Preparation method of piperacillin monoclonal antibody and application
A piperacillin monoclonal antibody technology, applied in the biological field, can solve the problems of delayed disease, insufficient guarantee of piperacillin drug efficacy, and influence on treatment effect, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment l
[0090] Embodiment 1: the preparation of piperacillin monoclonal antibody
[0091] 1.1 Preparation of feeder cells:
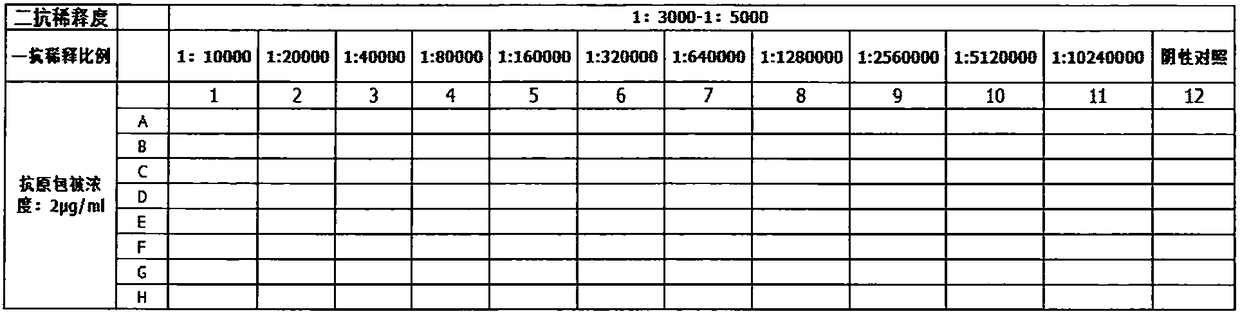
[0092] Take 6-10 week-old Balb / c mice, kill them by pulling their necks, soak them in 75% alcohol, and disinfect them for 3-5 minutes; transfer them to a sterile operating table, cut the skin with sterile scissors, and fully expose their peritoneum; Inject 10mL of pre-cooled serum-free 1640 culture solution into a sterile syringe, gently tap the mouse abdominal cavity with the bottom of the tweezers, suck out the culture solution, put it into a 10mL centrifuge tube, and centrifuge at 1200r / min for 10min; discard the supernatant, and use complete culture solution Resuspend the feeder cells, count, and adjust the number of cells to 2×105 / mL, add the feeder cells into a 96-well plate, 100 μL per well, and then put them in 37°C, CO 2 incubator cultivation;
[0093] 1.2 Hybridoma cell fusion and subcloning screening by limiting dilution method
[0094] a. Preparat...
Embodiment 22
[0108] Example 22: Antibody Purification
[0109] 2.1 Pretreatment of the column:
[0110] a. Take out the prepared Protein A / Protein G filler and transfer it into a 50ml centrifuge tube, add an appropriate amount of 20% (volume ratio) ethanol, mix it upside down, mix the filler and transfer it to an empty column;
