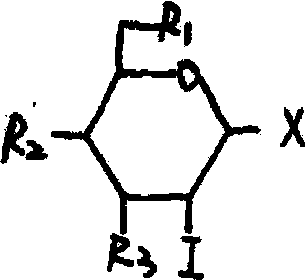
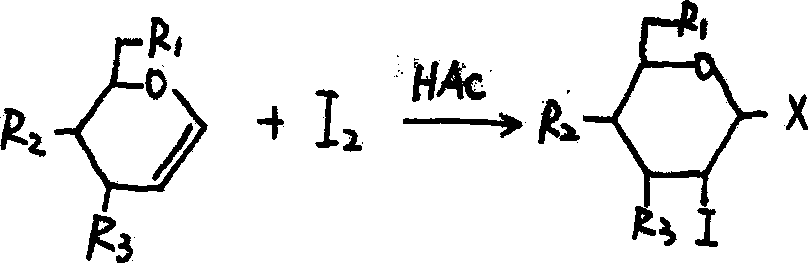Glucose iodine derivative, preparation method thereof and application thereof in pharmacy
A technology of glucose and derivatives, applied in the field of pharmaceutical preparations and sugar iodides, can solve problems such as activity decline, affecting genetic information proteins, cell death, etc., and achieve good clinical application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1 The preparation of 3,4,6-triacetyl-D-glucose iodine
[0034] Disperse 5.45g of 3,4,6-triacetyl-D-glucal, 4.39g of copper sulfate and 6.09g of iodine in 120ml of acetic acid and stir at 80°C for 4 hours. Cool to 25°C and concentrate under reduced pressure to 10ml. The residue was diluted with 250ml of ethyl acetate, and the solution was neutralized to pH=7 with saturated aqueous sodium bicarbonate solution, then washed with 50ml of saturated aqueous sodium thiosulfate solution, and finally washed with water. The organic phase was dried with anhydrous sodium sulfate, then filtered, concentrated under reduced pressure to a volume of 8 ml, and finally crystallized with 30 ml of ethanol at 5°C to finally obtain 7.74 g of 3,4,6-triacetyl-D-glucose iodine.
[0035] product testing:
[0036] 1. Melting point. The melting point is 107°C, which is consistent with the literature value (108°C);
[0037] 2. GC: 98.55%.
[0038] testing method:
[0039] Accurately w...
Embodiment 2
[0046] Example 2 Preparation of 3,4,6-triacetyl-D-glucose iodine spray
[0047] Dissolve 2 g of 3,4,6-triacetyl-D-glucose iodine in 500 ml of ethanol, fully dissolve, then add 200 ml of glycerin and 300 ml of water, mix well and put it into a 30 ml spray bottle to obtain 30 bottles.
Embodiment 3
[0048] Example 3 Preparation of 3,4,6-triacetyl-D-glucose iodine ointment
[0049] Oil phase components: 70g glyceryl stearate, 100g stearic acid, 100g liquid paraffin, 120g white vaseline and water phase components: 1g ethylparaben, 119g glycerin were dissolved in 480ml distilled water, heated to 80°C respectively, Add the melted oil phase components into the water phase components, stir for 30 minutes, cool to 25° C., add 10 g of 3,4,6-triacetyl-D-glucose iodine, and stir evenly to obtain 1000 g of ointment.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com