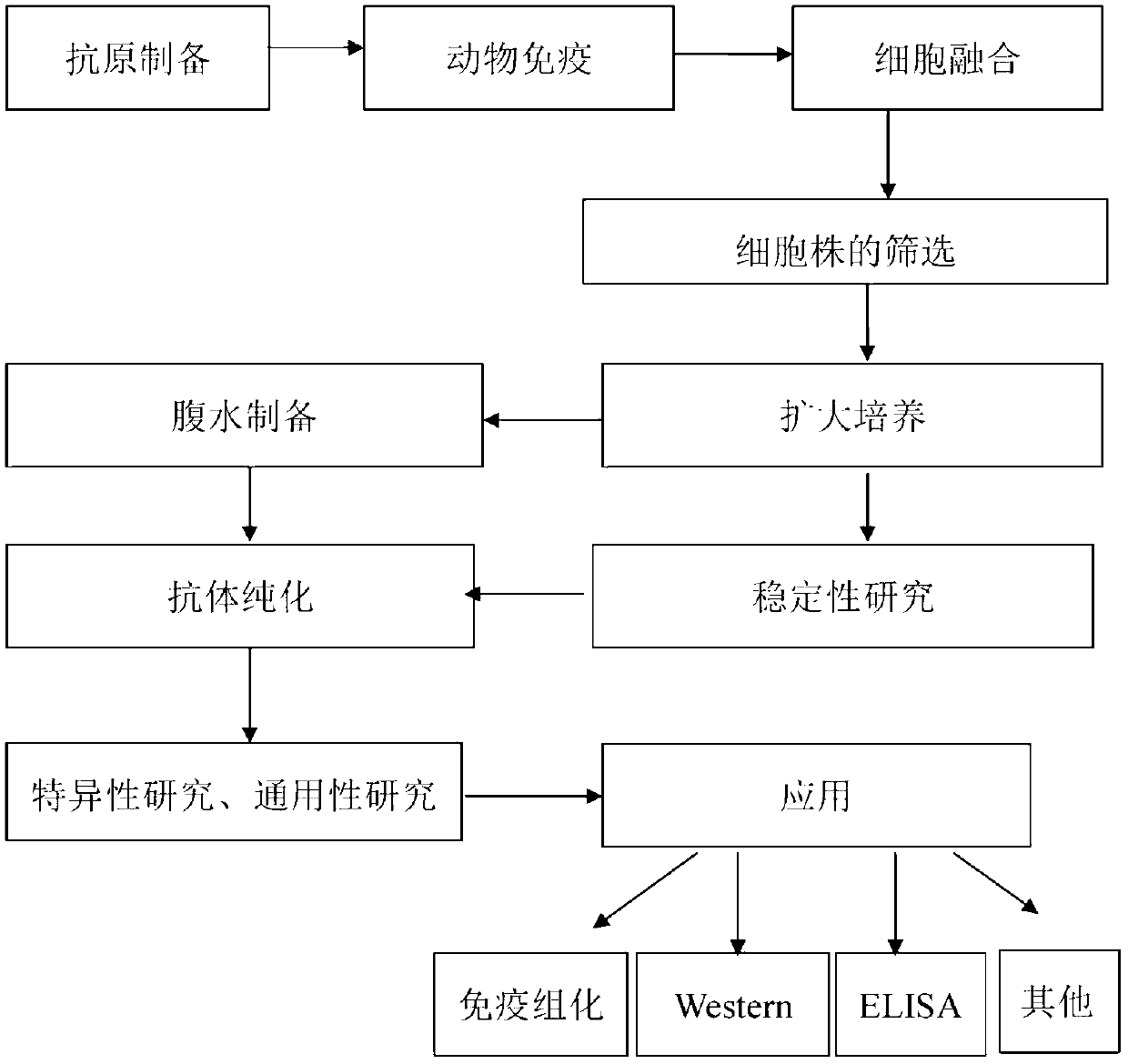
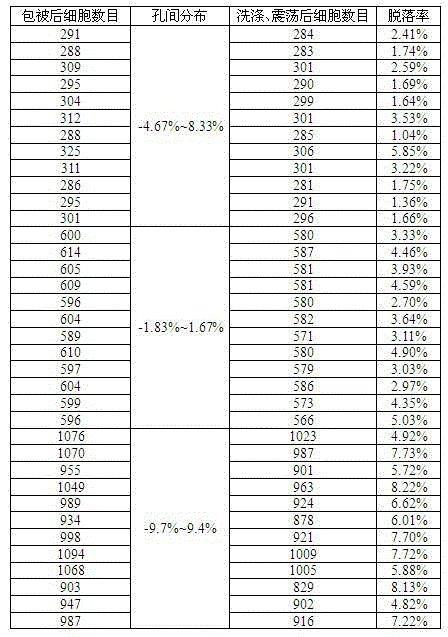
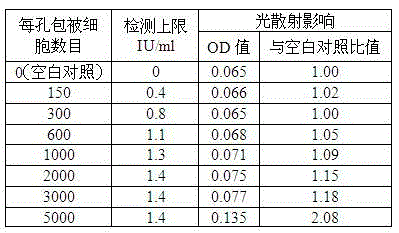
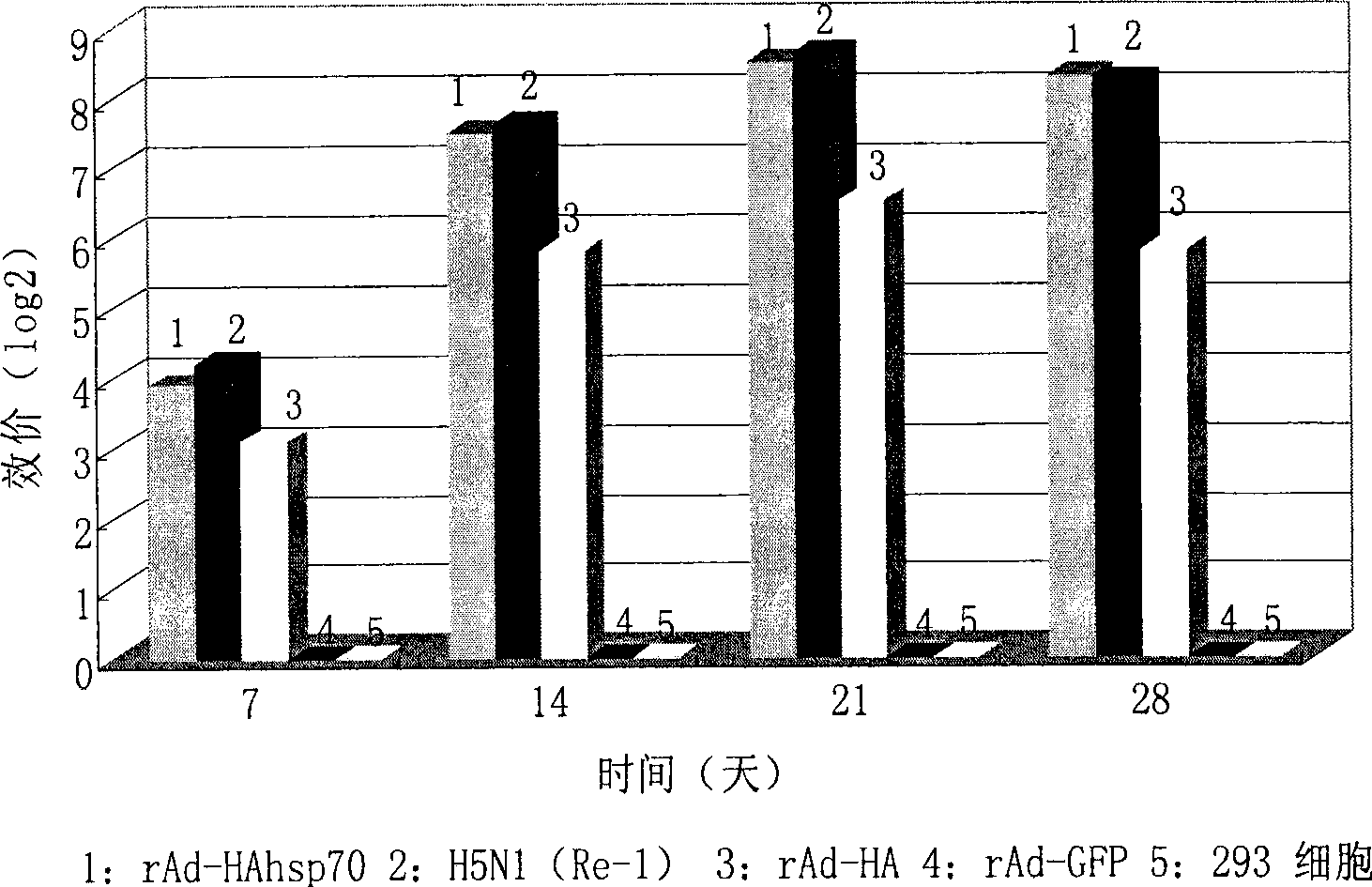Patents
Literature
175 results about "Titin Antibody" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
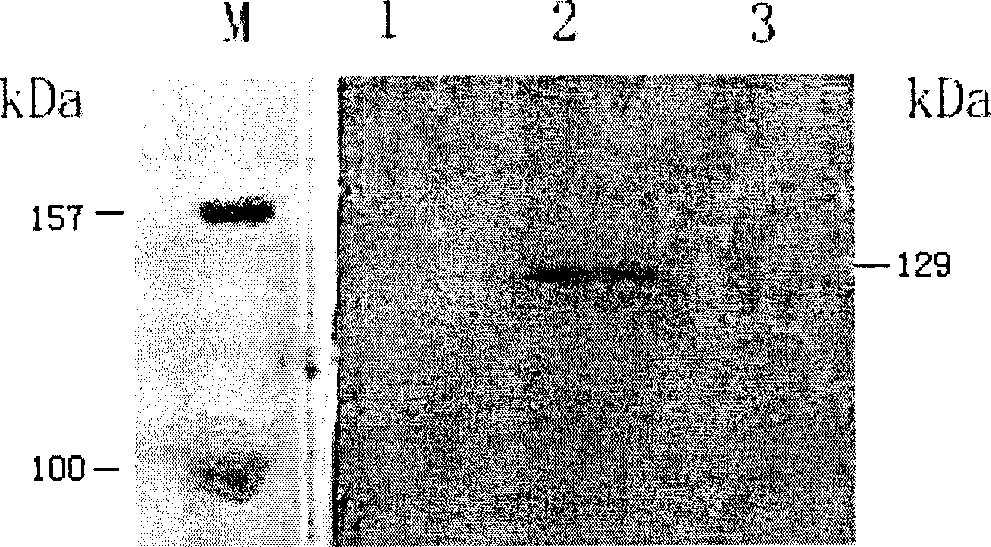
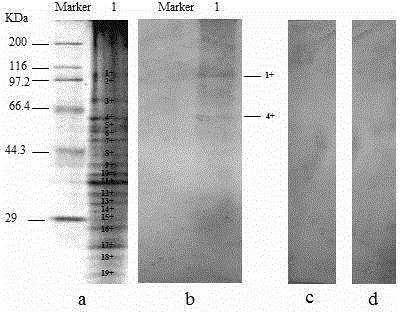
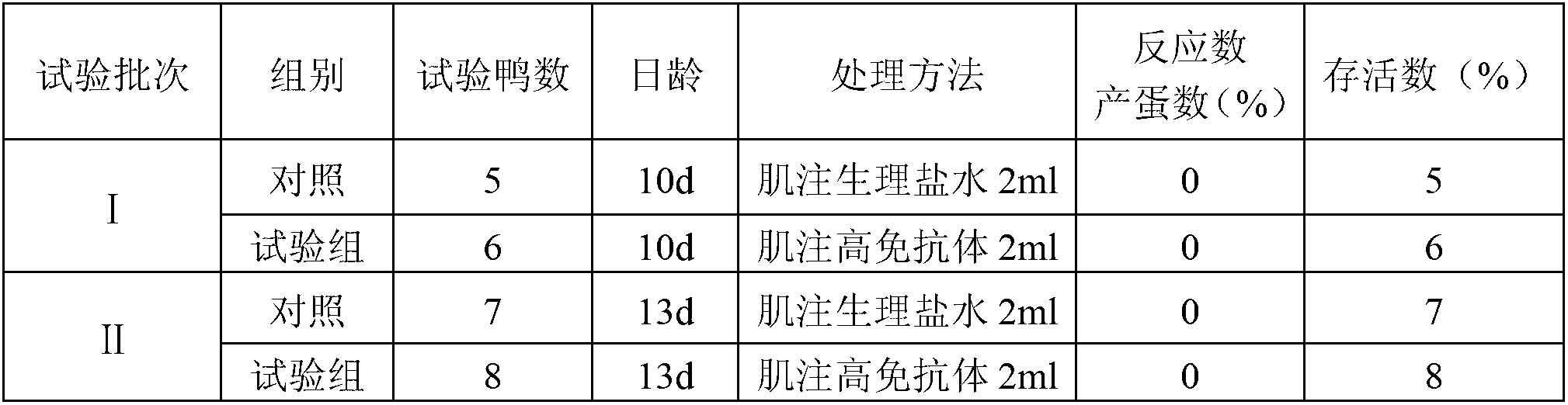
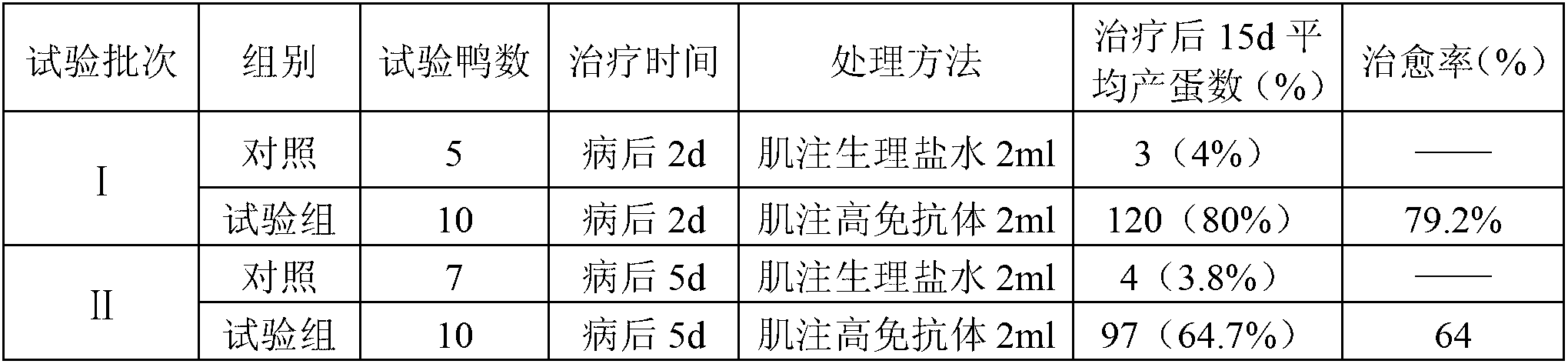
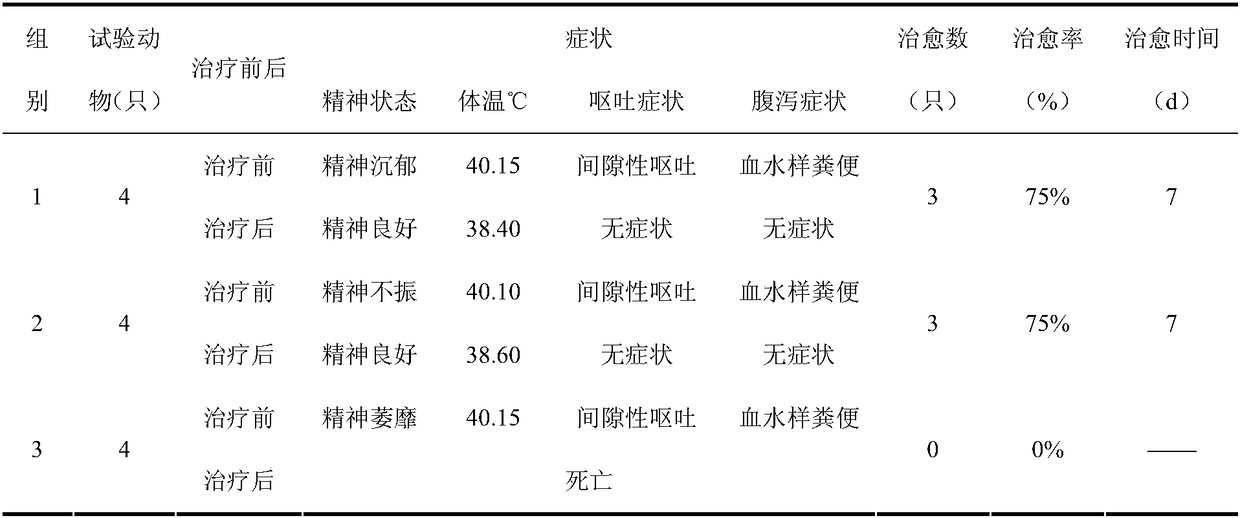
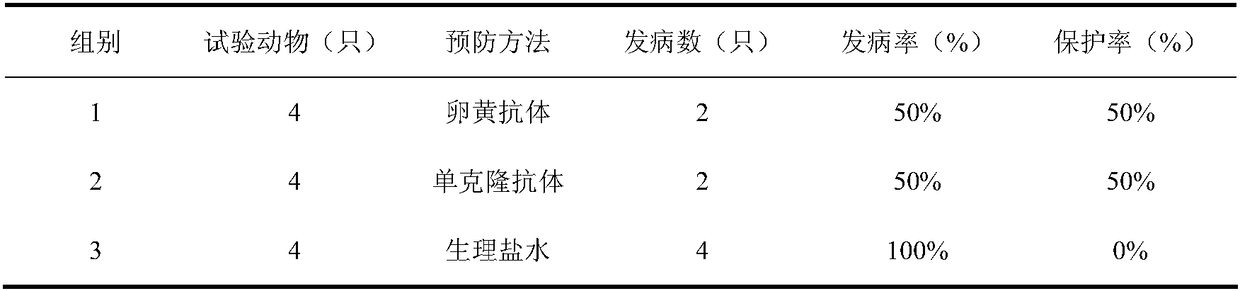
The antibody localizes titin (connectin) in skeletal and heart muscle of a wide variety of species from cold-blooded vertebrates to human. The antibody does not cross-react with nebulin.
Compound immunoenhancement agent, vaccine for birds and method for preparing compound immunoenhancement agent
ActiveCN102743750AShorten the immune window periodShort immune windowAntiviralsImmunological disordersBird fluDipeptide
The invention relates to a compound immunoenhancement agent and an application thereof on preparing a vaccine for birds. The compound immunoenhancement agent contains 5ng-10mg / mL of poly IC, 10ng-10mg / mL of muramyl dipeptide, 10ng-5mg / mL of levamisole, 10ng-5mg / mL of resiquimod and 10ng-5mg / mL of imiquimod. After the immunoenhancement agent and H5 hypotype bird flu inactivated vaccine are mixed together, antibody can be produced one week earlier, and the antibody titer is improved above 1.8log2. After chicken are immunized through H5 hypotype bird flu inactivated vaccine, H9 hypotype bird flu vaccine or infectious bronchitis vaccine which contains the compound vaccine immunoenhancement agent, the window phases of the antibody production are shortened, the antibody titer of the vaccine is improved, the immunization duration is prolonged, and the probability of infection disease is reduced.