[0111] b. After filling the empty column, tighten the connector, and manually discharge 20% of the filler from the connector until 1-2ml of 20% ethanol remains at the outlet.
[0112] c. Connect the prepared column to the AKTA system, wait for the system to balance and remove the air.
[0113] 2.2 Preparation of experimental reagents (all reagents should be sterilized with a 0.45 μm filter):
[0114] a.VBS buffer: pH7.2 barbiturate buffer
[0115] Accurately weigh 0.737g of barbiturate, 8.766g of sodium chloride, 0.197g of magnesium sulfate heptahydrate, and 0.033g of anhydrous calcium chloride, dissolve them in 1L of distilled water, adjust the pH to 7.2 with 1...
Embodiment 3
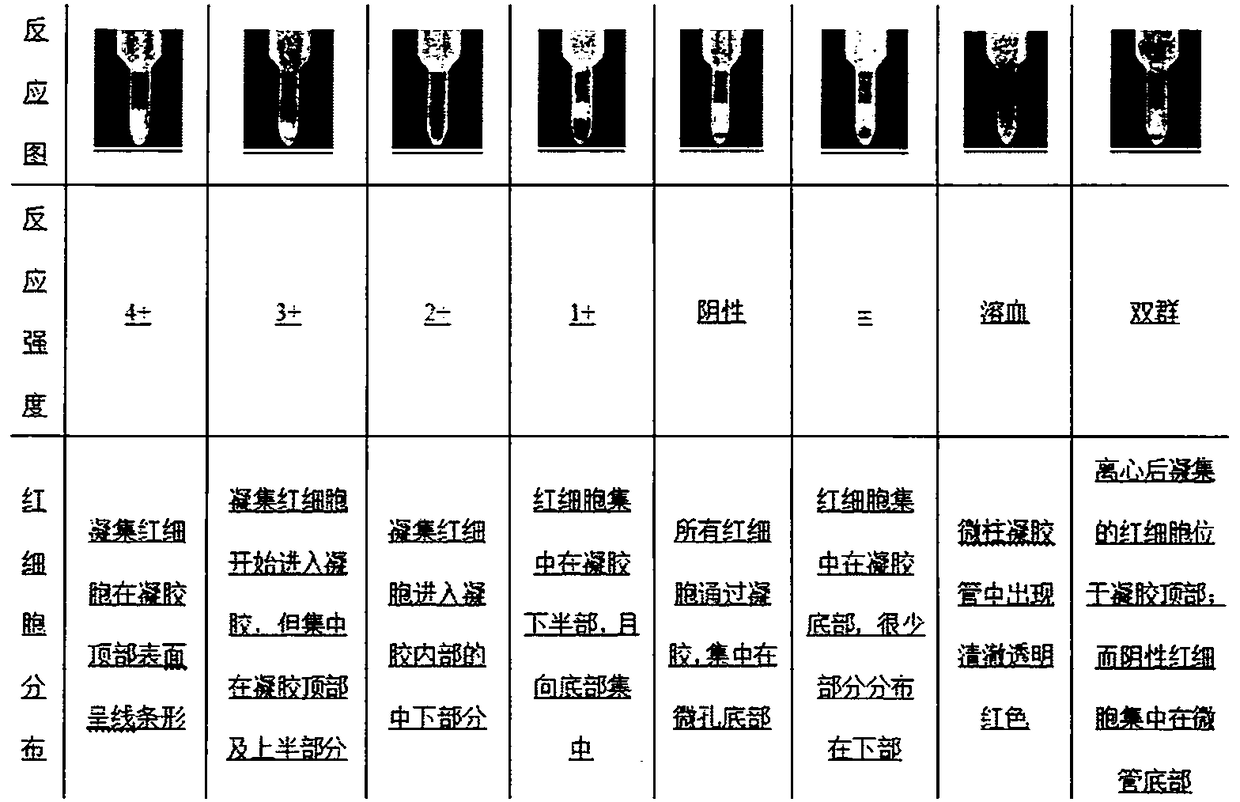
[0135] Example 3: Identification of antibody purity
[0136] 1. Experimental steps
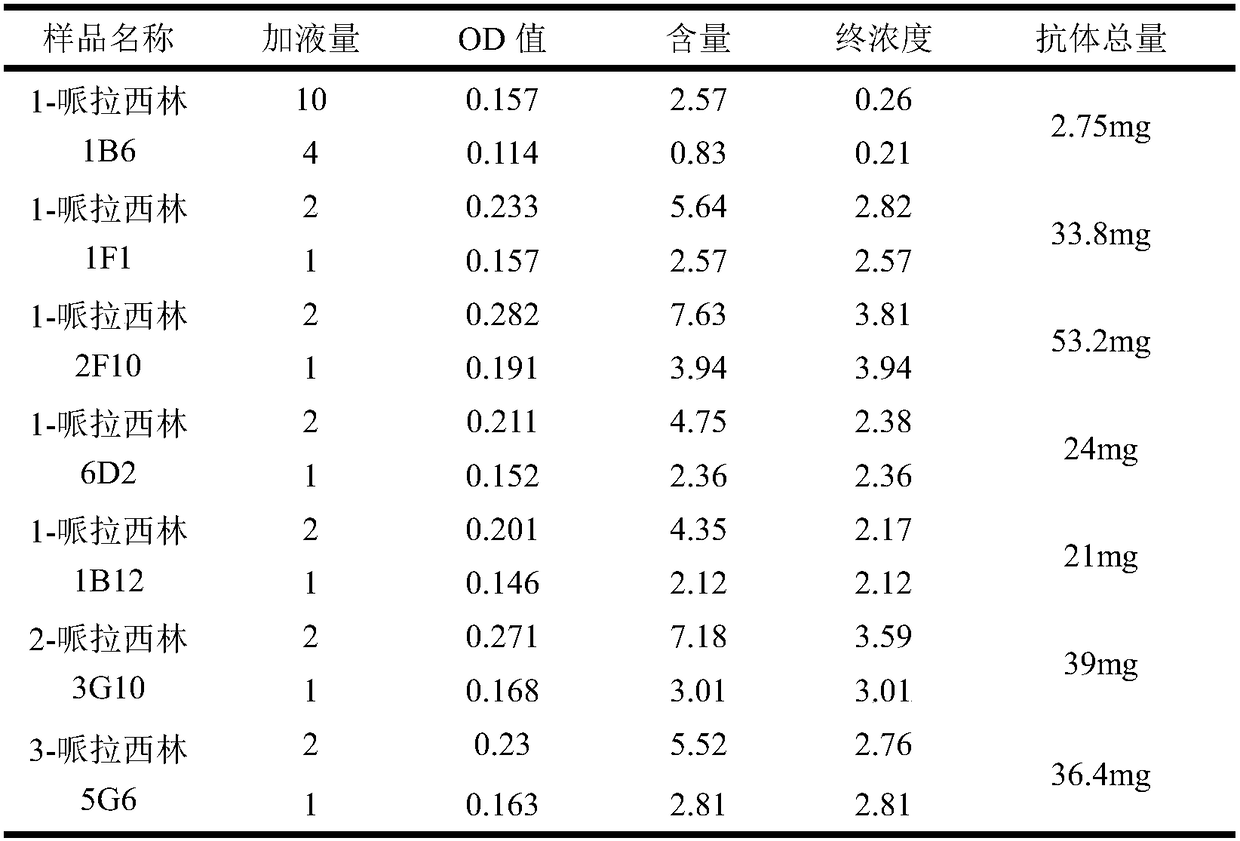
[0137] Take out the sample, add 0.1% proclin-300, filter it with a 0.22 μm filter, and use the BCA method to measure the antibody concentration. The specific operation is as follows:
[0138] a. According to the number of samples, prepare an appropriate amount of BCA working solution with 50 volumes of BCA reagent A plus 1 volume of BCA reagent B (50:1), and mix well. BCA working solution is stable at room temperature for 24 hours.
[0139] b. Completely dissolve the protein standard, take 10 μl and dilute to 100 μl, so that the final concentration is 0.5 mg / ml. What solution is the protein sample in, and what solution should the standard be diluted with. However, for simplicity, the standards can also be diluted with 0.9% NaCl or PBS.
[0140] c. Add 0, 1, 2, 4, 8, 12, 16, and 20 μl of the standard to the standard wells of the 96-well plate, and add the solution used to dilute the standar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com