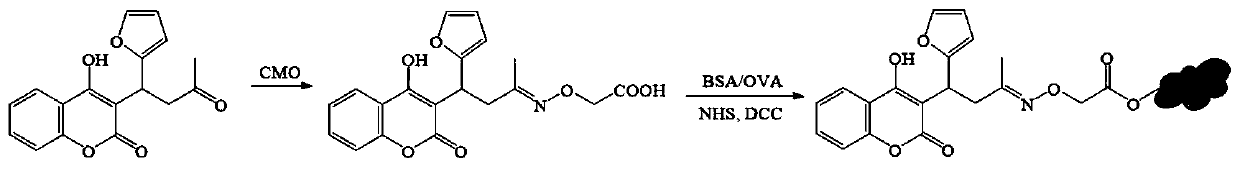
Synthesis method and application of coumafuryl hapten
A hapten and rodent control technology, which is applied in the field of public health, can solve the problems of complex sample processing, high detection cost, and inability to meet rapid screening, and achieve the effects of high sensitivity, high practical value, and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Example 1, the preparation and identification of the gram rodent hapten CF-CMO
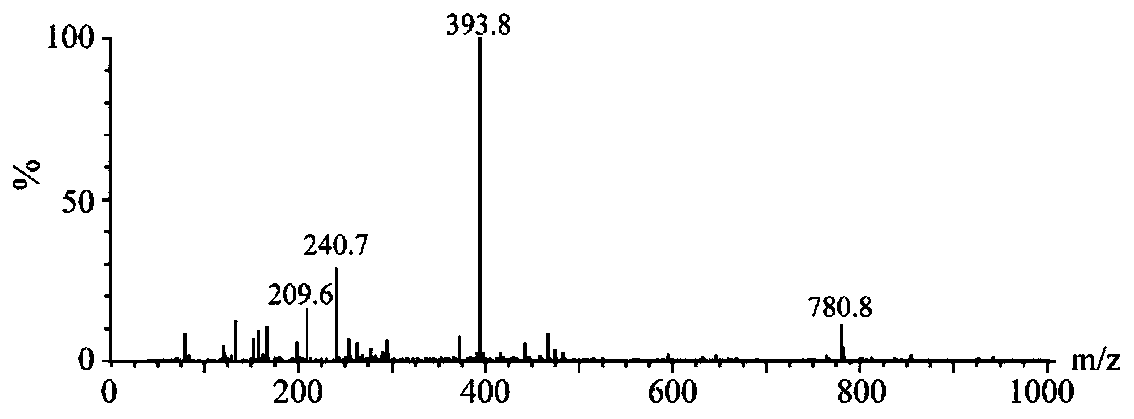
[0053] Weigh 20 mg of Kemieratine and 15 mg of carboxymethylhydroxylamine hemihydrochloride (CMO), dissolve them in 3 mL of pyridine solution, transfer them to a round-bottomed flask with a condensing device, and react with magnetic stirring at 60 ° C for 8 h. Blow dry (60° C.) under a water bath nitrogen instrument. Add 3 mL of 0.1M NaHCO 3solution, vortex to dissolve the residue. And extraction was performed 3 times with 5 mL of ethyl acetate. Afterwards, the aqueous phase was adjusted to pH=3 with 0.1M HCl, and extracted three times with 5 mL of ethyl acetate. The organic phase was blown dry (30° C.) with a water bath nitrogen apparatus to obtain the hapten CF-CMO (23 mg). (Synthetic route diagram see figure 1 )
[0054] The purified haptens were identified by mass spectrometry (see figure 2 ), CF-CMO: MS m / z 393.8[M-Na] + , the mass spectrometry data was consistent with the mol...
