Kit capable of determining titer of neutralizing antibodies of varicella-zoster viruses and production method thereof
A herpes zoster virus and kit technology, which is applied in the field of kits for determining varicella-zoster virus neutralizing antibody titers, can solve the problems of inability to achieve high-throughput determination, limited sample volume, high cost, and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
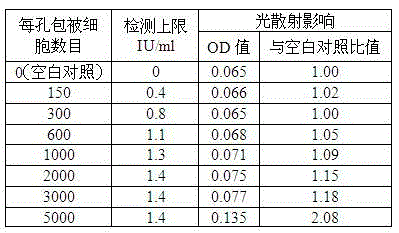
[0049] Example 1 Optimal well-coated cell number studies. If the number of coated cells per well is too small and the amount of antigen is insufficient, the detection limit of the kit will be too low; if the number of coated cells is too large, the cells will cause light scattering, which will affect the measurement results of the absorbance value during the measurement process.
[0050] Human embryonic lung fibroblasts grown as dense monolayers were inoculated with VZV seeds. The inoculation ratio was: 200 plaque-forming units (PFU) of VZV virus were inoculated per 1000 cells. Cultured at 37°C and 5% carbon dioxide for 60 hours, the cytopathic rate was 70%, the cells were digested into single cells with 0.25% trypsin, and bovine serum albumin (final concentration 5%) was added to adjust the cell density to 3×10 4 pcs / ml, 6×10 4 pcs / ml, 1.2×10 5 pcs / ml, 2×10 5 pcs / ml, 4×10 5 pcs / ml, 6×10 5 pcs / ml, 1×10 6 pieces / ml. The above-mentioned cells of different concentrations...
Embodiment 2
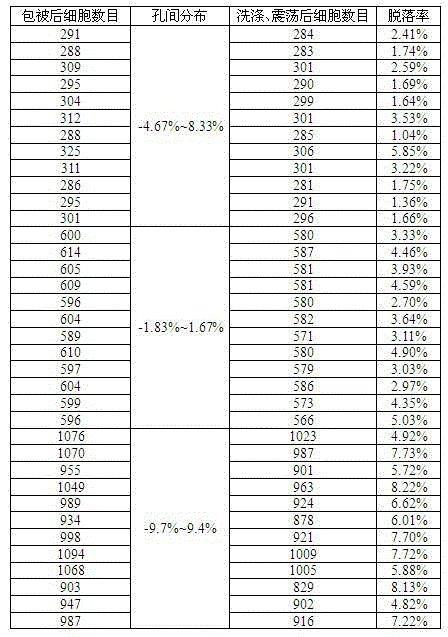
[0060] Example 2 Research on the distribution of coated cells between wells and the shedding of coated cells after washing and shaking.
[0061] Human embryonic lung fibroblasts grown as dense monolayers were inoculated with VZV seeds. The inoculation ratio was: 200 plaque-forming units (PFU) of VZV virus were inoculated per 1000 cells. Cultured at 37°C and 5% carbon dioxide for 60 hours, the cytopathic rate was 70%, the cells were digested into single cells with 0.25% trypsin, and bovine serum albumin (final concentration 5%) was added to adjust the cell density to 6×10 4 pcs / ml, 1.2×10 5 pcs / ml, 2×10 5 pieces / ml. The above-mentioned cells of different concentrations were thoroughly mixed and suspended, and added to different wells of a 96-well plate (12 wells for each concentration were repeated), and 5 microliters of cell suspension was added to each well. Shake the 96-well plate horizontally to distribute the cell suspension evenly at the bottom of the well. Add 5 m...
Embodiment 3
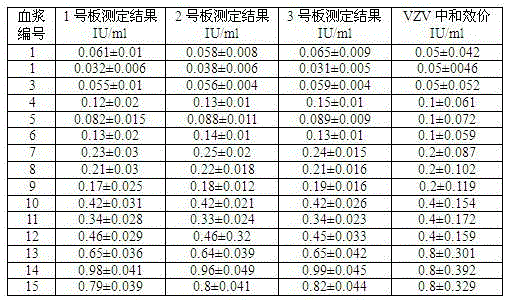
[0065] Example 3 Kit preparation and sample test result analysis
[0066] 1. 96-well plate preparation
[0067] A. Human embryonic lung fibroblasts (MRC5, 75cm) growing into a dense monolayer 2 , about 3 x 10 6 cells) inoculated with varicella-zoster virus (VZV, oka strain) 3×10 5 PFU, cultured at 37°C and 5% carbon dioxide for 3 days, the microscopic examination of the cell lesion reached 70%, the cells were digested with 0.25% trypsin, dispersed into individual cells by pipetting, centrifuged, and the cells were washed with PBS, and 20ml containing 5% The PBS solution of bovine serum albumin resuspended the cells, and the count was 1.6×10 with a cell counting plate. 5 pieces / ml.
[0068] B. Prepare 40 96-well plates, and add the above-mentioned mixed cells into 96-well plates at 5 μl / well using a 12-channel pipette. Shake the 96-well plate horizontally to distribute the cell suspension evenly at the bottom of the well. Add 5 microliters of 4% glutaraldehyde solution ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com