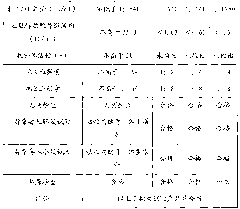Hand-foot-and-mouth disease resistant human immunoglobulin, and preparation and using methods and application thereof
An immunoglobulin, hand, foot and mouth technology, applied in the direction of antiviral immunoglobulin, antiviral agents, antibodies, etc., can solve the problem that the clinical application is still a long time, and achieve the effect of product safety and reliability, satisfying supply, and significant curative effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment 1
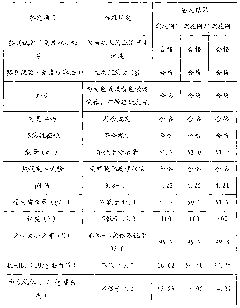
[0034] (1) Separation of components I+II+III.
[0035] Screen out 60L of raw plasma with an anti-EV71 antibody titer ranging from 1:8 to 1:16, soak and sterilize it in 75% ethanol solution for 2 to 3 minutes, rinse the surface of the plasma bag with purified water for 2 to 3 minutes, and use manual scissors Break the bags in a jacketed stainless steel tank, stirring gently. Simultaneously thaw by circulating water at 35°C. The plasma temperature was controlled at 0-2°C during the whole thawing process. Use 0.9% sodium chloride solution to adjust the plasma protein content to 50g / L, adjust the pH of the product to 5.7-6.3 with acetic acid / sodium acetate buffer solution with pH 4.0, stir and add 95% ethanol below -15°C to make the protein solution The final concentration of ethanol is 20%, the final temperature is controlled at -4~-6°C, and the reaction is stirred for 2 hours, and the filter aid diatomite and perlite are respectively added to the protein solution at 2 g / L, and...
specific Embodiment 2
[0054] (1) Separation of components I+II+III.
[0055] Screen out 50L of raw plasma with an anti-EV71 antibody titer of not less than 1:64, soak and disinfect in 75% ethanol solution for 2 to 3 minutes, rinse the surface of the plasma bag with purified water for 2 to 3 minutes, break the bag with manual scissors In a jacketed stainless steel tank, stir gently. Simultaneously thaw by circulating water at 20°C. The plasma temperature was controlled at 0-2°C during the whole thawing process. Use 0.9% sodium chloride solution to adjust the plasma protein content to 40g / L, adjust the pH of the product to 5.7 with acetic acid / sodium acetate buffer solution with pH 4.0, stir and add 95% ethanol below -15°C to make the ethanol in the protein solution final The concentration is 19%, the final temperature is controlled at -4~-6°C, and the reaction is stirred for 2 hours. The filter aid perlite is added to the protein solution at 5g / L, and the reaction is continued for 1 hour, and then...
specific Embodiment 3
[0071] (1) Separation of components I+II+III.
[0072] Screen out 70L of raw plasma with an anti-EV71 antibody titer ranging from 1:16 to 1:32, soak and sterilize it in 75% ethanol solution for 2 to 3 minutes, rinse the surface of the plasma bag with purified water for 2 to 3 minutes, and use manual scissors Break the bags in a jacketed stainless steel tank, stirring gently. Simultaneously thaw by circulating water at 20°C. The plasma temperature was controlled at 0-2°C during the whole thawing process. Use 0.9% sodium chloride solution to adjust the plasma protein content to 55g / L, adjust the pH of the product to 6.3 with acetic acid / sodium acetate buffer solution with pH 4.0, stir and add 95% ethanol below -15°C to make the ethanol in the protein solution final The concentration is 22%, the final temperature is controlled at -4~-6°C, and the reaction is stirred for 2 hours, and the filter aid diatomaceous earth is added to the protein solution at 10g / L, and the reaction is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com