EV71 subunit vaccine of mixed adjuvant and preparation method thereof
A subunit vaccine and adjuvant technology, applied in the field of biomedicine, can solve the problems of low titer of neutralizing protective antibodies, and achieve the effects of easy large-scale production, low cost and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
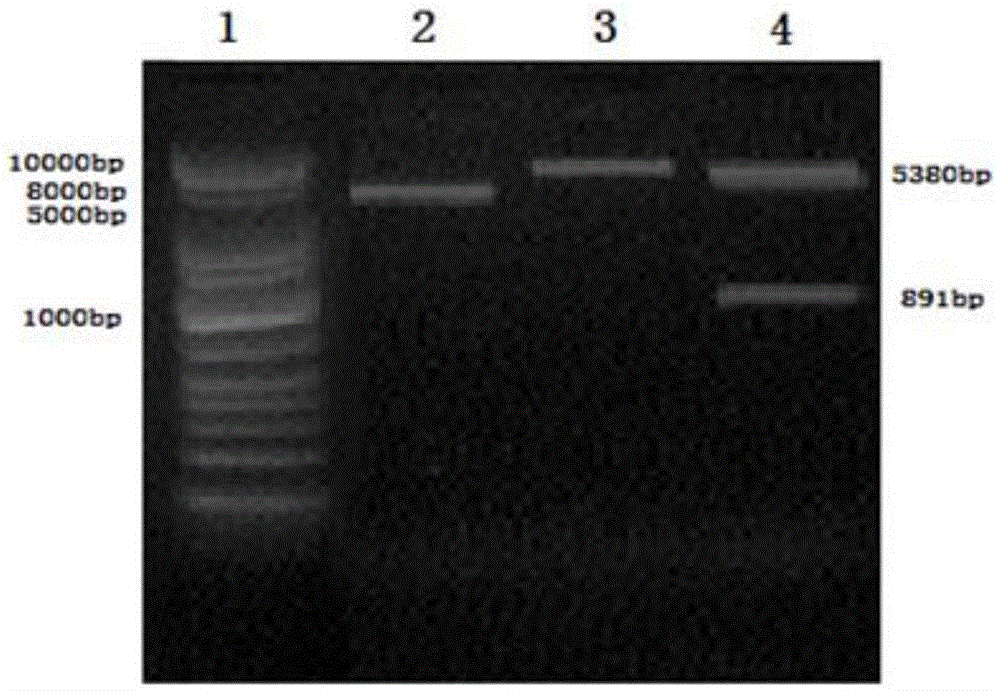
[0045] 1. Synthesis of VP1 gene and construction of recombinant vector pET22b-VP1
[0046] The EV71 virus strain derived from the C4 subtype was obtained from the Pasteur Institute in Shanghai, the viral RNA was extracted with a viral RNA extraction kit (Tiangen Biochemical Technology Co., Ltd.), and the viral cDNA was obtained by reverse transcription with a reverse transcription kit. Design primers to amplify the full sequence of the virus in segments, digest and link the segmented sequences into a complete sequence, and send it to Shanghai Yingjun Biotechnology Co., Ltd. for sequencing. The sequence of VP1 is finally determined as shown in SEQ ID NO.1, and the amino acid sequence is shown in SEQ ID NO.2 , and this full-length cDNA sequence has also repackaged a new infectious virus through in vitro transcription, so it is confirmed that the cDNA sequence is complete and the function is not lost.