Owner:南京国创生物技术研究院有限公司
Method for detecting valence of antibody
InactiveCN103439515AImprove linearityEasy accessCarrier-bound/immobilised peptidesBiological testingAntiendomysial antibodiesMicrosphere
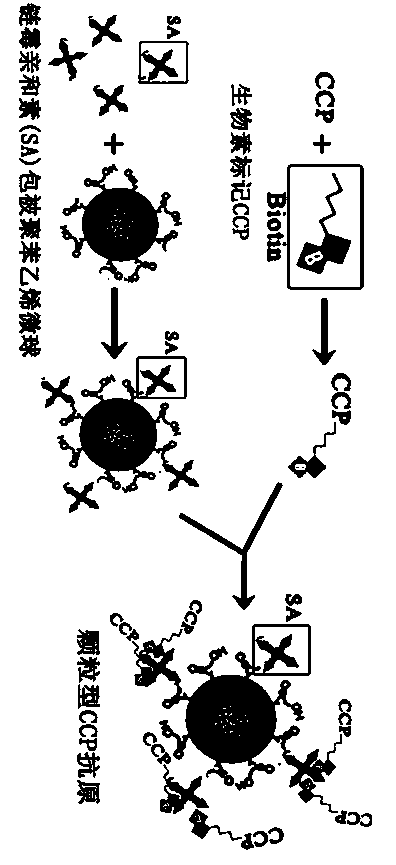
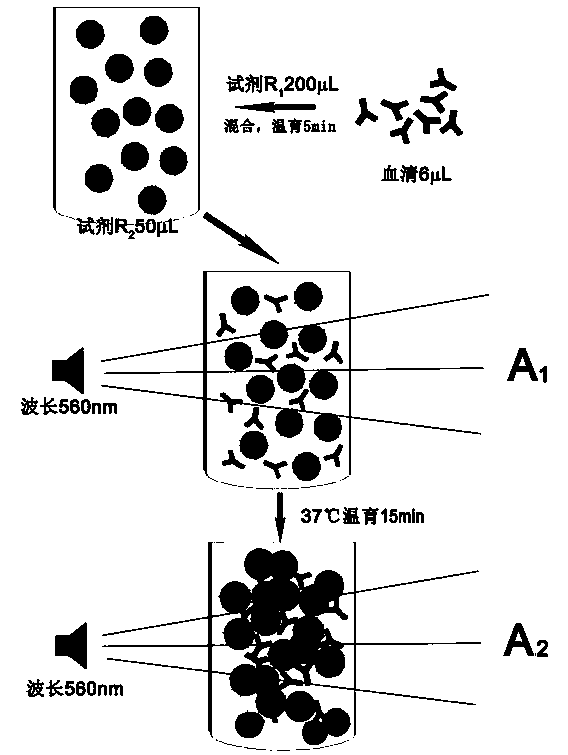
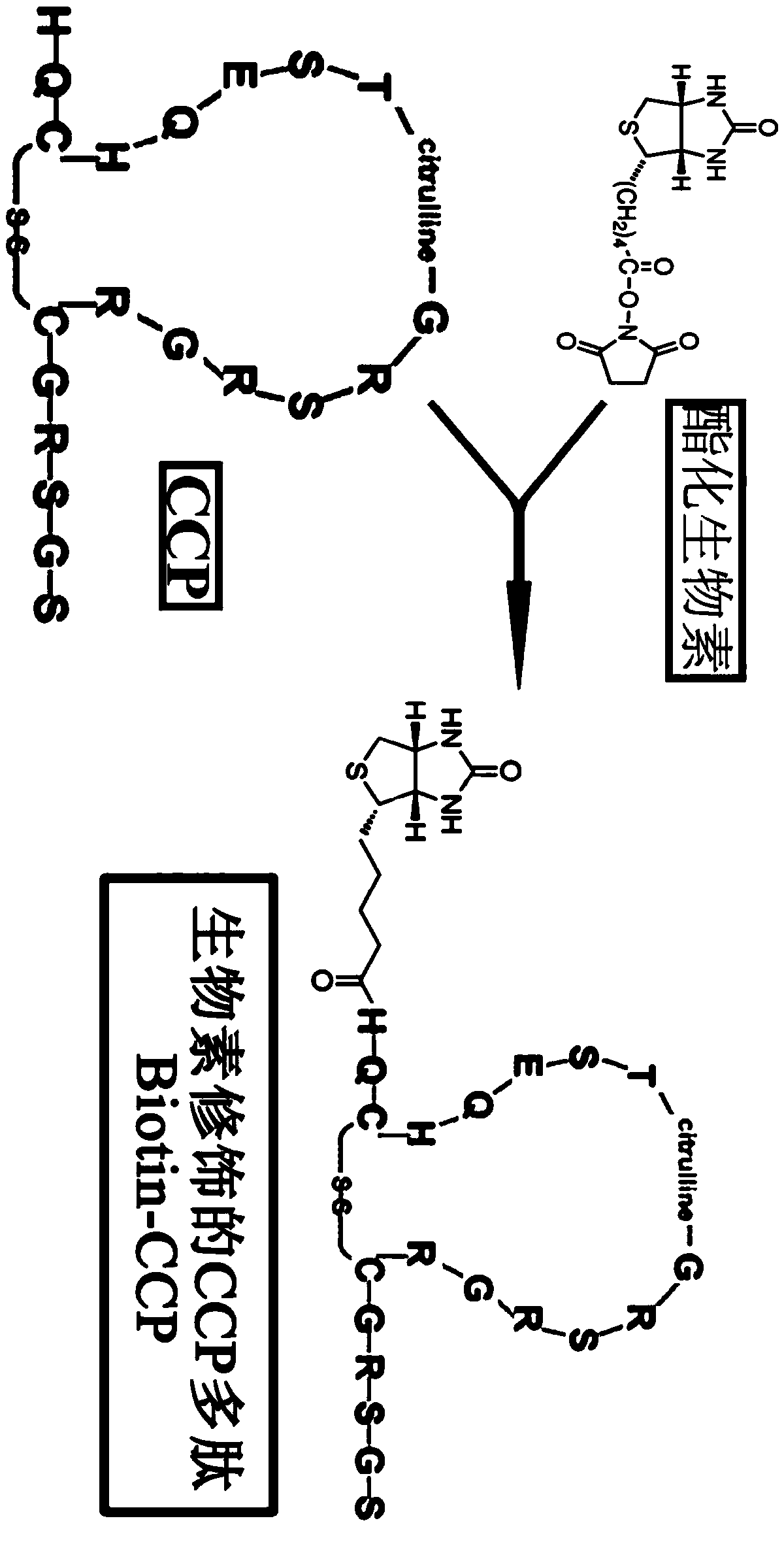
The invention discloses a method for detecting the valence of an antibody. The method comprises the following steps: 1), coupling an antigen on a microsphere to obtain a modified microsphere; 2), mixing and reacting the modified microsphere with an antibody solution; 3), measuring the turbidity of the reaction liquid, so as to determine the valence of the antibody. According to the method provided by the invention, CCP is modified on the surface of a polystyrene microsphere by adopting a vitamin H-streptavidin system, so as to prepare a granular CCP antigen successfully; in case that the granular CCP antigen prepared by adopting the method is used for detecting the valence of anti-CCP antibody in blood serum, the linearity of the detecting result is good, and the detecting process can be completed by 20 minutes, so that the method can be used for acquiring the detecting result more quickly as compared with the conventional ELISA detecting method consuming 2-3 hours.
Owner:SOUTHERN MEDICAL UNIVERSITY
Hand-foot-and-mouth disease resistant human immunoglobulin, and preparation and using methods and application thereof
InactiveCN102190725ASignificant effectImprove survival rateImmunoglobulins against virusesAntiviralsTotal proteinPasteurization
The invention relates to a hand-foot-and-mouth disease resistant human immunoglobulin, and preparation and using methods and application thereof in pharmacy. The preparation method comprises the following steps of: separating component I+II+III, I+III and II precipitates in turn by using a low-temperature methanol protein separation method; performing pasteurization on a component II precipitate; refining and purifying; performing dealcholization; preparing; and sterilizing, packaging and performing low-pH incubated inactivation. The product has antibody titer of not less than 1:640 for enteroviruses (including one or more of coxsackie virus, Echo and EV71), the immunoglobulin content is not less than 95.0 percent of the total protein content, and the sum of IgG monomer and dimer is not less than 95 percent; and the using method is that a specific antibody with titer of 160,000-320,000 is intravenously infused. The invention is suitable for industrial production; and the product has high-titer enterovirus resistant specificity, is safe and reliable, and can become an effective medicine for treating the hand-foot-and-mouth disease.
Owner:HUALAN BIOLOGICAL ENG INC
Monoclonal antibody of ractopamine and preparation method and application thereof
ActiveCN101921730ASensitive detectionHigh sensitivityMicroorganism based processesTissue cultureBALB/cOctanoic Acids
The invention relates to a monoclonal antibody of ractopamine and a preparation method and application thereof, which belongs to the field of immunochemical technology. In the invention, the ractopamine and carrier proteins BSA, HSA and OVA are coupled by a mixed anhydride method to synthesize artificial immunogens RAC-BSA and RAC-HSA and coating antigen RAC-OVA; a Balb / c mouse is immunized by the synthesized artificial immunogens RAC-BSA and RAC-HSA; the spleen cell of the immune mouse is fused with the SP2 / 0 myeloma cell and the two are coated by the coating antigen RAC-OVA; AND an indirect ELISA method and an indirect competition ELISA method are established to screen out a hybridoma cell strain (5D8) capable of stably excreting specific antibody. The Balb / c mouse is immunized by the obtained cell strain to prepare ascites which is purified by an octanoic acid-ammonium sulfate method and an ion exchange method, and the titer of the purified antibody is more than 5.12*10<5>. The monoclonal antibody has strong specificity, is used for preparing a ractopamine residue detection kit and colloidal gold test paper strips and can sensitively and quickly detect the ractopamine residue.
Owner:JIANGSU HUACHUANG MEDICINE RES & DEV PLATFORM MANAGEMENT CO LTD
ELISA kit of IBDV antibodies, test method and effective antibody titer determination method
The invention discloses an ELISA kit of IBDV antibodies, a test method and an effective antibody titer determination method. The ELISA kit comprises (1) an enzyme-labeled board coated with IBDV antigens and (2) goat anti-chicken Ig Y diluted according to the ratio of 1:3,000 and marked with HRP, wherein the enzyme-labeled board coated with the IBDV antigens is made by diluting the concentration ofthe IBDV antigens to 10ng / pore and then coating corresponding pores in a reaction board with diluent, the IBDV antigens are rVP2 proteins prepared through a baculovirus expression system, and a detection result is judged according to an S / P value obtained through an ELISA detection method. When the detection result is positive according to the S / P value obtained through the ELISA detection method, it is indicated that effective antibodies are in a high level and can neutralize IBDVs, and therefore it is needless to perform vaccine immunization again; and when the detection result is negativeaccording to the S / P value, it is indicated that the antibodies are in a low level without protective force and are prone to being infected by viruses, and therefore it is needed to perform vaccine immunization again. As a result, the established ELISA kit has a high antibody positivity detection rate and can guide vaccine immunization on production to reduce economic losses.
Owner:XINXIANG UNIV
Adenovirus vector avian influenza recombinant vaccine
ActiveCN101475641AImprove immunityGenetic material ingredientsAntiviralsProtective antigenHemagglutinin
The invention discloses an adenovirus carrier avian influenza recombined vaccine. The vaccine takes the hemagglutinin antigen gene of highly pathogenic avian influenza H5N1 as a main protective antigen gene which is fused with a braided mycobacterium tuberculosis heat shock protein coding gene. After being transferred into an adenovirus carrier to obtain a recombined adenovirus and immunize an animal, the fusion gene can produce a high titer antibody of the hemagglutinin antigen HA inside the animal and keep a high antibody titer inside the animal for a long period.
Owner:ZHEJIANG YEBIO BIOTECH +1
Indirectly competitive ELISA (Enzyme Linked Immunosorbent Assay) immune kit for detecting porcine reproductive and respiratory syndrome virus
InactiveCN103293311AAccurate quantitative determinationEasy to operateMaterial analysisAssayRespiratory syndrome virus
The invention discloses an indirectly competitive ELISA (Enzyme Linked Immunosorbent Assay) immune kit for detecting a porcine reproductive and respiratory syndrome virus. The kit comprises an enzyme marker for resisting a monoclonal antibody of the porcine reproductive and respiratory syndrome virus. The kit has the beneficial effects that the antibody valence of the porcine reproductive and respiratory syndrome virus is detected accurately and quantitatively; the kit is operated simply and short in required operation time. Thus, the kit can be used for detecting a large number of samples and surveying epidemiology, and is well consistent with a testing method recommended by WTO (World Health Organization) and OIE (Office International Des Epizooties).
