Dimethomorph hapten, and preparation method and application thereof
The technology of dimethomorph and mesomorpholine is applied in the field of dimorpholine hapten and its preparation, which can solve the problems of many pretreatment steps, low recovery rate and the like, and achieves simple synthesis method, fast detection method, high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1: the synthesis of dimethomorph hapten
[0038] Step 1) p-nitrobenzoyl chloride 50.0g (purchased from Shanghai McLean Biochemical Technology Co., Ltd., article number N814650), with absolute ethanol as solvent, p-nitrobenzoyl chloride: chlorobenzene: aluminum trichloride (purchased from Shanghai McLean Biochemical Technology Co., Ltd., product number A822326) with a molar ratio of 1:2.5:1.5, a reaction temperature of 75° C., and a reaction time of 1.5 h. The reaction solution became viscous, reddish brown, stopped the reaction, poured into 2M sulfuric acid aqueous solution for treatment, suction filtered and washed with water, added the filter cake into dilute sodium hydroxide solution, heated and stirred, washed with water until neutral, and dried to obtain a yellow crude product. After dissolving in 95% ethanol, filtering and recrystallizing, 46.1 g of white solid, product 1, was obtained, with a yield of 92.2%.
[0039] Step 2) Dissolve 29.5 g of triethy...
Embodiment 2
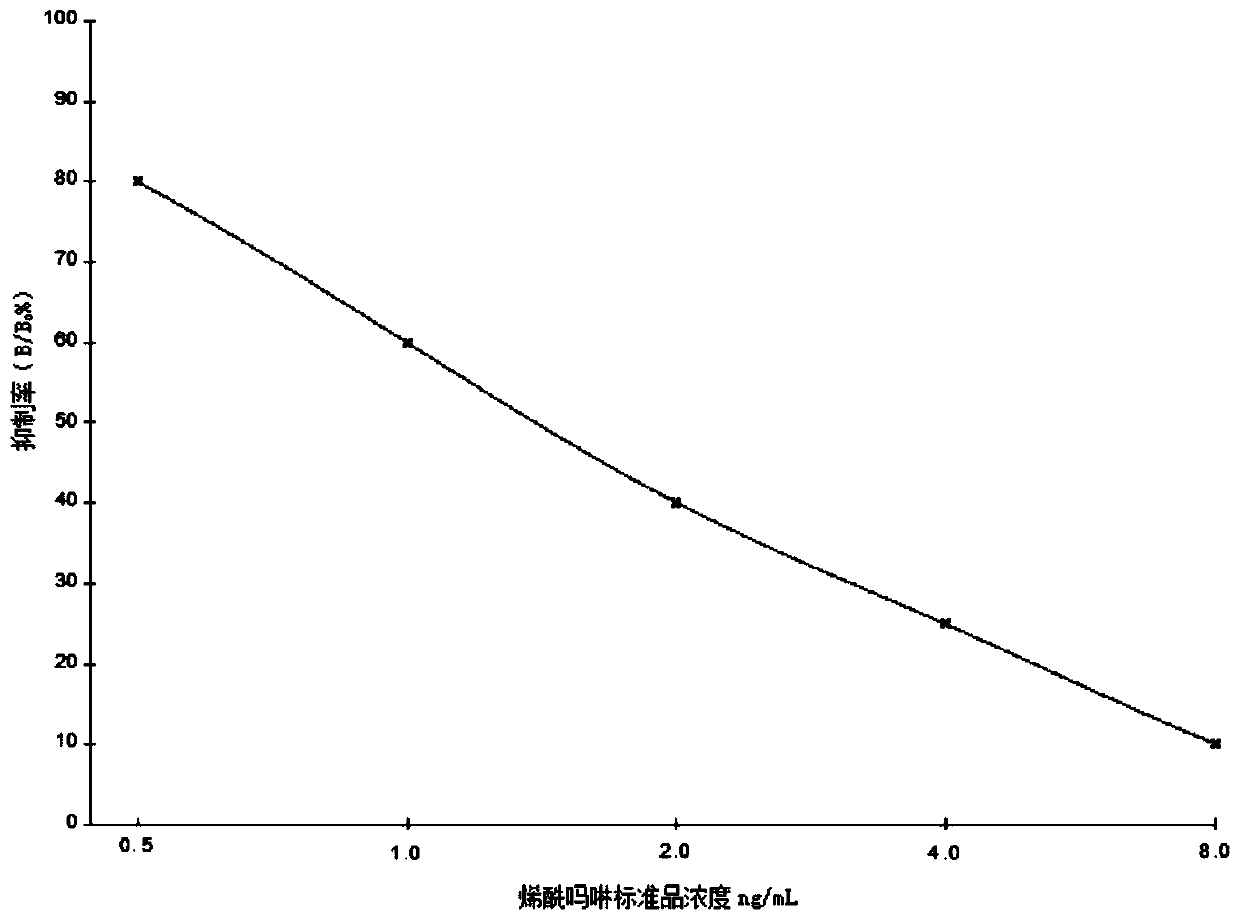
[0043] Example 2: Identification of dimethomorph haptens
[0044] Samples of product 3, product 4, and product 5 in Example 1 were sent to Beijing Zhongke Huiren Technology Co., Ltd. for NMR identification. The specific results are as follows:
[0045] Get the product 3 structure of above-mentioned embodiment 1 and be characterized as:
[0046] 1 H NMR: δ6.16 (1H, s), 6.99 (2H, ddd, J = 8.0, 1.3, 0.6Hz), 7.38 (2H, ddd, J = 8.5, 1.5, 0.5Hz), 7.50-7.61 (4H, 7.53(ddd,J=8.0,1.5,0.6Hz),7.58(ddd,J=8.5,1.6,0.5Hz)).
