A pertussis toxin detection kit and its application
A detection kit, a technique for pertussis, which is applied in measurement devices, immunoassays, instruments, etc., can solve the problems of long test period, high cost, and inability to quantify, and achieve the effect of effective immune effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The preparation of embodiment 1 hybridoma cells
[0033] (1) Inoculate pertussis working seeds into fermentation medium after resuscitation and shaking flask culture, culture at 35.0±2.0°C for 44-46 hours, and harvest at the late stage of exponential growth phase.
[0034] (2) After harvesting, it is sterilized, clarified, rough and pure.
[0035] (3) The purified solution is obtained, and the PT stock solution is obtained after glutaraldehyde detoxification.
[0036] (4) Mix and emulsify equal volumes of the vaccine stock solution and Freund's adjuvant (Freund's complete adjuvant for immunization on day 0, incomplete Freund's adjuvant for immunization on day 14 and 28) BALB / c mice were immunized subcutaneously on the back with 0.2ml / mouse.
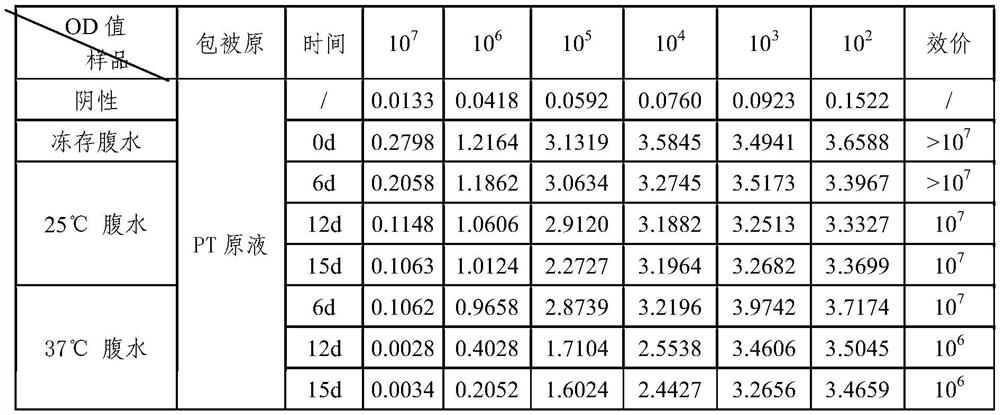
[0037] (5) One week after the last immunization, blood was collected to detect the antibody titer, and the serum was diluted 10 4The OD value of the indirect ELISA of the PT-detox solution immunized mouse serum was 1.126. Two w...
Embodiment 2
[0038] Example 2 Cell Fusion and Strain Construction
[0039] (1) Resuscitate and culture the SP2 / 0 cell line before cell fusion, expand the culture 3 days before fusion, remove RPMI1640 cell culture medium (Gibco) 1 day before fusion, and add culture medium again to prepare SP2 / 0 cells.
[0040] (2) The immunized mice were sacrificed, and the mouse splenocyte suspension was prepared according to conventional methods.
[0041] (3) Add an appropriate amount of incomplete IMDM culture medium (Gibco) according to the counting results of splenocytes and SP2 / 0 cells, shake and mix the SP2 / 0 cells, and pipette the splenocytes evenly. Then the splenocytes and SP2 / 0 cells were mixed in a 50ml centrifuge tube at a ratio of 1:2 to 10:1, and mixed well.
[0042] (4) Add incomplete IMDM culture medium to 50ml, centrifuge for 5-10 minutes, and pour out the supernatant. Lightly tap the bottom of the fusion tube to loosen and evenly precipitate the cells, and place the centrifuge tube in a...
Embodiment 3
[0051] Example 3 Stability Detection of Monoclonal Antibody Secreted by Hybridoma Cell Lines
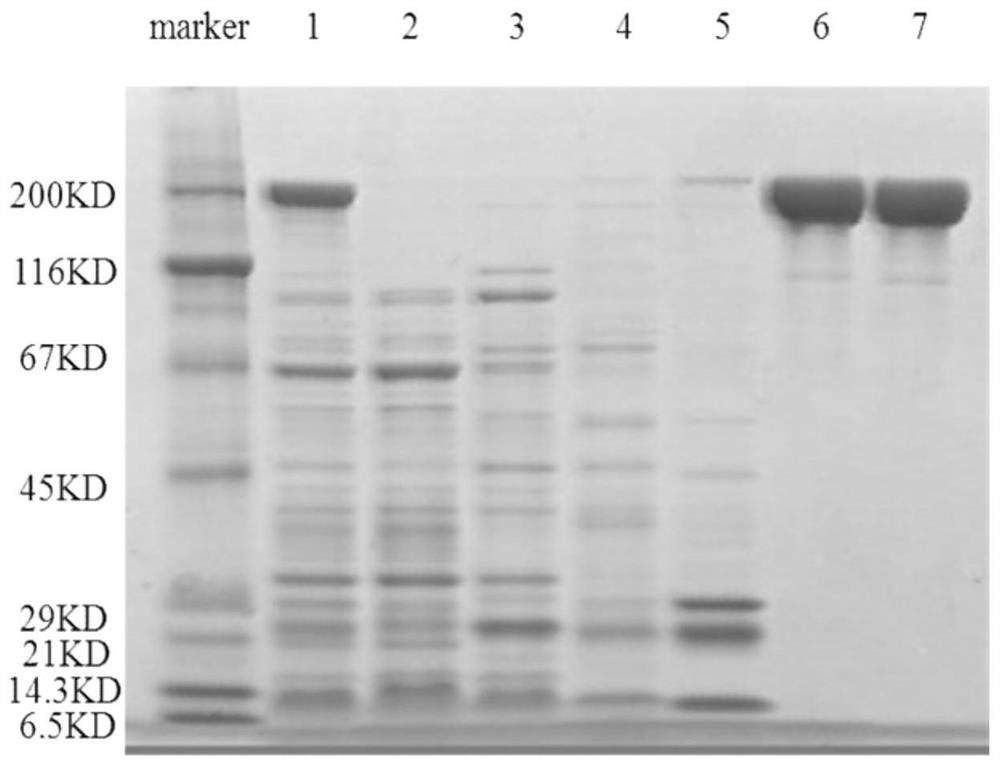
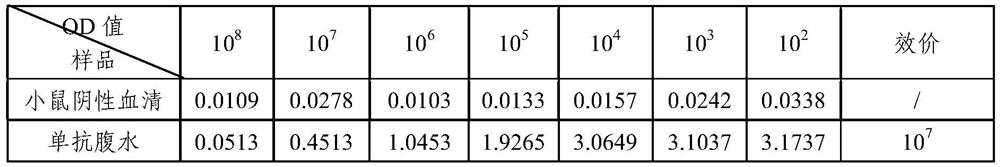
[0052] After the 3rd month and the 9th month after the preparation of the hybridoma cells, the frozen PT mouse monoclonal antibody hybridoma cell line with the preservation number CGMCC No. 15288 was taken out from liquid nitrogen for recovery and expanded culture. The ascites was prepared, and the antibody titer was detected by indirect ELISA, and the ascites prepared in the previous stage was used as a control for detection at the same time. As a result, the monoclonal antibody ascites titer prepared by the hybridoma cell line of the present invention reaches 10 7 Above, there is no difference with the titer of ascites in the previous stage, indicating that the titer of ascites prepared after cell preservation has not decreased. Therefore, the antibody secretion activity of the monoclonal cell line is not reduced, and the stability is good. The monoclonal antibody ascitic fluid p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com