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Application of Shuanghuanglian oral liquor in preparation of medicine for treating and preventing poultry virosis
The invention belongs to the technical field of veterinary medicines, particularly relates to an application of Shuanghuanglian oral liquor in preparation of a medicine for treating and preventing poultry virosis, and in particular relates to an application of Shuanghuanglian oral liquor in preparation of a medicine for treating and preventing newcastle disease or infectious bronchitis. As the experiments show, the Shuanghuanglian oral liquor has an excellent effect on immunological enhancement respect to newcastle disease and infectious bronchitis bivalent inactivated vaccines (LaSota-M41 strains), can be used for obviously improving the antibody level as well as the immune protective effects of the newcastle disease and infectious bronchitis bivalent inactivated vaccines (LaSota-M41 strains), and also can be used for improving the infectious bronchitis virus serum antibody titer, and as a result, the anti-excitability ability of the poultry organisms is improved.
Owner:HENAN XINZHENGHAO BIO ENG
Competitive enzyme-linked immunosorbent assay method of EV71 neutralizing antibody, kit or reagent and prepration method thereof
ActiveCN101609097AGuaranteed specificityOvercome the inability to carry out the shortcomingsFermentationMaterial analysisAntigenSerum reaction
The invention discloses a competitive enzyme-linked immunosorbent assay method of an EV71 neutralizing antibody, a kit or a reagent and a preparation method thereof. The kit utilizes the labeled monoclonal antibody which has higher neutralizing potency on three genes of A, B and C of EV71 virus, a reference material of the EV71 neutralizing antibody and a coated plate and can simply, rapidly and accurately carry out qualitative and quantitative assay on the content of the EV71 neutralizing antibody in a human sample or an animal sample. EV71 virus particles are coated on the ELISA plate, the enzyme-labeled EV71 neutralizing antibody is diluted according to a certain proportion, then respectively mixed with the sample to be assayed and the reference material of the EV71 neutralizing antibody and reacted with an EV71 antigen coated on the ELISA plate, a semi-logarithmic standard curve is drawn according to the absorptance percentage of OD value of the reference serum reaction and the logarithm of the potency of the reference material of the EV71 neutralizing antibody after color development, and the absorptance percentage of the OD value of the sample to be assayed is substituted ina standard curve equation to calculate the corresponding neutralizing potency.
Owner:SINOVAC BIOTECH
Haemophilus parasuis outer membrane protein P5 (OMP5) resistant monoclonal antibody, hybridoma cell strain and application
InactiveCN102876635ANo cross reactionStrong specificityImmunoglobulins against bacteriaMicroorganism based processesEscherichia coliBordetella
The invention discloses a haemophilus parasuis outer membrane protein P5 (OMP5) resistant monoclonal antibody, a hybridoma cell strain and an application. The hybridoma cell strain is preserved in the China center for type culture collection (CCTCC), and the preservation serial number is CCTCCC2012135. The monoclonal antibody prepared by the hybridoma cell strain is good in specificity, high in valence, high in generality, free from cross reaction with swine Escherichia coli, swine pasteurella, swine pleuropneumonia actinobacillus, streptococcus suis and swine bordetella bacilli, capable of detecting haemophilus parasuis with different serotypes and widely applicable to etiology diagnosis, serology detection and immunology detection and prevention of haemophilus parasuis diseases, and the enzyme-linked immuno sorbent assay (ELISA) antibody valence can reach 1:204800 after purification.
Owner:广东省农业科学院兽医研究所
ETEC (enterotoxigenic escherichla coli) yolk antibody powder and preparation method thereof
InactiveCN105713088AHigh potencyImprove high temperature stabilityEgg immunoglobulinsImmunoglobulins against bacteriaEscherichia coliYolk
The invention discloses ETEC (enterotoxigenic escherichla coli) yolk antibody powder and a preparation method thereof. The method comprises following steps: escherichia coli strains are cultured in a solid culture medium at 35-40 DEG C for 18-24 h; after culture, pilin is extracted coarsely from the solid culture medium and is purified; the purified pilin and equal volume of a freund's adjuvant are mixed, an oil-emulsion vaccine is formed and used for immunizing a primiparous hen, and serum and a yolk antibody are collected after immunization; the yolk antibody is subjected to spray drying through a powder sprayer, and the yolk antibody powder is prepared. Analysis of the titer of the immunized yolk antibody with ELISA (enzyme-linked immunosorbent assay) discovers that higher-titer yolk antibody can be obtained by using purified pilin as an antigen for immunization. After the yolk antibody powder is prepared from the yolk antibody through spray drying by the powder sprayer, the antibody titer is only reduced by one degree of multiple proportions, the yolk antibody has very good high-temperature stability, and a power preparation process adopting a spray drying method is applicable to production of the yolk antibody powder.
Owner:FOSHAN UNIVERSITY
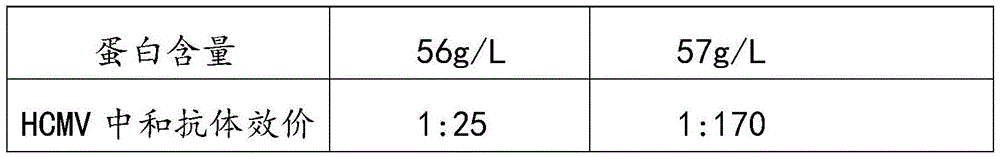
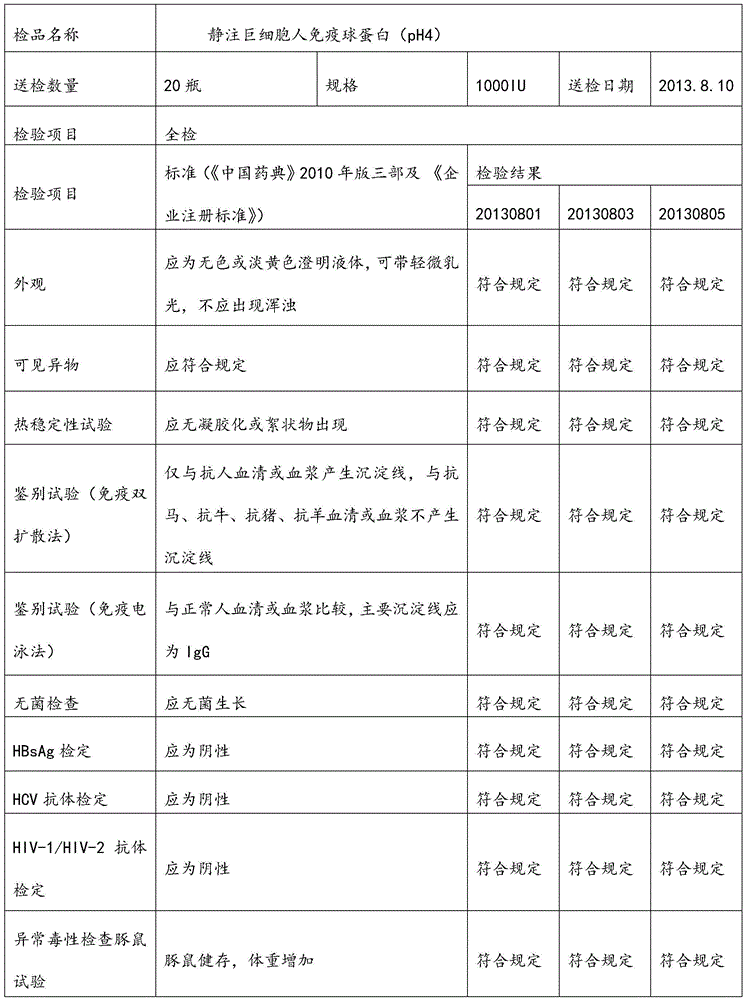
Intravenously injected cytomegalovirus human immune globulin and preparation method thereof
ActiveCN105601735AEffectively treat severe infectionsSuitable for industrial productionImmunoglobulins against virusesPeptide preparation methodsFiltrationUltrafiltration
The invention relates to intravenously injected cytomegalovirus human immune globulin and a preparation method thereof, and the titer of a cytomegalovirus neutralizing antibody of the human immune globulin is larger than or equal to 1:500. The preparation method comprises the steps that 1, positive plasma with the titer of the cytomegalovirus neutralizing antibody larger than or equal to 1:20 is screened out; 2, the screened-out efficient positive plasma is mixed; 3, the mixed plasma is separated through a low-temperature ethanol filter-pressing method, an immune globulin component II is separated and purified by combining an ion-exchange column chromatography method, viruses are removed through filtration, chromatography, ultrafiltration, preparation, low-pH incubation virus inactivation and nanofilm filtration, the immune globulin with the purity of 98.5%-100% is obtained through subpackaging, and the antibody titer is not lower than 1:500. The cytomegalovirus human immune globulin prepared through the preparation and production method is high in antibody titer, purity and recovery rate and capable of conducting targeted treatment on cytomegalovirus, is an effectively drug for treating recessive and dominant infection caused by the cytomegalovirus in people, is safe and reliable and has larger social benefits and economic benefits.
Owner:哈尔滨派斯菲科生物制药有限公司
Bivalent freeze-dried egg yolk antibody for infectious feline rhinitis-conjunctivitis and feline panleukopenia and preparation and application thereof
InactiveCN110240648ANo costIncrease productionEgg immunoglobulinsSenses disorderFeline panleukopeniaFreeze-drying
The invention discloses a bivalent freeze-dried egg yolk antibody for infectious feline rhinitis-conjunctivitis and feline panleukopenia and a preparation method and an application thereof, and belongs to the technical field of veterinary biopharmaceuticals. The bivalent freeze-dried egg yolk antibody for the infectious feline rhinitis-conjunctivitis and the feline panleukopenia can be directly prepared by bivalent vaccine immunized laying hens without hurting animals, and is simple in process method and low in cost; and the obtained bivalent antibody is high in titer, good in stability, quick to take effect, free of producing drug resistance, capable of being used for rapid treatment or emergency prevention of the infectious feline rhinitis-conjunctivitis and the feline panleukopenia and high in protection rate. Compared with vaccines and antibiotics, the invention has wider and more practical application prospects.