[0047] Get the product 4 structure of above-mentioned embodiment 1 and be characterized as:
[0048] 1 H NMR: δ3.51-3.67 (8H, 3.59 (ddd, J = 15.2, 10.2, 3.2Hz), 3.62 (ddd, J = 12.2, 3.2, 2.4Hz)), 6.35 (1H, s), 6.82 (2H ,ddd,J=8.4,1.2,0.5Hz),7.31-7.40(4H,7.34
[0049] ddd,J=8.4,1.9,0.5Hz),7.37(ddd,J=8.4,1.4,0.5Hz)),7.57(2H,ddd,J=8.4,1.7,0.5Hz).
[0050] Get the product 5 structure of above-mentioned embodiment 1 and be characterized as:
[0051] 1 H NMR: δ3.51-3....
Embodiment 3
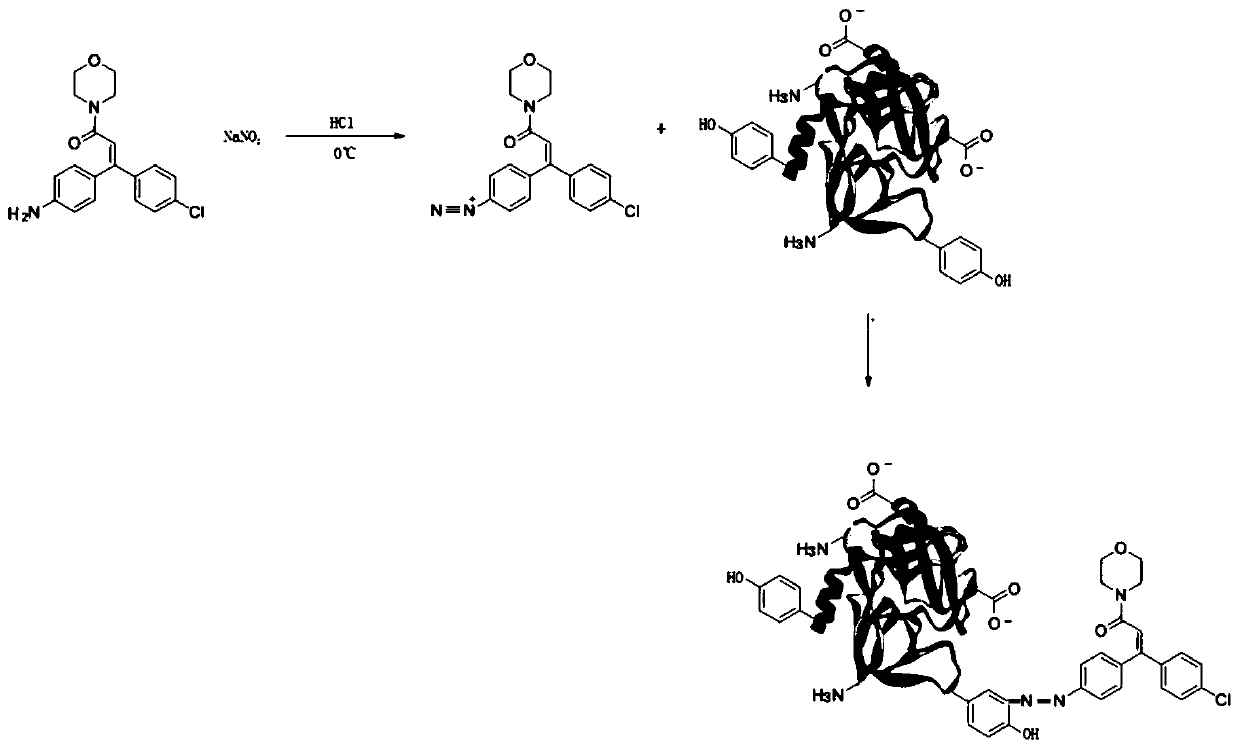
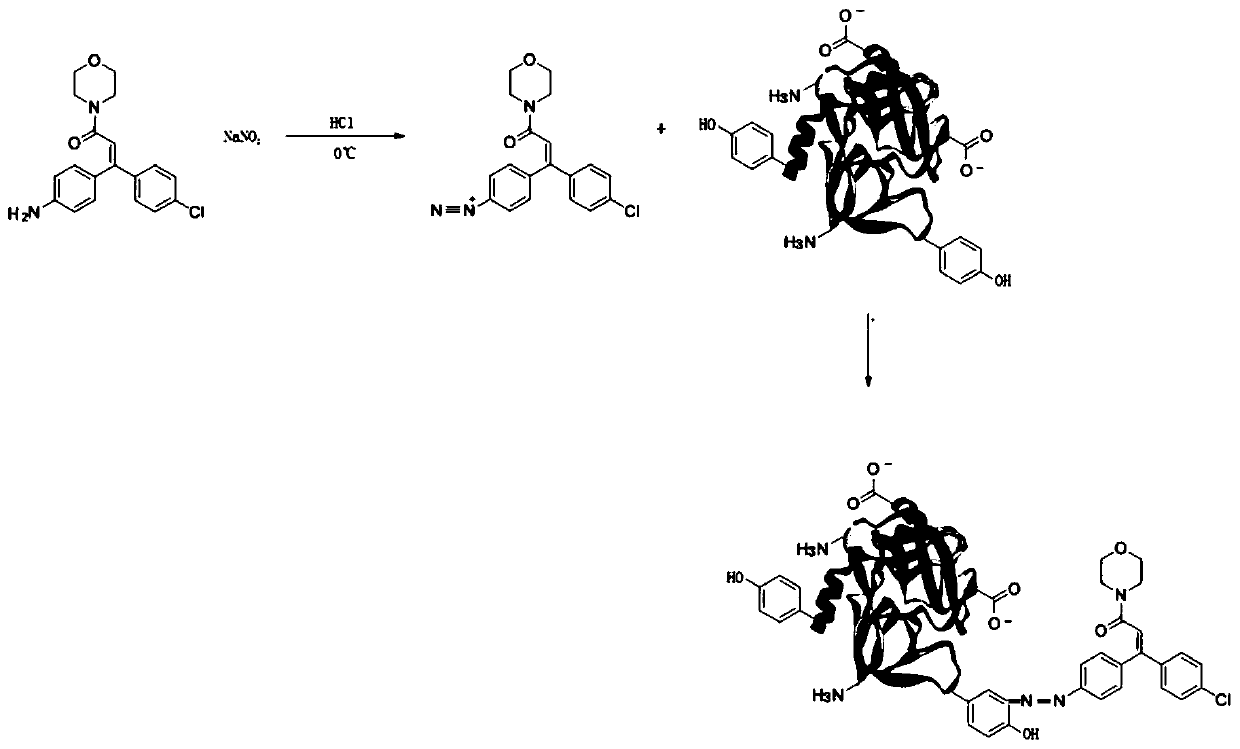
[0052] Example 3: Dimethomorph antigen
[0053] Step 1) Dissolve 50 mg of dimethomorph hapten with dilute hydrochloric acid, add dropwise 1.5% sodium nitrite solution, and keep stirring under ice bath. After the solution turned blue-black, stop adding the sodium nitrite solution and continue the reaction for 15 minutes.
[0054] Step 2) One portion was added dropwise to bovine serum albumin solution (pH 8.3, 0.2M carbonate buffer solution) (purchased from sigma company, product number 5479) with a concentration of 20 mg / mL, and the other portion was added dropwise to 10 mg / mL In mL ovalbumin solution (purchased from sigma, product number A5503) (pH 8.3, 0.2M carbonate buffer solution), stir, adjust the pH to 10, react at 2-8°C for 3h, and use 0.01M PBS Dialyze for 3 days, aliquot and store at -20°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com