Human enterovirus 71 type specific polypeptide and application thereof
A human enterovirus, specific technology, applied in the field of medical immunity, can solve problems such as false positives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Synthesis of a polypeptide
[0043] The method used to synthesize the polypeptides of SEQ ID NO. 1 to SEQ ID NO. 6 is a conventional chemical synthesis method.
[0044] b peptide screening
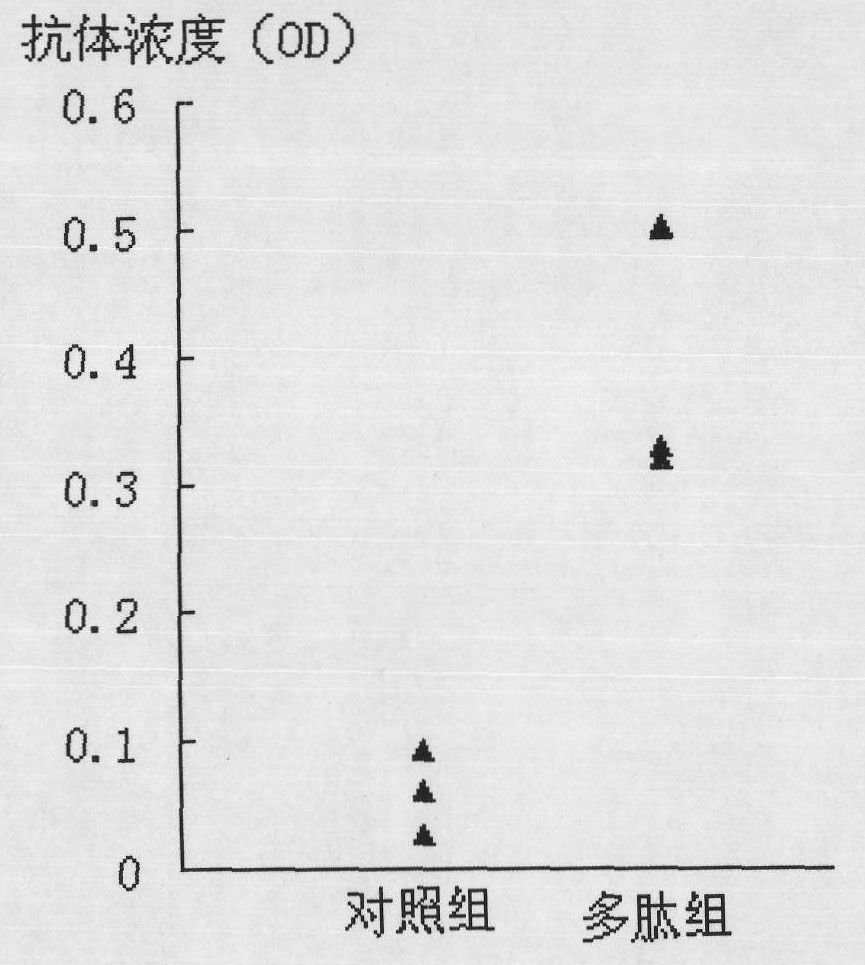
[0045] According to the amount of 10μg of the above polypeptide per well, dissolved in 100μl of coating solution (1.59g Na 2 CO 3 , 2.93g NaHCO 3 ) And coat the ELISA plate for 12 hours, discard the coating solution, wash with deionized water twice, pat dry on absorbent paper each time; add 250μl blocking solution (PBS, 0.05% Tween-20, 5%, skimmed milk powder) placed at room temperature for 2 hours; discard the blocking solution, add serum diluted 1:50 with 100μl blocking solution, the serum used is the mouse antiserum immunized with EV71 VP1 protein, Immune mouse antiserum with PV VP1 protein and unimmunized control serum; leave at room temperature for 1 hour; discard the liquid; wash 5 times with deionized water; add 100μl of blocking solution 1:5000 diluted biotin-labeled anti-mouse Ig...
Embodiment 2
[0048] Example 2 Genetic engineering expression of polypeptide.
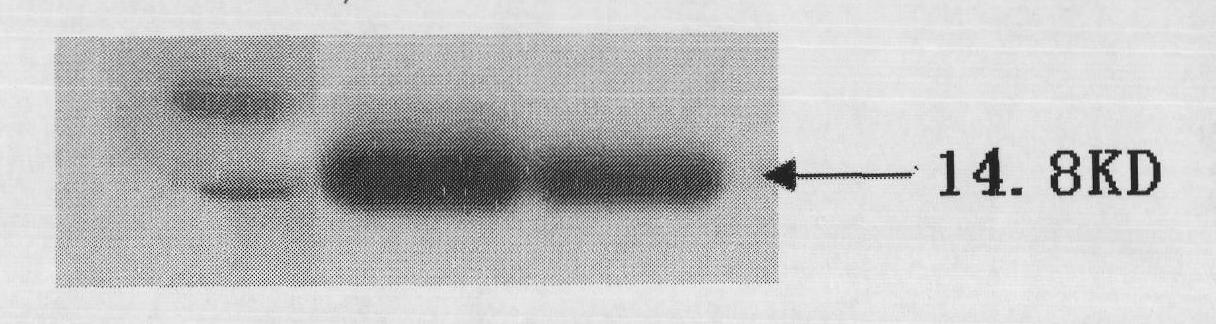
[0049] SEQ ID NO. 8 is the nucleotide sequence encoding the polypeptide 1 of the present invention. The target gene is synthesized with this sequence, and the restriction sites of BamHI and HindIII are introduced respectively at both ends. The gene was inserted into the BamHI and HindIII sites of the vector pQE-80L (Qiajie Biotechnology (Shanghai) Co., Ltd.). The vector pQE-80L contains the gene sequence encoding the polypeptide of SEQ ID NO.7. The recombinant vector capable of accurately expressing the fusion protein of SEQ ID NO1 and SEQ ID NO7 is transformed into E. coli BL21 (DE3), and the obtained genetically engineered strain can be used to express the polypeptide of the present invention.