Owner:CHANGCHUN SR BIOLOGICAL TECH
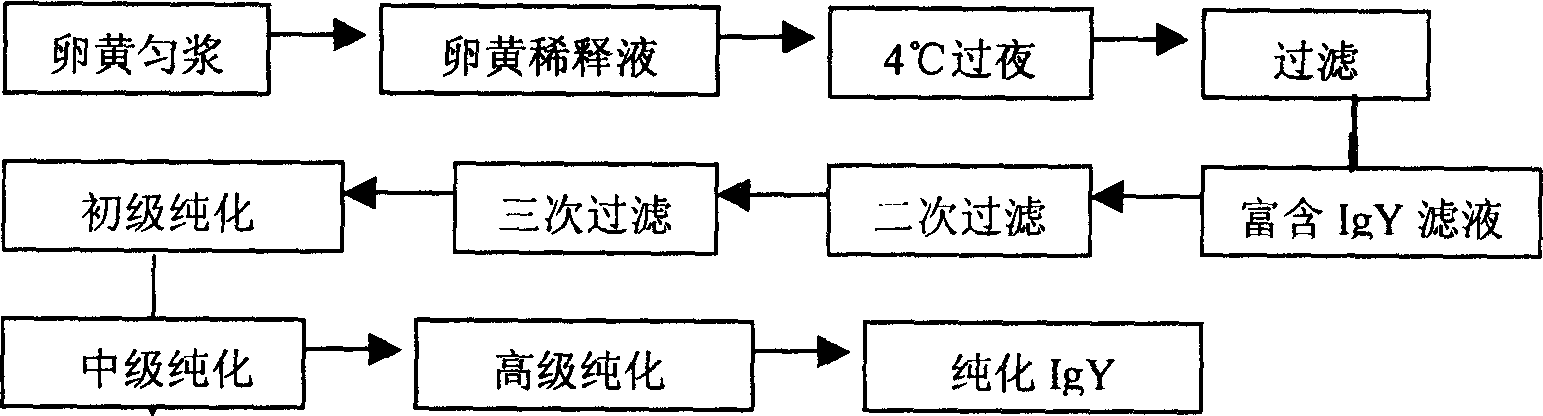
Method for preparing IgY for SARS
InactiveCN1546526ALong-term titer maintenanceEasy to feedEgg immunoglobulinsAntiviralsSerum igeNeutralizing antibody
The invention discloses a method for preparing IgY for SARS, which belongs to a process for manufacturing anti-SARS biological pharmaceuticals. The process comprises the steps of making vaccinogen, preparing antibody, homogenating vitelline, diluting, low-temperature stewing, filtering, purifying, and pulverizing. The said antibody preparation process is disclosed in the invention. The process according to the invention can be applied in preparing anti-SARS medicament.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Hydrophobia, tetanus double-titer human immunoglobulin and preparation method thereof
InactiveCN101502651AEasy to useHigh economic and social benefitsAntibacterial agentsAntiviralsProtein contentTetanus vaccine
The invention relates to a rabies and tetanus double valence human normal immunoglobulin, the rabies valence of antibody is not less than 100IU / mL, the tetanus valence of antibody is not less than 60IU / mL, the content of protein is not more than 175g / L, the immunoglobulin purity is not lower than 92% of the total protein content. The preparation method of source plasma of the protein is to select plasma supplier to be inoculated with rabies vaccine and then with adsorbed tetanus vaccine at least 1-5 days later to obtain the source plasma with rabies valence of antibody being not less than 6IU / mL and the tetanus valence of antibody being not less than 5IU / mL and ensure that the rabies valence of antibody of the source plasma is not less than 10IU / m and the tetanus valence of antibody is not less than 8IU / mL after mixture. The immunoglobulin of the invention can be used to immunize patients suffering exposed wounds caused by biting and scratching of a mad dog or other mad animals.
Owner:SHANXI KANGBAO BIOLOGICAL PROD
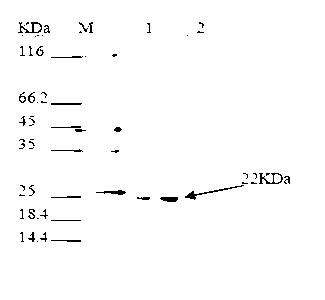
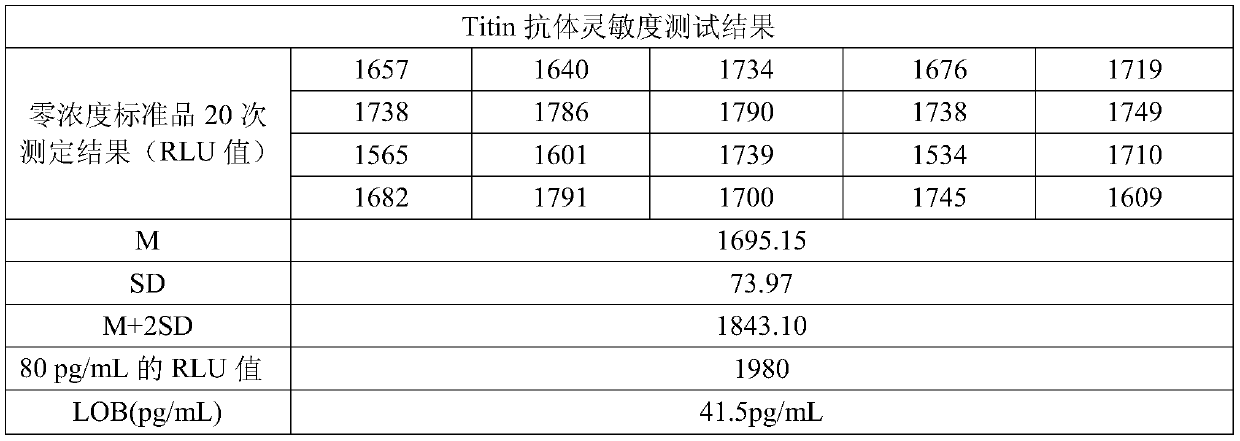
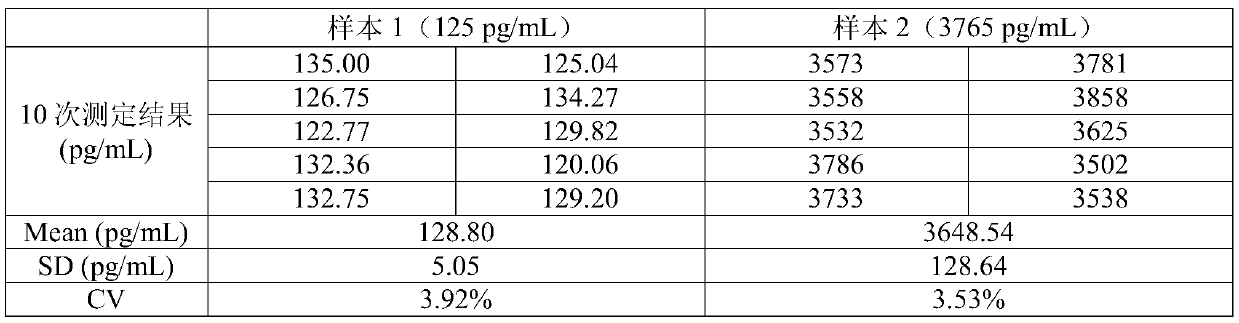
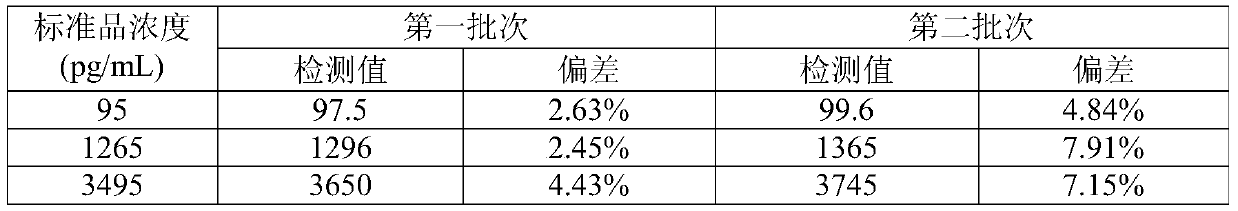
Electrochemiluminescence kit for detecting Titin antibody and preparation method of kit
PendingCN111044719AHigh detection sensitivityWide linear rangeChemiluminescene/bioluminescenceDisease diagnosisAntigenAntiendomysial antibodies
The invention relates to an electrochemiluminescence kit for detecting a Titin antibody in blood and a preparation method of the electrochemiluminescence kit. The prepared kit comprises a streptavidincoupled magnetic particle working solution, a biotin labeled Titin antigen working solution, a ruthenium terpyridyl labeled anti-human IgG antibody working solution, a working solution of a calibrator and / or a quality control product of the Titin antibody, a tripropylamine-containing electrochemiluminescence substrate solution and a cleaning solution. The kit adopts electrochemiluminescence, a streptavidin-biotin signal amplification system is utilized, the detection sensitivity is high, the linear range is wide, the detection result repeatability is high, and accurate quantification of the Titin antibody can be realized.
Owner:江苏三联生物工程股份有限公司
Use of immunological stimulant compound(ISCOMs)in fish immunity by oral administration and dipping bath method
InactiveCN1879880AImprove the ability of proliferation and transformationHigh antibody titerImmunological disordersAntibody medical ingredientsStimulantT lymphocyte
The invention relates to a bath or oral application of immune activate compound in the fish immunity, wherein the invention has the advantages that: said compound ISCOMs is used to bath immunity to improve the antibody level and improve the increment transformation ability of T-lymphocyte, to induce the immune protection; when in the same dosage, the antibody level is higher than non-adjuvant group and the ISM1312 group; and the ISCOMs used in oral can improve the antibody level and improve the increment transformation ability of T-lymphocyte, to induce the immune protection.
Owner:BIOLOGICAL TECH INST OF FUJIAN ACADEMY OF AGRI SCI +3
Dimethomorph hapten, and preparation method and application thereof
ActiveCN111138381AThe synthesis method is simpleGood potencyOvalbuminSerum albuminChemical synthesisChemical structure
The invention relates to the technical field of biology, and discloses a dimethomorph hapten, and a preparation method and an application thereof. The dimethomorph hapten reserves the chemical structure of dimethomorph to the greatest extent, a nitrogen-containing group capable of being coupled with proteins is introduced through chemical synthesis, the synthesis method is simple, and the productpurity and yield are high; and the hapten is used as a raw material to prepare an antigen system suitable for animal immunization, and a prepared monoclonal antibody has good specificity, affinity and antibody titer, and can be used for preparing an enzyme linked immunosorbent assay kit, and the kit has the advantages of low detection cost, and fast, efficient and accurate detection method, and is suitable for on-site monitoring of dimethomorph residues in vegetables and fruits and screening of a large number of samples.
