Neutralizing epitope from varicella-zoster virus (VZV) gE protein and antibody aiming the same
A protein and antibody technology, applied in the field of molecular virology and immunology, which can solve the problems of insufficient specificity of polyclonal antibodies and unsatisfactory therapeutic effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0170] The invention will now be described with reference to the following examples, which are intended to illustrate the invention, but not to limit it.
[0171] Unless otherwise specified, the molecular biology experiment methods and immunoassay methods used in the present invention are basically with reference to J.Sambrook et al., Molecular Cloning: A Laboratory Manual, 2nd Edition, Cold Spring Harbor Laboratory Press, 1989, and F.M.Ausubel et al., Refinement Molecular Biology Experimental Guide, 3rd Edition, John Wiley & Sons, Inc., 1995 The method described in; the use of restriction endonucleases was in accordance with the conditions recommended by the product manufacturer. The reagents whose sources are not indicated in the examples are all conventional reagents in the art or commercially available reagents. Those skilled in the art understand that the examples describe the present invention by way of example and are not intended to limit the scope of the claimed inven...
Embodiment 1
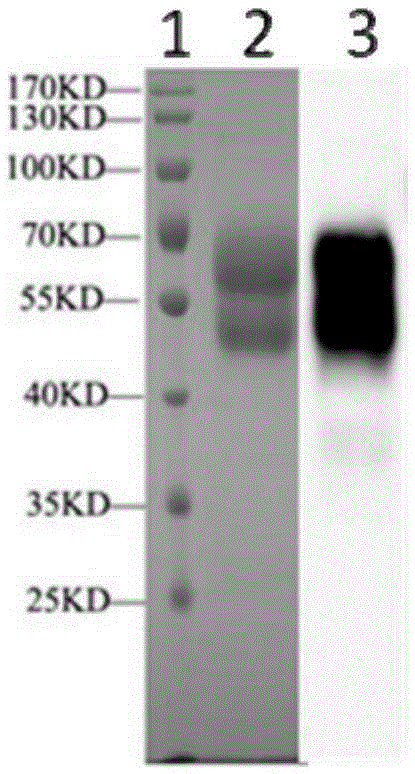
[0172] Embodiment 1: Preparation of recombinant VZV protein rgE
[0173] The invention uses an insect-baculovirus expression system to express and purify the extracellular segment of VZVgE glycoprotein. Specific steps are as follows:
[0174] 1. Construction of recombinant baculovirus
[0175] The present invention uses the p-Oka virus genome as a template, and uses the primers shown in Table 2 to perform PCR, thereby obtaining the gene encoding the gE glycoprotein extracellular segment (1-538aa). The amplified PCR product and pFastBac (pFB) vector were subjected to double enzyme digestion with BamHI and HindIII (both purchased from Japan TAKARA Company), respectively, and then the digested PCR product and vector were recovered and ligated. The ligation product was used to transform DH5α competent strains, and then the clones were selected for PCR, enzyme digestion and sequencing identification, and a positive clone containing the correct sequence was obtained, which was n...
Embodiment 2
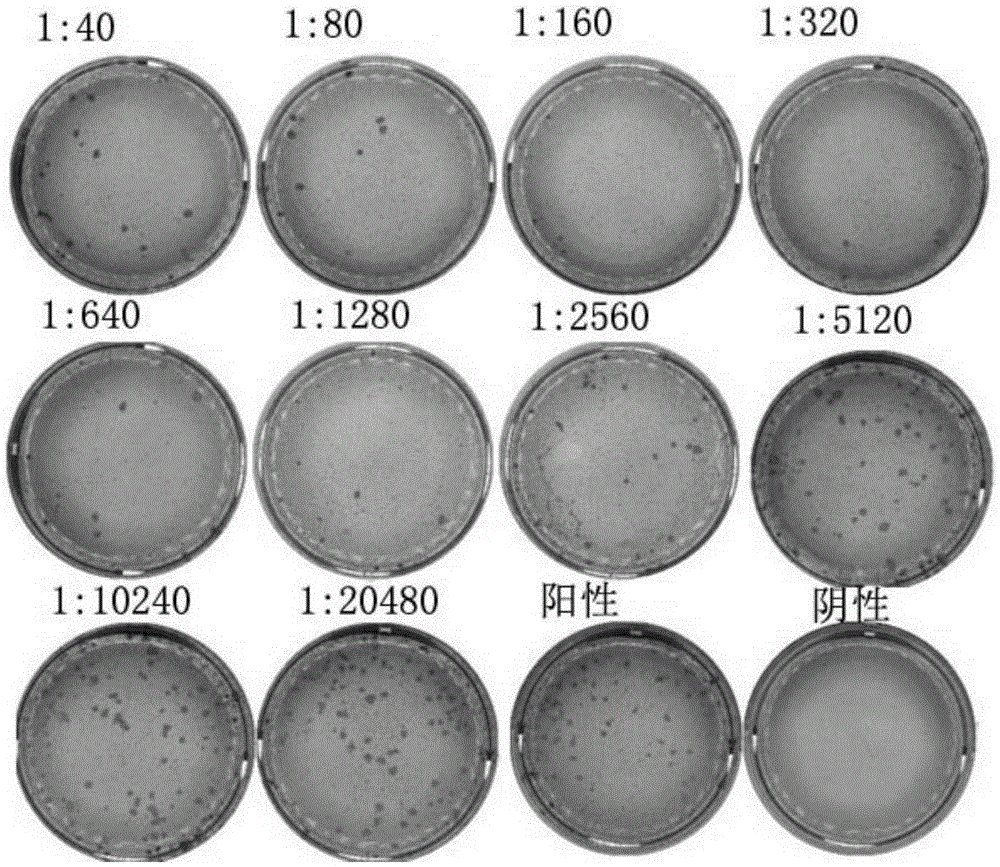
[0183] Embodiment 2: Preparation of anti-VZVgE protein monoclonal antibody
[0184] mouse immunization
[0185] 1. Preparation of immunogen: The recombinant protein rgE (rgE) antigen was obtained by referring to the preparation method of the recombinant protein in Example 1. The 6-week-old BALB / c female mice were immunized with the purified rgE protein, and 5 female mice (numbered: mouse 1 to mouse 5) were immunized with an immunization dose of 100 μg / mouse. The antigen used for primary immunization was mixed with Freund's complete adjuvant (purchased from Sigma). After the initial immunization, booster immunization was performed once every two weeks, and the antigen used in the booster immunization was mixed with Freund's incomplete adjuvant (purchased from Sigma), and the booster immunization was performed three times in total. On day 11 after the third booster immunization, blood was collected. The last booster immunization was carried out 72 hours before the fusion, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com