Filamentous hemagglutinin detection kit and its application
A filamentous hemagglutinin and kit technology, applied in applications, biological tests, measurement devices, etc., can solve problems such as differences in registered quality standards and no FHA toxins, and achieve effective immune effects, high and high neutralization titers.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Example 1 Preparation of hybridoma cells
[0061] (1) After the cyclopathy seeds were recovered, and the shake bottle was cultured in fermentation medium, 35.0 ± 2.0 degreulation was cultured for 44-46 hours, and the index growth period was gains.
[0062] (2) After harvest, it is sterilized, clarified, crude, and pure.
[0063] (3) Get purified, the FHA stock solution is obtained after detoxification of formaldehyde.
[0064] (4) After mixing emulsification after mixing emulsification of FHA stock solution and Frego adjuvant (0 day immunization is complete adjuvant, 14 days, 28 days immunized into Fernant Adjuvant), etc., 14 days, 28 days Multi-point immunized BALB / C mice in the back is 0.2 ml / only.
[0065] (5) Last immunization first blood test antibody titer, serum dilution 10 4 The indirect ELISA of the immunized mouse serum was 1.311 with FHA stock solution. After two weeks of last immunization, the selected mice were shocked by the harvest solution, and the splee...
Embodiment 2
[0066] Example 2 Cell fusion and strain
[0067] (1) Cell fusion recovery culture SP2 / 0 cell strain was expanded 3 days before the fusion, and the RPMI1640 cell culture medium was removed 1 day before fusion, and the culture solution was re-added, and SP2 / 0 cells were prepared.
[0068] (2) Immunization mice were sacrificed, and the spleen cell suspension of mice was prepared according to conventional methods.
[0069] (3) According to the results of splenocytes and SP2 / 0 cell counts, the amount of incomplete IMDM culture solution (GIBCO), SP2 / 0 cells are shaken, and the splenocytes are blown well with a pipette. The spleen cells were then mixed with SP2 / 0 cells from 1: 2 to 10: 1 in 50 ml of centrifuge tubes.
[0070] (4) Add incomplete IMDM culture solution to 50ml, centrifuge for 5-10 minutes, and pour a clean. Tap the fusion tube, so that the precipitated cell is loose, and the centrifugal can be placed 37 ° C water bath, ready to fusion.
[0071] (5) 1 ml of 50% of ...
Embodiment 3
[0078] Example 3 Sequence and Stability Detection of Hybridoma Cell Series Secrecy
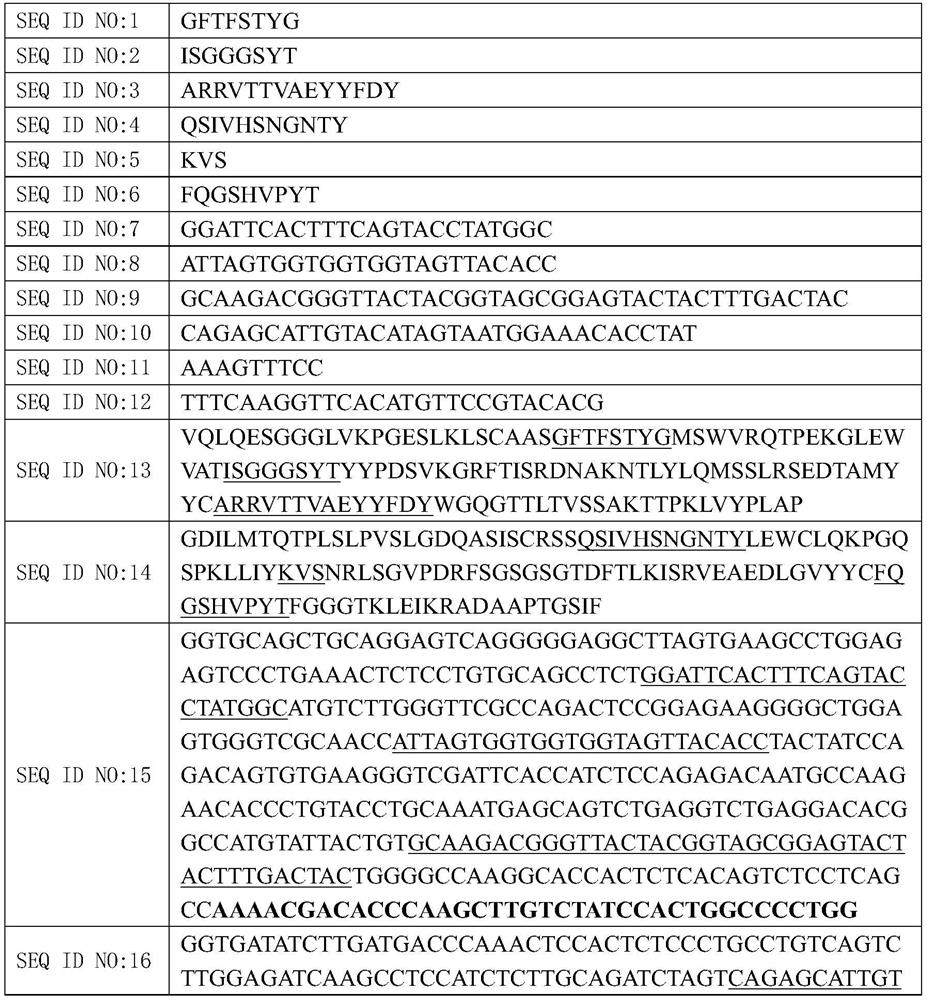
[0079] Remove the frozen FHA mouse monoclonal hybridoma cell line from liquid nitrogen to expand cultivation, 10 6 The total nucleic acid is extracted at the above number, entrusted Beijing Liuhehua Great Technology Co., Ltd. via the PCR amplification of the heavy chains and light chains and sequencing, and then determines the corresponding amino acid sequence by nucleotide sequence, determined polynucleotides measured. Sequences and amino acid sequences for sequence lists.