Owner:NATIONAL MARINE ENVIRONMENTAL MONITORING CENTRE
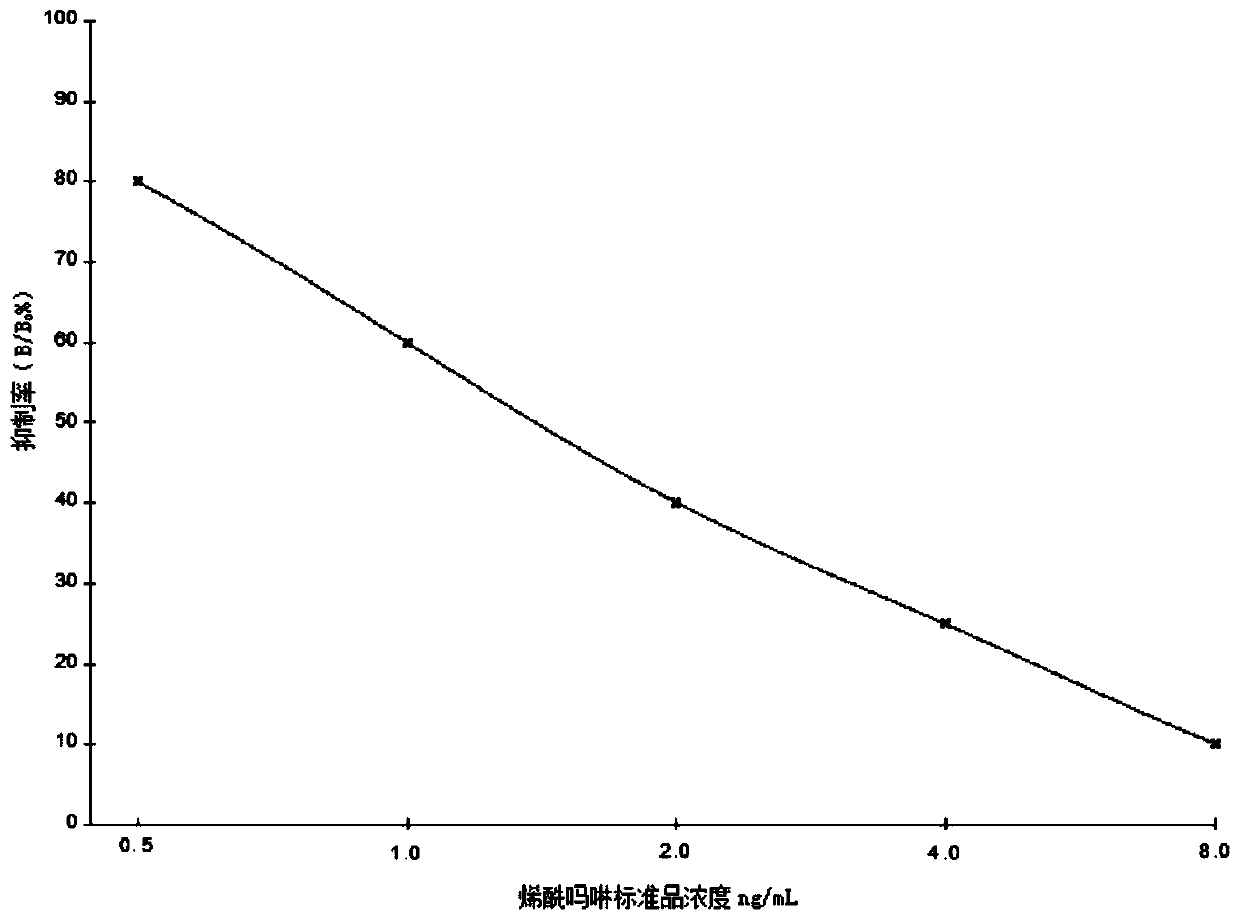
Synthesis method and application of coumafuryl hapten
InactiveCN110563712AHigh sensitivityHigh utility valueOvalbuminSerum albuminPolyclonal antibodiesCoumafuryl
The invention provides a synthesis method and an application of a coumafuryl hapten. The structural formula of the coumafuryl hapten is shown in the description. The coumafuryl hapten is prepared by acondensation reaction of coumafuryl and carboxymethyl hydroxylamine semi-hydrochloride. The invention also provides a coumafuryl artificial antigen. The coumafuryl artificial antigen is prepared by coupling the coumafuryl hapten with a carrier protein. The complete antigen of the coumafuryl hapten prepared by the synthesis method of the coumafuryl hapten can expose the chemical structure of the coumafuryl as an antigen determinant, so a foundation is laid for the preparation of a high-sensitivity anti-coumafuryl antibody. Finally, an antibody CF-CMO-BSA-3 # with optimal properties is obtainedthrough screening, the highest titer can reach 3.2 * 10<4>, and the sensitivity is 0.32 ng / mL. A coumafuryl polyclonal antibody prepared from the coumafuryl hapten has the unique advantages of high sensitivity, high practical value and the like, and has a good application prospect in public health safety detection.
Owner:CHINA AGRI UNIV
Anti-cryptococcal capsular polysaccharide monoclonal antibody and preparation and application of hybridoma cell strain thereof
ActiveCN109879961ANo cross reactionHigh affinityTissue cultureImmunoglobulins against fungi/algae/lichensColloidCell strain
The invention relates to an anti-cryptococcal capsular polysaccharide hybridoma cell strain and an anti-cryptococcal capsular polysaccharide monoclonal antibody secreted thereof. The antibody can be specifically bound to cryptococcal capsular polysaccharide and can be used for in vitro detection of cryptococcal infection. The antibody titer is up to more than one million, and the antibody has excellent affinity and good specific binding capability. The detection limit of a colloidal gold-labeled immunodiagnostic reagent developed with the antibody as the raw material is 0.5 ng / ml, and the antibody has excellent performance in sensitivity, specificity, stability and all other aspects.
Owner:GENOBIO PHARM CO LTD
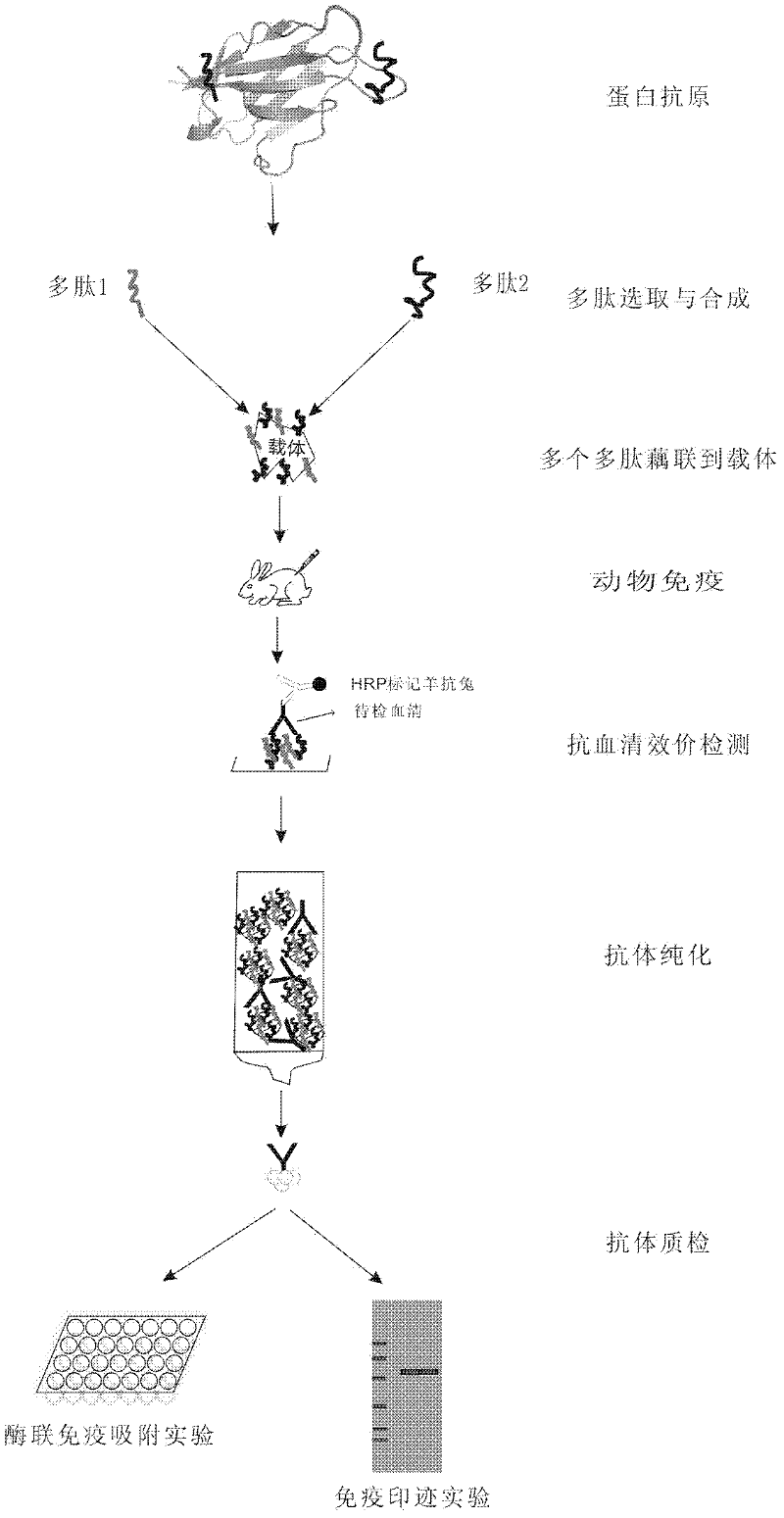
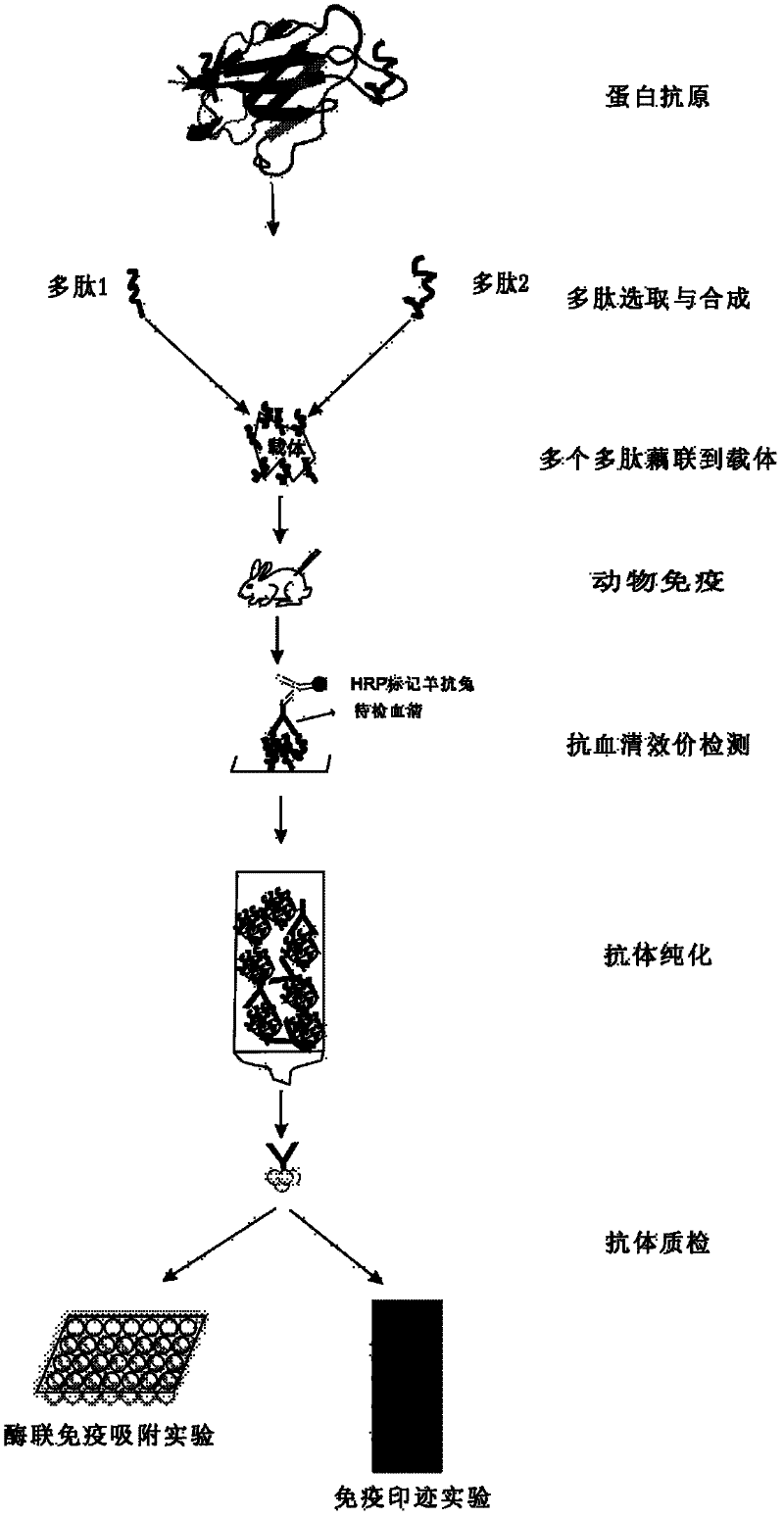
Method for producing antibody by coupling multi-polypeptide epitope of protein antigen with carrier
InactiveCN102391375AHigh potencyEasy to manufactureSerum immunoglobulinsPeptide preparation methodsEpitopeProtein antigen
The invention relates to a method for producing an antibody by coupling multi-polypeptide epitope of protein antigen with a carrier and solves the problems such as high production cost, difficulty in purification, or long period, complex preparation technology and low valence in the existing antibody producing method. The antibody is obtained by selection and synthesization of epitope, coupling of various polypeptides with the carrier, animal immunology and detection, and an antigen affinity chromatography extraction method. The method has the advantages of being simple, low in cost, short in period and high in valence of the prepared antibody.
