Bovine rotavirus recombinant VP8 protein and application
A bovine rotavirus and recombinant protein technology, applied in the fields of molecular biology and genetic engineering, can solve the problems of inability to reduce the incidence of virus transmission and high risk of intussusception, achieve good commercial development prospects, improve immune efficacy, The effect of easy purification preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
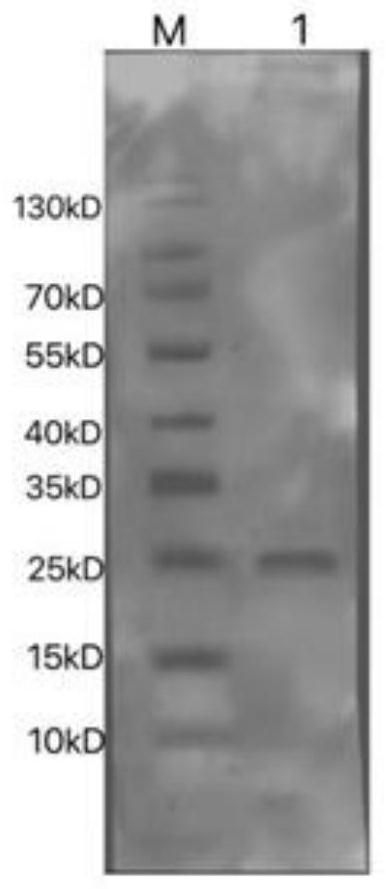
[0036] A method for preparing cow rotavirus recombinant VP8 gene protein, comprising the following steps:
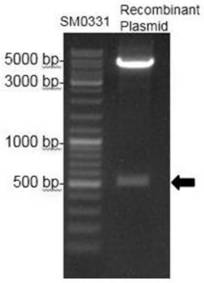
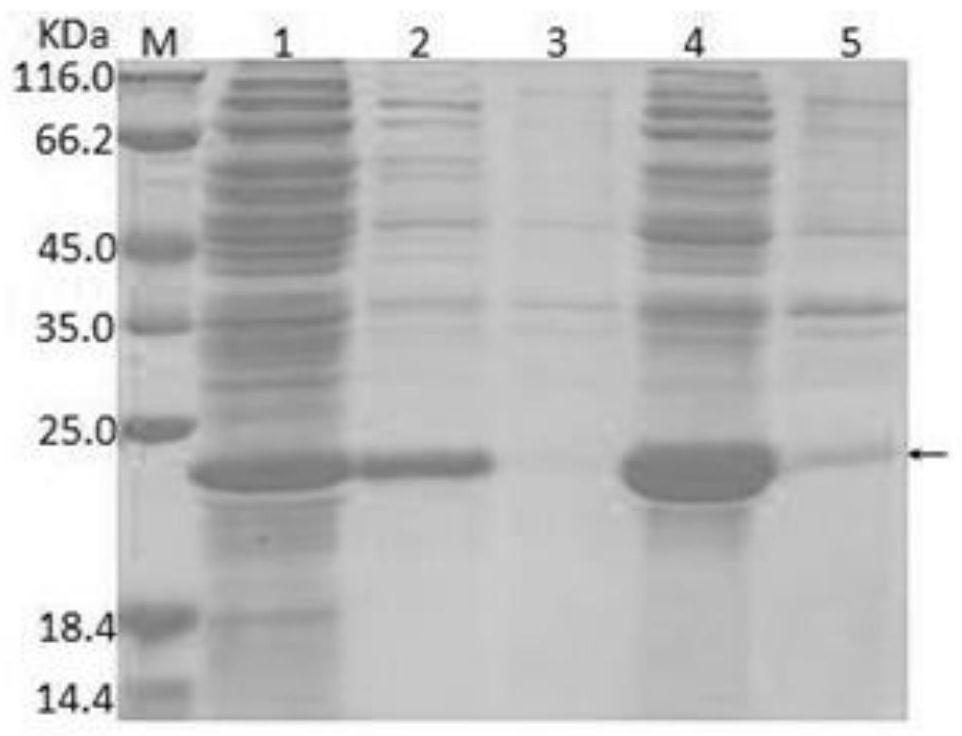
[0037] (1) Amplification of BRV G6P[1]VP8 gene: The molecular epidemiological survey of dairy cows in some areas of my country and yak rotavirus in the Qinghai-Tibet Plateau region showed that SDA2 rotavirus was the dominant genotype of bovine rotavirus . Take an appropriate amount of bovine rotavirus P[1] strain virus nucleic acid-positive fecal samples stored in the Animal Medicine Laboratory of Southwest University for Nationalities, extract total RNA according to conventional methods, reverse-transcribe and synthesize cDNA, and conduct RT-PCR amplification. Primers: F: GATGACACCATATGGAGCCTATCCTGGACG; R: GACACCTCGAGCTGGATCGGCGGCAGGCC. Using biological software to splicing the gene fragments, a complete ORF sequence of VP8 gene was obtained, the size was 501bp, and its amino acid sequence was shown in SEQ ID NO: 1, encoding 170 amino acids, and its nucleic acid sequenc...
Embodiment 2
[0044] Preparation of rotavirus recombinant VP8 protein subunit vaccine
[0045] (1) After taking the purified recombinant VP8 protein in Example 1 and diluting it to 1 mg / mL, it was heated to 31° C. in a water bath with 201 adjuvant simultaneously. After the vaccine was placed at 4°C overnight, the quality of the vaccine was tested.
[0046] (2) Sterility test: The VP8 recombinant protein subunit vaccine was spread on LB medium, and no bacteria grew after culturing at 37°C for 24 hours.
[0047] (3) Dosage form detection; a small amount of VP8 subunit vaccine was dropped into deionized water, the vaccine was evenly mixed with deionized water, and the vaccine was a water-in-oil-in-water recombinant protein subunit vaccine.
[0048] (4) Preliminary stability test: Pipet 1 mL of vaccine into a 1.5 mL centrifuge tube, and centrifuge at 3000 r / min and 4°C for 15 min. The vaccine has no stratification.
[0049] (5) Safety test (animal inoculation method): The VP8 recombinant prot...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com