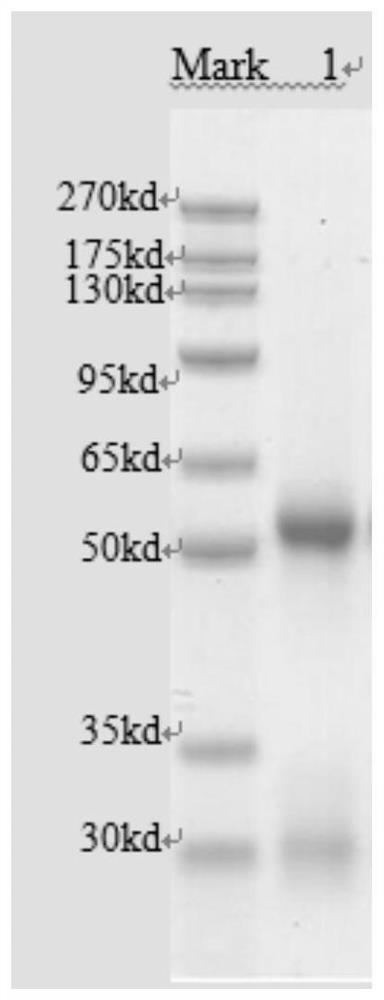
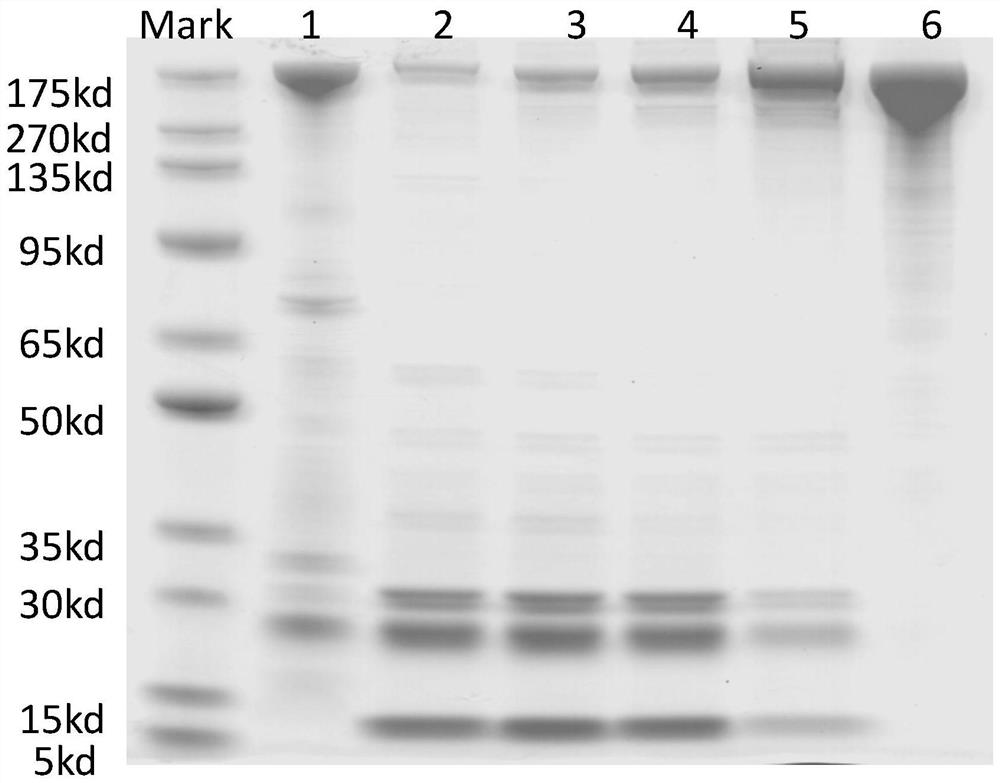
[0080] After the completion of hybridoma cells, the freezed FHA mice monoclonal hybridoma cell line was removed from liquid nitrogen in the preparation of hybridoma cells. After expanding the culture, the antibody effectiveness of indirect ELISA was detected. Price, the ascites prepared in the previous period were tested simultaneously. The results showed that the monoclonal antibody of the hybridoma cell line was reached in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com