Owner:伊艾博(武汉)科技股份有限公司
A-type foot-and-mouth disease targeting composite epitope protein mediated by pig chemotactic factors and vaccine
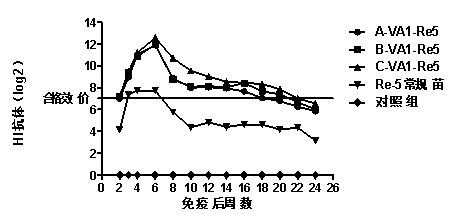
ActiveCN105777909AEnhance humoral immunityEnhance cellular immunitySsRNA viruses positive-senseChemokinesDendritic cellTitin Antibody
The invention provides A-type foot-and-mouth disease targeting composite epitope protein mediated by pig chemotactic factors.The amino acid sequence of the protein is as shown in SEQ ID No.2 in a sequence list.The invention further provides a vaccine with the pig A-type foot-and-mouth disease targeting composite epitope protein.An FMDV specific antibody can be detected one week after an animal is immunized by the vaccine, and the valence of the antibody is continuously improved and reaches the highest four weeks after immunization.After 1000 pigs of the HNXX strain of the A / SEA / 97 series of the pig media infective dose (PID50) adapt to toxin attack, the pigs in the vaccine immunity group can all completely resist strong toxin attack, and no pigs present clinical symptoms within 10 days after toxin attack.It is shown that after an A8 antigen targets an XCR1 receptor through XCL1 and dendritic cells are activated by CpG, the body fluid immunity and cell immunity level of the antigen can be remarkably improved, and thus the vaccine is an effective vaccine with the A-type foot-and-mouth disease targeting composite epitope protein mediated by pig chemotactic factors.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Method for efficiently preparing Enterobacter sakazakii polyclonal antibody
InactiveCN105693855AEfficient preparationNovel methodSerum immunoglobulinsImmunoglobulins against bacteriaImmune profilingNew Zealand white rabbit
The invention belongs to the technical field of immunoassay, and concretely relates to a method for efficiently preparing an Enterobacter sakazakii polyclonal antibody. The method comprises the following steps: immunizing New Zealand white rabbits with Enterobacter sakazakii granular antigens through adopting a subcutaneous multi-point injection and ear vein venous injection combination technology, and further purifying antiserum by a Protein A affinity chromatography column to rapidly obtain the high-tilter Enterobacter sakazakii polyclonal antibody. Compared with traditional immunization methods, the method disclosed in the invention has the advantages of shortening of the immunization time by 7-14d, and great increase of the antibody tilter. A West-blotting and IFAT combination technology is used to detect the specificity of the antibody, and a result shows that the prepared antibody has very high specificity to Enterobacter sakazakii. The method provided by the invention is simple and efficient, provides technical support for preparation of the Enterobacter sakazakii polyclonal antibody, and is helpful for developing the immunodetection technology of the Enterobacter sakazakii.
Owner:OCEAN UNIV OF CHINA
Solid-phase competition ELISA (Enzyme-Linked Immuno Sorbent Assay) detection kit of duck tembusu virus antibody
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Duck tembusu virus (DTMUV) disease immunotherapy preparation and preparation method thereof
The invention relates to a duck tembusu virus (DTMUV) disease immunotherapy preparation and preparation method thereof, which belong to the field of bio-pharmaceuticals. The preparation method of the DTMUV disease immunotherapy preparation comprises the following steps of: culturing seed viruses for producing a tembusu virus strain on a large scale, and harvesting a virus liquid; deactivating the collected virus liquid, concentrating, adding the deactivated and concentrated virus liquid into an adjuvant, and emulsifying to obtain an immune antigen; immunizing laying ducks by using the immune antigen, randomly collecting blood and separating serum after last immunization, and measuring the valence of an anti-DTMUV antigen through an Agar Gel Precipiti (AGP) test till the valance of the antigen is qualified; and collecting eggs laid by an immunized qualified duck group, and preparing into a high-immunity egg yolk antibody with the conventional method. The DTMUV disease immunotherapy preparation contains a DTMUV high-immunity egg yolk antibody. The DTMUV disease immunotherapy preparation prepared with the method has the advantages of good treatment effect, safety, reliability and low cost, and is suitable for large-scale industrial production.
Owner:GUANGZHOU GREAT BIOLOGICAL TECH +1
Specific egg-yolk antibody for preventing and treating feline distemper and preparation method of specific egg-yolk antibody
ActiveCN109111518AStrong specificityHigh antibody titerEgg immunoglobulinsImmunoglobulins against virusesYolkInoculation methods
The invention discloses a specific egg-yolk antibody for preventing and treating feline distemper. The specific egg-yolk antibody is prepared by taking FPV inactivated vaccine and FPV subunit vaccineas immunogens and using immune laying hens. Accordingly, a corresponding preparation method is established and comprises the following steps: creatively inoculating laying hens with two different FPVimmunogens, taking the FPV inactivated vaccine as a basic immunogen and the FPV subunit vaccine as a strengthened immunogen; optimizing an immunization method and inoculation dose to produce high antibody titer; immunizing the laying hens, then harvesting eggs from hype-immunized chickens, separating egg yolk, and extracting and purifying a coarse product of the yolk antibody, thus obtaining the specific egg-yolk antibody for preventing and treating the feline distemper. The test shows that the yolk antibody disclosed by the invention has the advantages of good safety, high specificity, high antibody titer, low cost and high yield; the specific egg-yolk antibody can be used for treatment and emergency prevention of the feline distemper and enhancing the resistance of sick cats and has a good popularization and application prospect.
Owner:GUANGXI VETERINARY RES INST
Novel duck reovirus compound vaccine and preparation method of egg yolk antibody
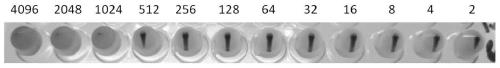
ActiveCN111440815AGood clinical protective effectReduce manufacturing costEgg immunoglobulinsViral antigen ingredientsBiotechnologyAdjuvant
The invention aims to provide a novel duck reovirus compound vaccine and a preparation method of an egg yolk antibody. The preparation method comprises the following steps: mixing recombinant PVAX1-sigma B plasmid containing a novel duck reovirus sigma B protein gene and a novel duck reovirus sigma C protein according to a certain ratio, and performing emulsifying with a white oil adjuvant to prepare the compound vaccine. The average antibody titer of eggs collected 7-150 days after the three times of immunization reaches 1:1024 or above, and the highest antibody titer can reach 1:4096. The egg yolk antibody product prepared by extracting and purifying hyper-immune eggs can provide complete protection for ducks infected with the novel duck reovirus. The novel duck reovirus egg yolk antibody prepared by the method disclosed by the invention is definite in effect and low in cost, and has remarkable economic and social benefits.
Owner:WEIFANG HUAYING BIOTECH CO LTD
Monoclonal antibody hybridoma cell line 1H6 for resisting infectious bursal disease virus VP2 protein
ActiveCN110373393AStrong specificityHighly competitiveVirus peptidesImmunoglobulins against virusesBALB/cTitin Antibody
The invention relates to a monoclonal antibody hybridoma cell line 1H6 for resisting infectious bursal disease virus VP2 protein, and belongs to the technical field of biology. In the invention, BALB / c mice are immunized with purified IBDV QL strain antigen, splenocytes are prepared and fused to an SP2 / 0 myeloma cell line, double screening is carried out by IBDV recombinant VP2 (rVP2) protein expressed by prokaryote and purified IBDV QL strain to obtain the monoclonal antibody hybridoma cell line 1H6 which not only can react with rVP2 protein expressed by prokaryote, but also can react with IBDV, the antibody subclass is IgG1 kappa, and the antibody titers of induced ascites are 108 respectively. Sandwich ELISA assay shows that 1H6 has no cross-reaction with other four avian viruses; indirect immunofluorescence test proves that 1H6 has good specific reaction; and immunoblot analysis shows that 1H6 can produce specific protein bands with rVP2 protein and IBDV.
