African swine fever neutralizing epitope subunit vaccine
A subunit vaccine, African swine fever technology, applied in the field of vaccines, can solve the problems of insignificant increase in antibody levels and inability to quickly produce antibodies, and achieve the effects of good immunity, avoiding accelerated virus infection and improving safety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
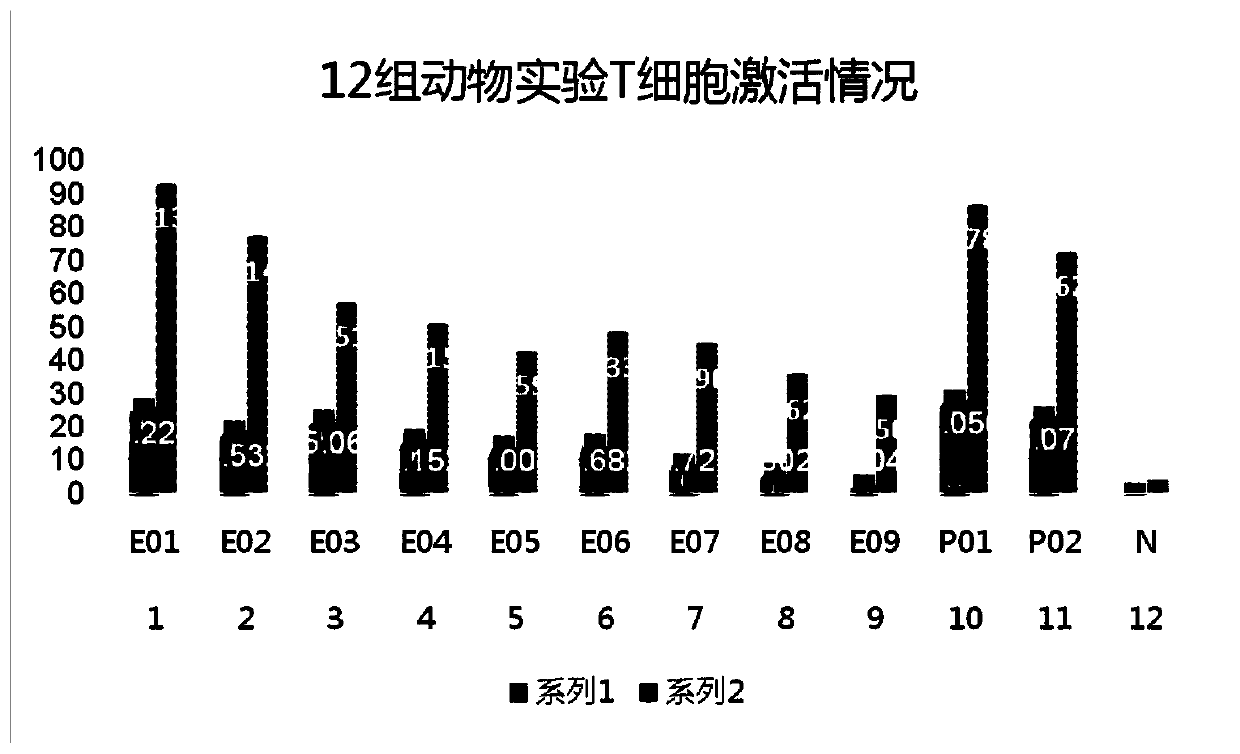
Examples
Embodiment 1
[0038] Example 1. Obtaining neutralizing epitope peptides of p72 protein, p54 protein, p30 protein, CD2v protein, C-type lectin protein, PP62 protein, p17 protein or p12 protein:
[0039] Using http: / / tools.iedb.org / bcell / , http: / / crdd.osdd.net / raghava / abcpred / ABC_submission.html, SYFPEITHI and BIMAS, etc., combined with existing literature analysis and design, the obtained African swine fever virus The potential epitopes of neutralizing antibodies are as follows:
[0040] 1.1 p72 protein neutralizing epitope peptide:
[0041] SEQ ID NO. 1: MASGGAFCLIANDGKADKI;
[0042] SEQ ID NO. 2: NVNKSYGKPDPEPTLSQIEETHLVHFNAHFKPYVPVGFEYNKVRPHTGTPTLGNKLTFGIPQYGDFFHD;
[0043]SEQ ID NO. 3: HSSWQDAPIQGTSQMGAHGQLQTFPRNGYDWDNQTPLEGAVYTLVDPFGRPIVPGTKNAYRNLVYYCEYPGERL;
[0044] SEQ ID NO. 4: VSVEGTSGPLLCNIHDLHKPHQSKPILTDENDTQRTCSHTNPKFLSQHFPENSHNIQTAGKQDITPITDA;
[0045] SEQ ID NO.5: RRNIRFKPWFIPGVINEISLTNNELYINNLFVTPEIHNLFVKRVRFSLIRVHKTQ;
[0046] 1.2 p30 protein neutralizing epitope peptid...
Embodiment 2
[0078] Example 2. The specific amino acid sequence of the African swine fever neutralizing epitope fusion protein of the present invention is exemplified:
[0079] 2.1 When explaining the African swine fever neutralizing epitope fusion protein, the sequence number representing the amino acid sequence of the neutralizing epitope peptide is used for characterization, and the connecting arm is indicated in bold, NP 147-155 The amino acid sequence of TYQRTRAV (in italics) and the immunoactive peptide tuftsin are used TKPR (underlined) indicates that different constituents are connected by connecting arms and "-".
[0080] For example: (1) The amino acid sequence of the African swine fever neutralizing epitope fusion protein represented by SEQ ID NO.1-KK-SEQ ID NO.5-KK-SEQ ID NO.10-GSSSSGSSSG is: MASGGAFCLIANDGKADKI-KK-MEVIFKTDLRSSSQVVFHAG- KK-MDSEFFQPVYPRHYGECLSPVTTPSFFSTHMY-GGGSGGG;
[0081] (2) TKPR The amino acid sequence represented by -KK-SEQ ID NO.6-KK-SEQ ID NO.3-KK-SE...
Embodiment 3
[0115] Example 3 According to the amino acid sequence of the African swine fever neutralizing epitope fusion protein, the method of genetic engineering is used to express and purify the fusion protein
[0116] 3.1 Expression and purification of fusion protein by prokaryotic expression system
[0117] 3.1.1 According to the amino acid sequence of the designed fusion protein (including the purification tag His if necessary), Shanghai Sangong will carry out the synthesis of the nucleic acid molecule encoding the fusion protein and the construction of the vector (according to the amino acid sequence of the fusion protein and the fusion protein encoding the fusion protein). The expression system of nucleic acid molecule application of protein carries out codon optimization, and synthesizes this nucleic acid molecule), prokaryotic expression vector selects pET28a, inserts the nucleic acid molecule of synthesis between NocI and XhoI, and host bacterium selects Bl21(DE)3.
[0118] 3.1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com