[0050] The expression conditions are as follows: single clones are selected and inoculated in 10 mL of LB medium, and cultured overnight at 37°C in a shaker. The bacterial solution was inoculated into an auto-induction me...
Embodiment 3
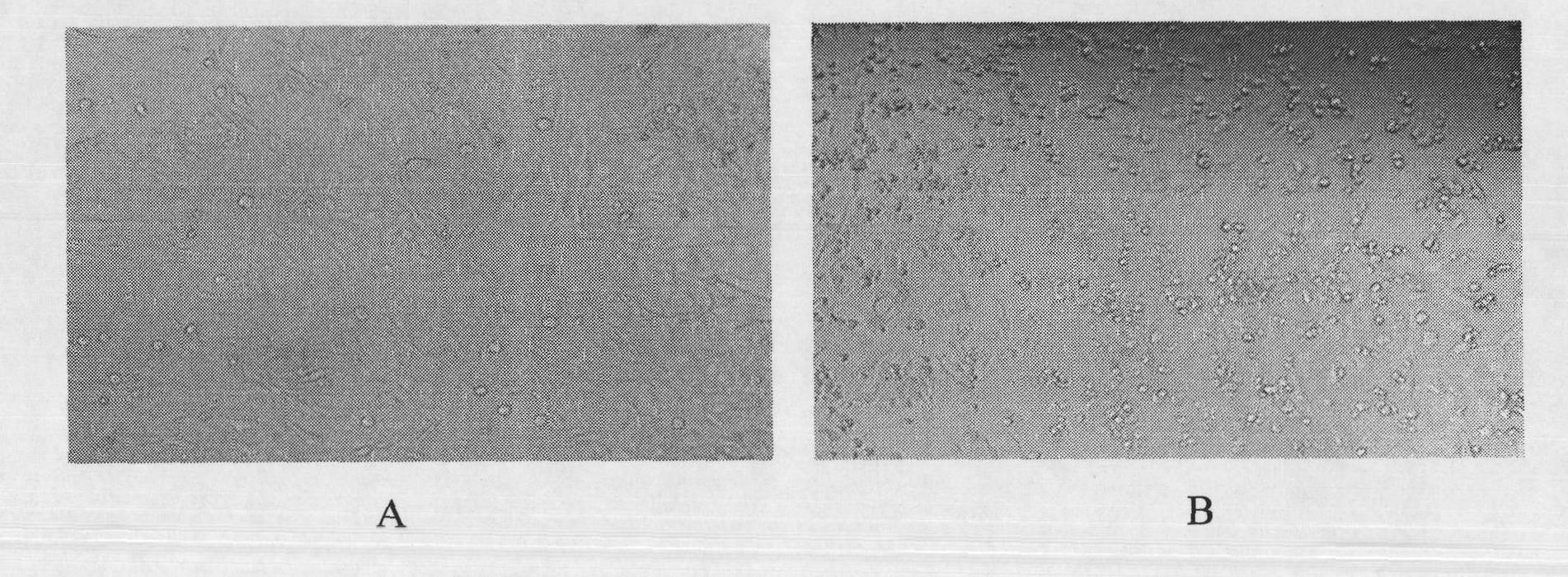
[0053] Polypeptide 1 was dissolved in 100μl coating solution (1.59g Na 2 CO 3 , 2.93gNaHCO 3 ) Coated ELISA plate for 12 hours, discard the coating solution, wash 2 times with deionized water, pat dry on absorbent paper each time; add 250μl blocking solution (PBS, 0.05) to each well %Tween-20, 5%, skimmed milk powder) placed at room temperature for 2 hours; discard the blocking solution, and add serum diluted with 100μl blocking solution 1:50. The serum used is the serum of the patient with EV71 infection and the normal serum. Healthy human serum, pregnant woman serum and control serum (a known healthy human serum); placed at room temperature for 1 hour; discarded the liquid; washed 5 times with deionized water; added 100μl of blocking solution diluted 1:5000 biotin label Anti-human IgM antibody secondary antibody, put it at room temperature for half an hour; discard the liquid, add 100μl of blocking solution 1:5000 diluted AP-labeled streptavidin, place at room temperature for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com