Owner:JIANGSU ACAD OF AGRI SCI
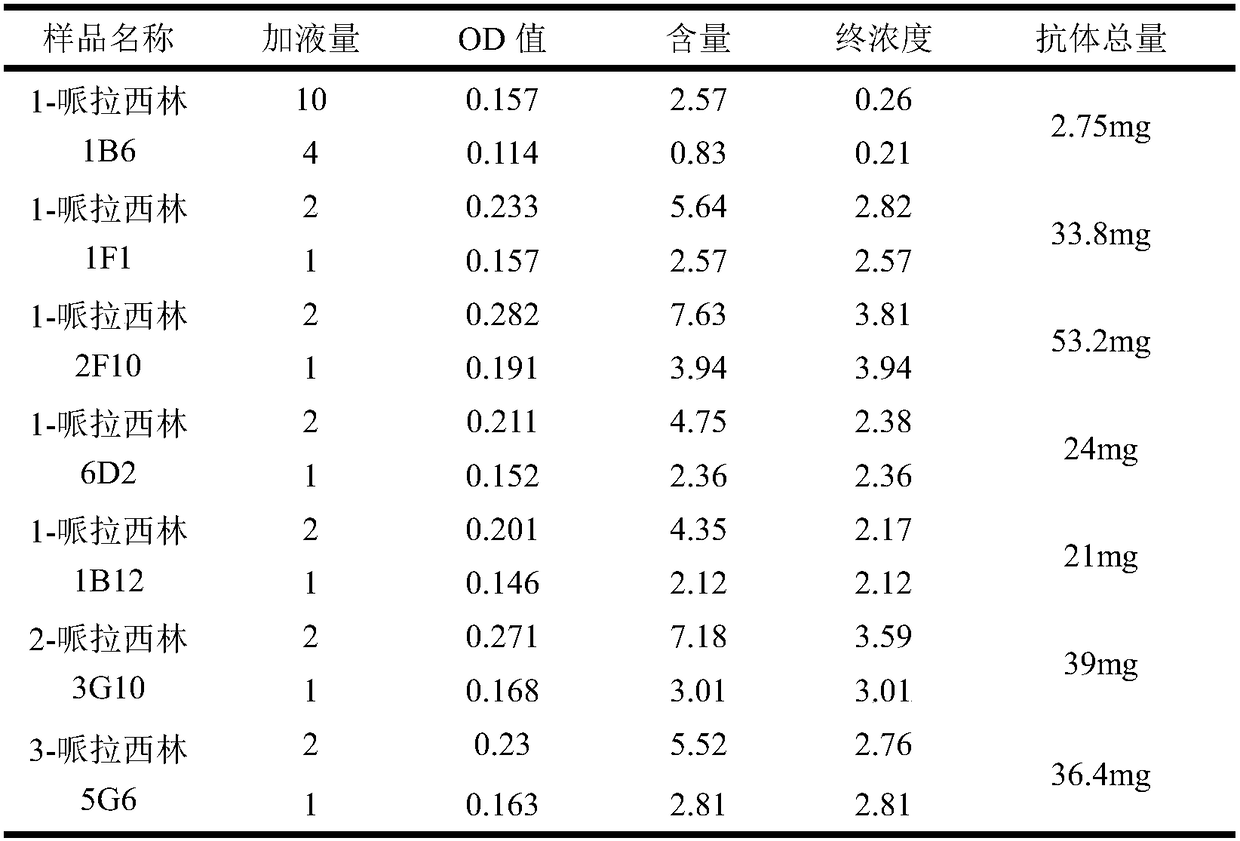
Preparation method of piperacillin monoclonal antibody and application
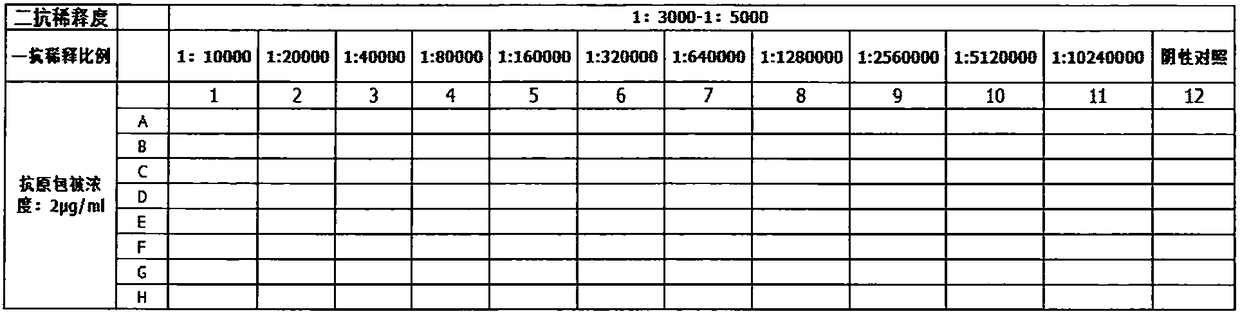
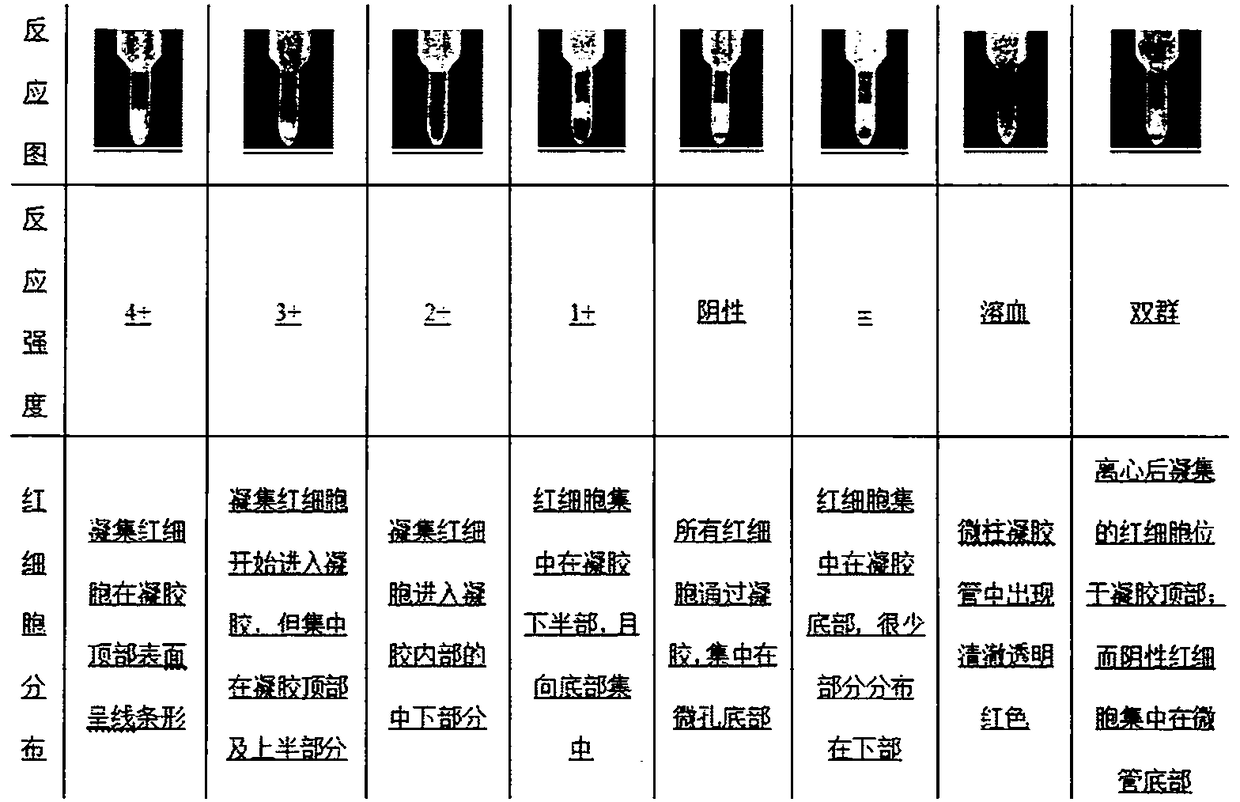
InactiveCN108467434AThe development process is safe and reliableGood value for moneyBiological material analysisPeptide preparation methodsMicro columnAgglutination
The invention relates to a preparation method of a piperacillin monoclonal antibody and application. A hybridoma cell strain is utilized for preparation. The preparation method comprises the followingsteps: preparing a piperacillin monoclonal antibody, performing antibody purification, performing antibody purity identification and performing antibody titer identification. The prepared piperacillin monoclonal antibody is used for detecting piperacillin drug antibodies. Blood of a detected object serves as a detection sample, if the piperacillin antibody exists in plasma, the antibody is boundto a piperacillin drug antigen on piperacillin treated cells by virtue of anti-IgG+G3d bridging so as to produce agglutination, the antibody cannot pass through a gel space and remains on the upper layer of the gel or is dispersed into the gel under the effect of centrifugal force, and presents a positive reaction; and if any antibody does not exist in the plasma or the cell surface does not contain any drug antigen, agglutination is not produced, and the antibody can pass through the gel space to deposit at the bottom of a micro-column gel hole under the effect of the centrifugal force and presents a negative reaction.
Owner:江苏中济万泰生物医药有限公司
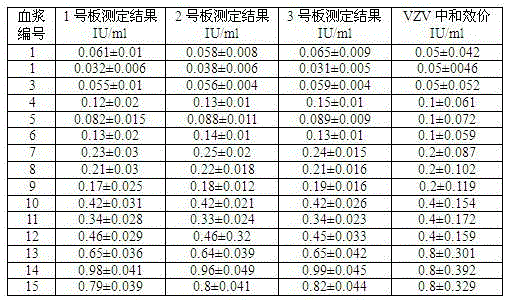
Kit capable of determining titer of neutralizing antibodies of varicella-zoster viruses and production method thereof
ActiveCN104360055AIntegrity guaranteedGuaranteed validityMaterial analysisViral glycoproteinChickenpox
The invention relates to the technical field of in-vitro diagnostic reagents and particularly relates to a kit capable of determining the titer of neutralizing antibodies of varicella-zoster viruses and a production method thereof. The kit adopts cells infected by VZV as antigens, is coated and fixed in holes of a 96-pore plate, the neutralizing antibodies in the sample to be determined are captured by using a large amount of virus glycoprotein (neutralizing glycoprotein) carried on the surfaces of the cells, simultaneously the integrity of the cells is maintained; and the other virus antigens inside are not contacted with the sample, so that the specificity of the neutralizing antibodies in determination can be guaranteed. The kit can be directly used for fast determination of the titer of the neutralizing antibodies in VZV on an automatic biochemical analyzer or an enzyme labeling instrument; the determination process is simple and fast, the flux of the determined sample is high and quantitative determination can be realized.
Owner:SHANDONG TAIBANG BIOLOGICAL PROD CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com