Vaccine for preventing and treating diseases caused by Coxsackie virus and preparation method and application thereof
A coxsackie virus and vaccine technology, applied in chemical instruments and methods, antiviral immunoglobulins, biochemical equipment and methods, etc., can solve the development constraints of anti-CVA6 drugs and vaccines, and the lack of stable animals for anti-CVA6 drugs and vaccines. Infection model, unclear pathogenesis of hand, foot and mouth disease, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1. Isolation and identification of Coxsackie virus A6 (CVA6) strain WF057R
[0056] The inventors isolated a Coxsackievirus A6 (Coxsackievirus) strain from the feces of a child with hand, foot and mouth in Shandong Province in 2015, and named the strain WF057R. Coxsackievirus A6 (Coxsackievirus) strain WF057R was deposited on December 26, 2016 in the General Microbiology Center of the China Microbial Culture Collection Management Committee (abbreviated as CGMCC, address: No. 3, Beichen West Road, Chaoyang District, Beijing) No.), the deposit number is CGMCC No.13393.
Embodiment 2
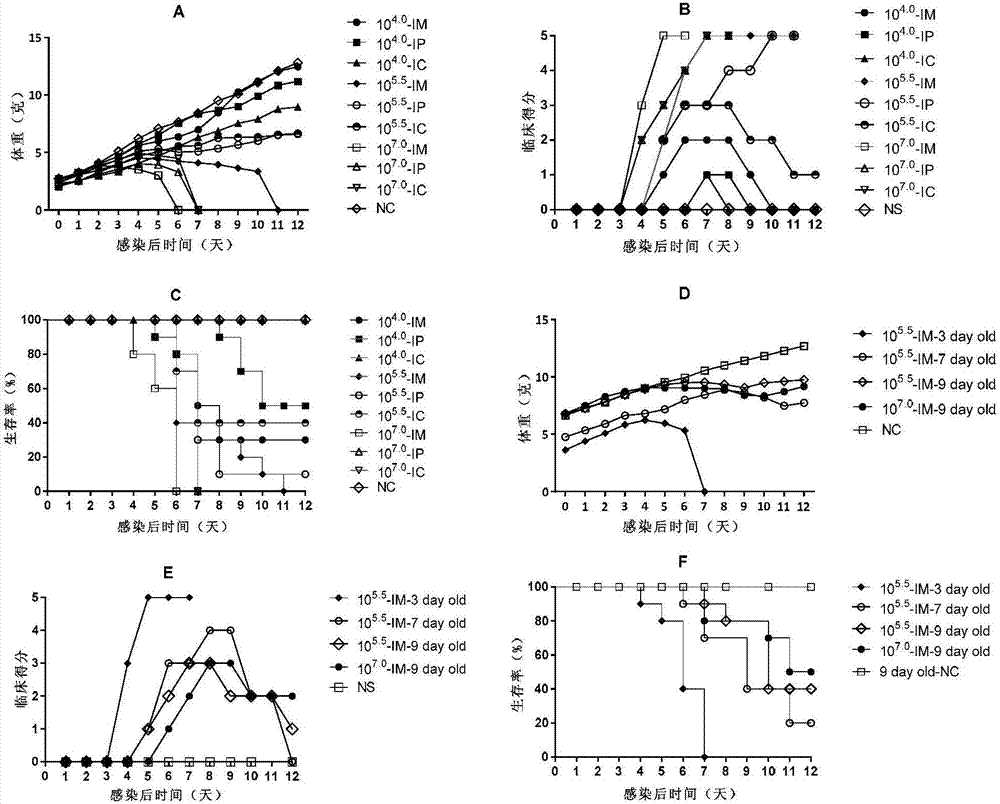
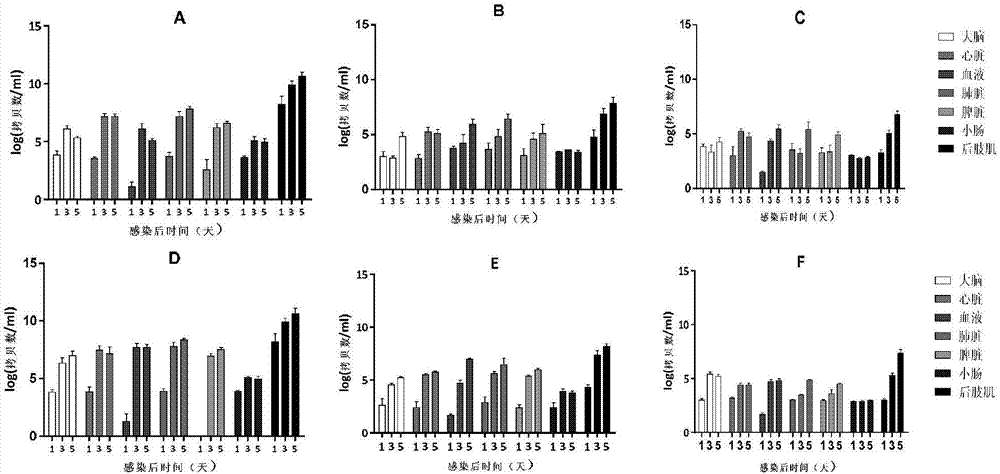
[0057] Example 2: Preparation of CVA6 infection animal model using Coxsackie A6 virus strain WF057R
[0058] 1. Cultivation of WF057R
[0059] Inoculate RD cells in medium 1 (medium 1 is the liquid obtained by adding fetal bovine serum, penicillin and streptomycin to the MEM maintenance solution, wherein the mass percentage of fetal calf serum is 10%, and the mass percentage of penicillin The concentration is 1%, the mass percentage concentration of streptomycin is 1%), the temperature is 37℃, the concentration of 5% CO 2 Cultivate in a cell incubator to obtain RD cell culture solution. The Coxsackie A6 virus strain WF057R of Example 1 was inoculated on the RD cells in the RD cell culture medium, and when the area of the cells with cytopathic effect (CPE) exceeded 80%, the cell culture medium was collected to obtain CVA6 strain WF057R virus solution, quantify the virus in a 96-well plate by limiting dilution method, and determine the virus TCID 50 Afterwards, it is divided into ...