Embodiment 2
[0055] Embodiment 2, the preparation of gram rodent artificial antigen
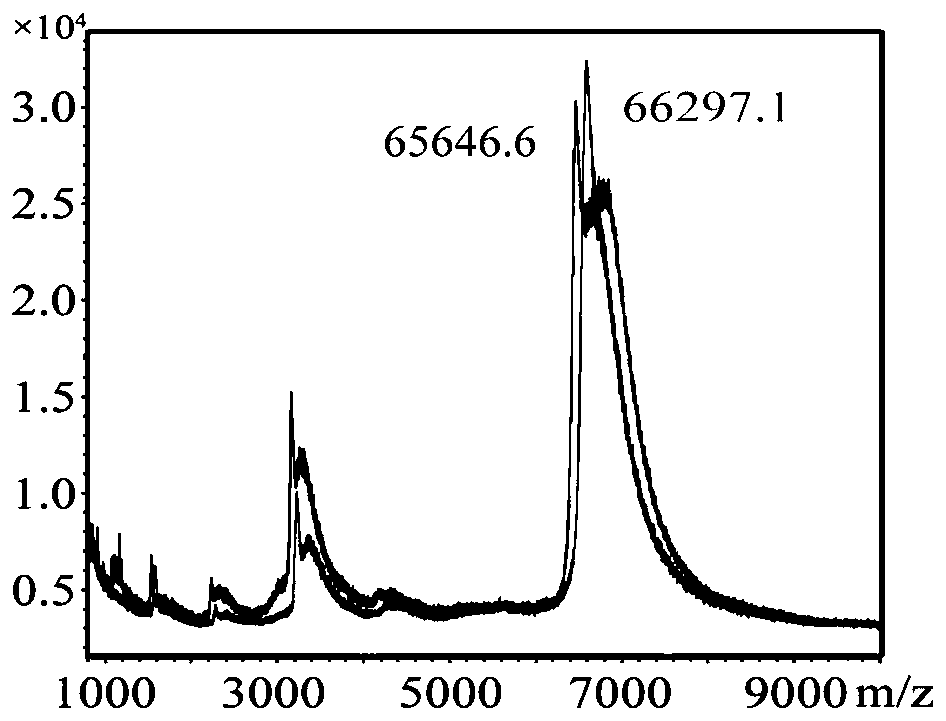
[0056] Weigh 10 mg of gram murine hapten (CF-CMO) and dissolve it in 300 μL of DMF to obtain a hapten solution. Add 5mg EDC and 3mg NHS in the prepared hapten solution again (flow chart sees figure 1 ), placed on a magnetic stirrer, 300rpm room temperature reaction 5h. The reacted solution was centrifuged (5000 rpm), and the supernatant (280 μL) was collected. Get 40mg BSA and 50mg OVA afterward, be dissolved in the PBS damping fluid that contains 5% (volume percentage composition) DMF respectively in 7mL and 9mL contain in the PBS buffer fluid of 5% (volume percentage composition) DMF, obtain two kinds of albumen solutions. 200 μL and 180 μL of the above supernatant were added dropwise to the two protein solutions respectively, and placed in a magnetic stirrer for 5 hours of reaction to obtain a conjugate (artificial antigen) of the hapten and the carrier protein. The protein conjugate prepared above ...
Embodiment 3
[0057] Embodiment 3, the preparation and identification of the polyclonal antibody of Kemomurine
[0058] 1. Preparation of murine polyclonal antibody
[0059] A total of 6 mice were immunized with the CF-CMO-BSA prepared above as the immunogen, and CF-CMO-OVA was used as the coating agent for antiserum detection. The concentration of the complete antigen was determined by the Bradford method, and the concentrations of CF-CMO-BSA and CF-CMO-OVA were both 5 mg / mL.
[0060] For the first immunization, dilute the immunogen to 1 mg / mL (dilute with 0.01mol / L PBS), mix the diluted immunogen with Freund’s complete adjuvant in equal volume, and fully emulsify it, and inoculate it subcutaneously at multiple points on the back of the neck for 6-8 days. Week-old BALB / c mice (6 mice), the dose of inoculation immunogen was 100 μg / mouse, and the injection dose was 0.2 mL / mouse. Afterwards, booster immunization was performed every 3 weeks, and the immunogen was emulsified with an equal vol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com