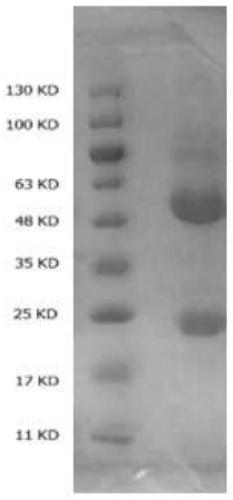
Anti-cryptococcal capsular polysaccharide monoclonal antibody and preparation and application of hybridoma cell strain thereof
A hybridoma cell line, monoclonal antibody technology, applied in application, antifungal/algae/lichen immunoglobulin, botanical equipment and methods, etc., can solve the problem of high price and achieve good affinity and good specificity The effect of binding capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Example 1: Preparation method of monoclonal antibody against cryptococcal capsular polysaccharide
[0085] 1. Polysaccharide extraction
[0086]Cultivate cryptococcus (the strain number is ATCC208821) with liquid Sabouraud medium, culture at 20-25°C for 3-4 days, centrifuge the supernatant after the bacteria solution is inactivated, add sodium acetate to the supernatant to a final concentration of 5 %, adjust the pH=5.0; add 3 times the volume of absolute ethanol, overnight at 4°C, a creamy precipitate at the bottom can be seen; after discarding the supernatant, the precipitate is cryptococcal capsular polysaccharide, air-dry the precipitate, and weigh (or calculate the mass by concentration) The precipitate was dissolved in 0.2M NaCl, dialyzed in a 50KD dialysis bag for 24 hours, and concentrated by suspension at 4°C; slowly added 0.3% CTAB which was three times the weight of the polysaccharide precipitate, and centrifuged after standing, the obtained precipitate was w...
Embodiment 2
[0103] Embodiment 2 colloidal gold immune test strip preparation
[0104] The anti-cryptococcus capsular polysaccharide monoclonal antibody was labeled with colloidal gold to prepare a double-antibody sandwich method immune colloidal gold test strip, which was named as the cryptococcal capsular polysaccharide detection card (colloidal gold method). The specific process is as follows:
[0105] Step 1 Gold-labeled antibody preparation
[0106] Measure 50mL colloidal gold solution, add it to a sterilized and dried beaker, place it on an electric heating magnetic stirrer, 500-600rpm, add 0.1M K while stirring 2 CO 3 solution, adjust the pH to 9.5; labeling: add 0.3 mL (2 mg / mL) of anti-cryptococcal capsular polysaccharide monoclonal antibody, and stir for 1 h at the same speed as above; sealing: add 5 mL of 10% bovine serum albumin (BSA) solution, 2 %PEG20000 2.5ml, the same speed as above, stir for 1h; low-speed centrifugation: 1500 rpm, 4 ° C for 30 min, discard the precipitate,...
Embodiment 3
[0113] Embodiment 3 colloidal gold immune test strip clinical sample comparison
[0114] The cryptococcal capsular polysaccharide detection card (colloidal gold method) prepared by Example 2 is used as an assessment reagent to carry out clinical trials, and the cryptococcal antigen detection kit (colloidal gold immunochromatography method) of the U.S. Immuno-Mycologics company is selected as a comparison reagent , Blind detection of serum and cerebrospinal fluid samples into the group, statistical analysis of the detection results, and investigation of the consistency of the detection results of the above test strips and contrast reagents. Among the cerebrospinal fluid samples, 321 cases had the same positive test results of the assessment reagent and the comparison reagent, 22 cases had positive test results of only the test reagent, 10 cases had positive test results of only the comparison reagent, and 10 cases had negative test results. The number of cases with consistent r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| aggregation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com