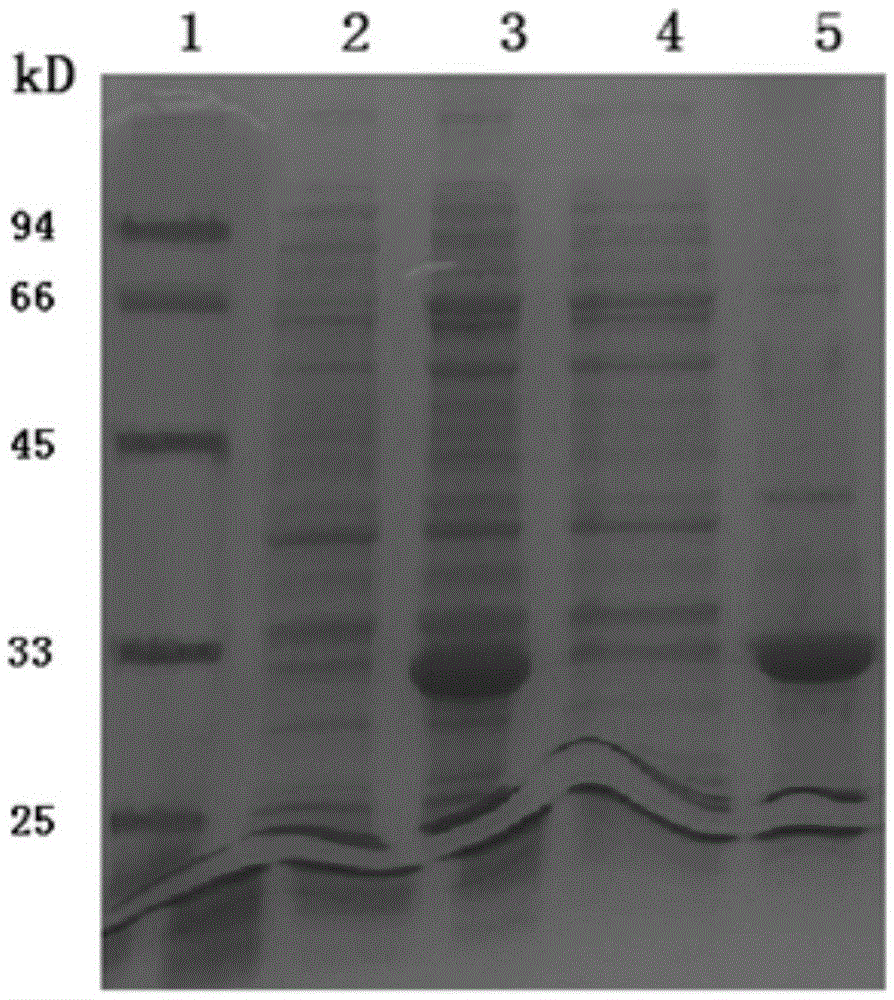
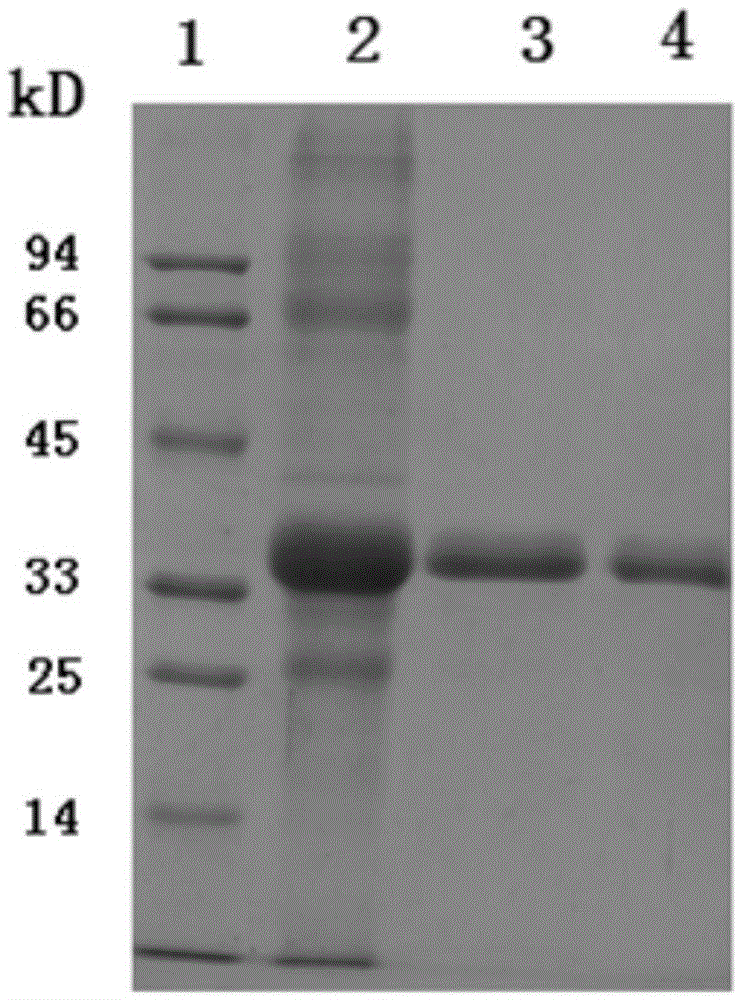
[0047] Based on the VP1 sequence obtained by the above sequencing as a standa...
Embodiment 2
[0071] 1. Mix different immune adjuvants and VP1 antigen protein
[0072] The protein sample collected through the gel filtration chromatography column was first combined with aluminum hydroxide adjuvant, and the solution of the protein sample was (20mmol / LTris-HClpH8.0, 8mol / L urea, 70mmol / Lβ-mercaptoethanol), the protein The concentration is about 0.5mg / ml, the volume is about 200μl, the amount of aluminum hydroxide adjuvant is 200μg, mix and stand at room temperature for 30min, mix with a pipette tip in the middle, centrifuge at 3000rpm, remove the supernatant, and buffer with 10mmol / LpH7.0HEPES The antigenic protein coated with aluminum hydroxide adjuvant was obtained, which was the immunogen ①; then the coated protein was mixed with 20 μg MPLA (Invivogen) adjuvant, and supplemented with 10 mmol / L pH7.0 HEPES buffer to 200 μl , as the immunogen for mice ②; the protein sample purified by gel filtration chromatography was dialyzed and freeze-dried to obtain 100 μg protein sa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com