Embodiment 3
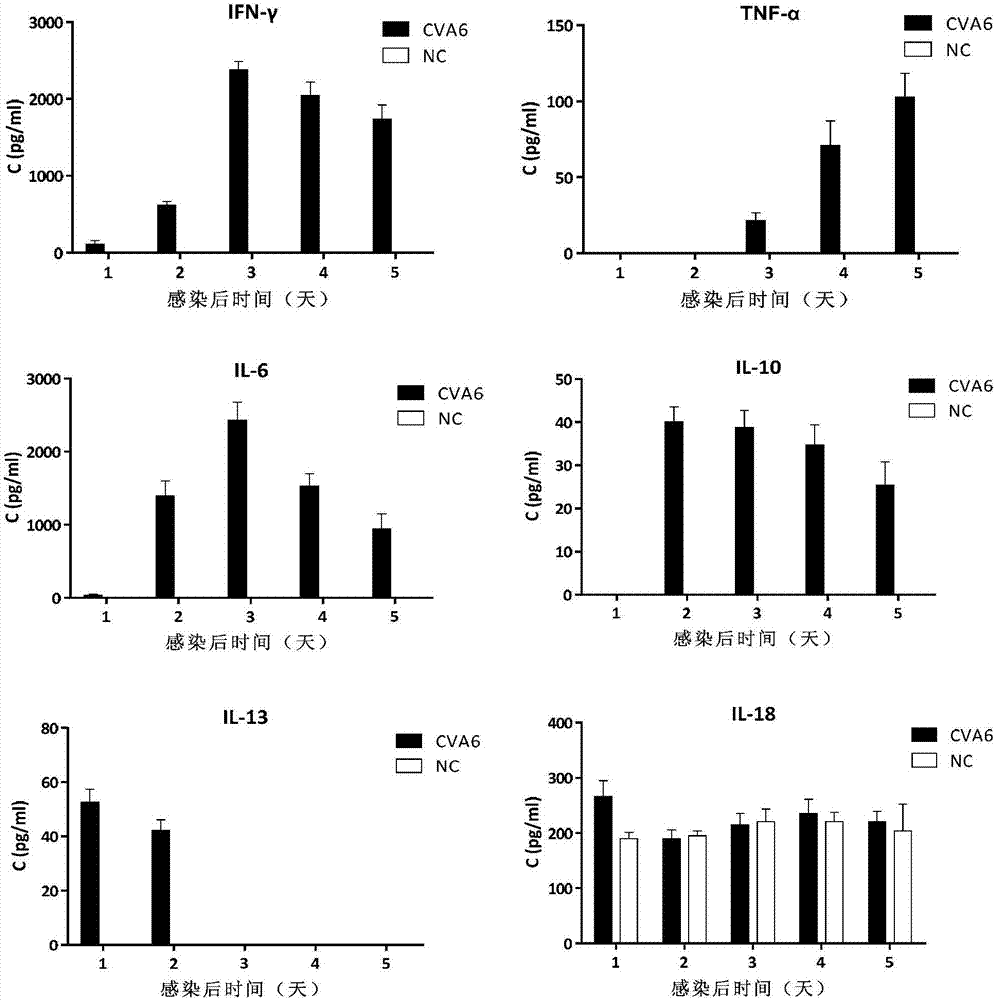
[0092] Example 3. The antiserum produced by the CVA6 vaccine with inactivated WF057R as the activity can protect mice and treat diseases caused by CVA6
[0093] 1. Preparation of inactivated CVA6 vaccine
[0094] The diluted CVA6 strain WF057R virus liquid (10 5 TCID 50 / ml) Dilute with formalin at a ratio of 1:4000 (V / V) to obtain a virus dilution. Incubate the virus dilution at 37°C to inactivate the virus for 72 hours to obtain an inactivated virus solution; Mix the same volume with complete Freund’s adjuvant to make a complete emulsion, which is the CVA6 vaccine. The content of inactivated virus in the CVA6 vaccine is 5×10 4 TCID 50 / ml. The infectivity titer of CVA6 vaccine was detected by microtitration to observe its inactivation effect.
[0095] Virus microtitration method: press 1×10 per well 4 Cells / 100μl culture medium (the cells in the culture medium are RD cells, and the medium is the medium 1 of Example 2). Inoculate RD cells on a 96-well flat-bottom cell culture plate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com