epitopes bound by those antibodies. The antibodies and derivatives thereof and / or other antibodies that specifically bind to the neutralizing epitopes provided herein can be used to neutralize botulinum neurotoxin and are therefore also useful in the treatment of botulism.
Owner:RGT UNIV OF CALIFORNIA
Human papillomavirus multiplexed assay
InactiveUS7067258B2Inducing high titres of neutralizing antibodiesAnimal cellsMicrobiological testing/measurementHuman papillomavirusType specific
The present invention relates to an immunoassay for simultaneously measuring the presence of antibodies to a plurality of HPV types that utilizes particle-based flow cytometric analysis. The presence and / or titre of neutralizing antibodies in a test sample are determined in a competitive format, where known, type-specific, fluorescently labeled neutralizing monoclonal antibodies compete with antibodies within a test sample for binding to conformationally sensitive, neutralizing epitopes on specific HPV-VLPs. The invention also provides a microsphere complex comprising a microsphere coupled to an HPV VLP.
Owner:MERCK SHARP & DOHME CORP
PEDV S gene major antigen epitope serial connection recombination gene, and preparation method and application thereof
ActiveCN105925597ARapid diagnosisMonitoring and Assessing ProtectionSsRNA viruses positive-senseVirus peptidesAntigen epitopeImmunogenicity
The invention discloses a PEDV S gene major antigen epitope serial connection recombination gene, and a preparation method and an application thereof, and belongs to the field of a PEDV S protein major neutralizing epitope region efficient expression method. The PEDV S gene major antigen epitope serial connection recombination gene is obtained through connecting three major antigen epitopes of a PEDV S gene in series; every two major antigen epitopes are connected through a flexible amino acid base sequence; the name is S123; and the sequence of the base sequence is shown as SEQ ID NO:1. The recombination gene contains most PEDV antigen epitopes; the selected fragments avoid a high-variation region; the expression quantity is efficient and stable; the immunogenicity is high; when the application of PEDV S gene major antigen epitope serial connection recombination protein to a PEDV indirect ELISA detection kit is used, the PEDV can be fast diagnosed; and the swinery neutralization protection can be well monitored and evaluated.
Owner:SOUTH CHINA AGRI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com