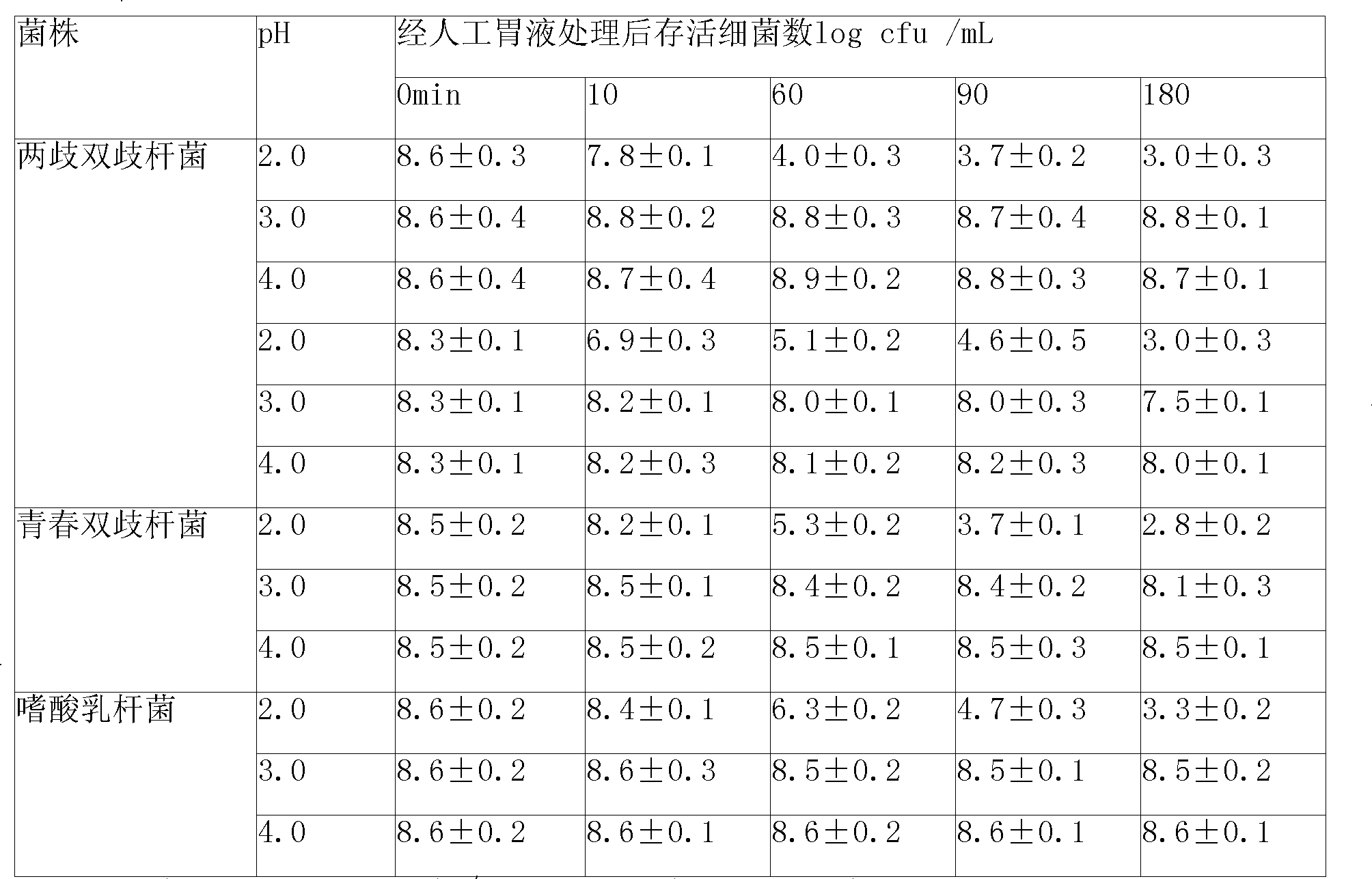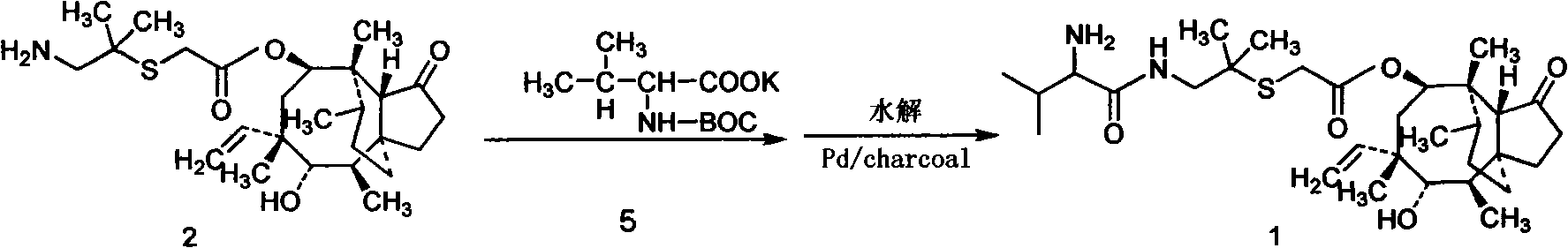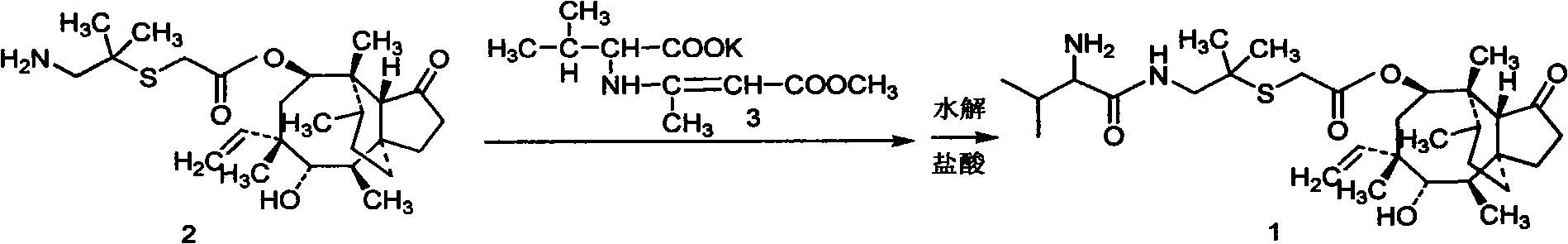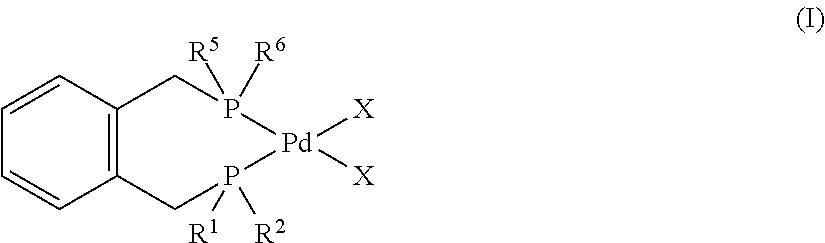Patents
Literature
197results about How to "Reduced purification steps" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
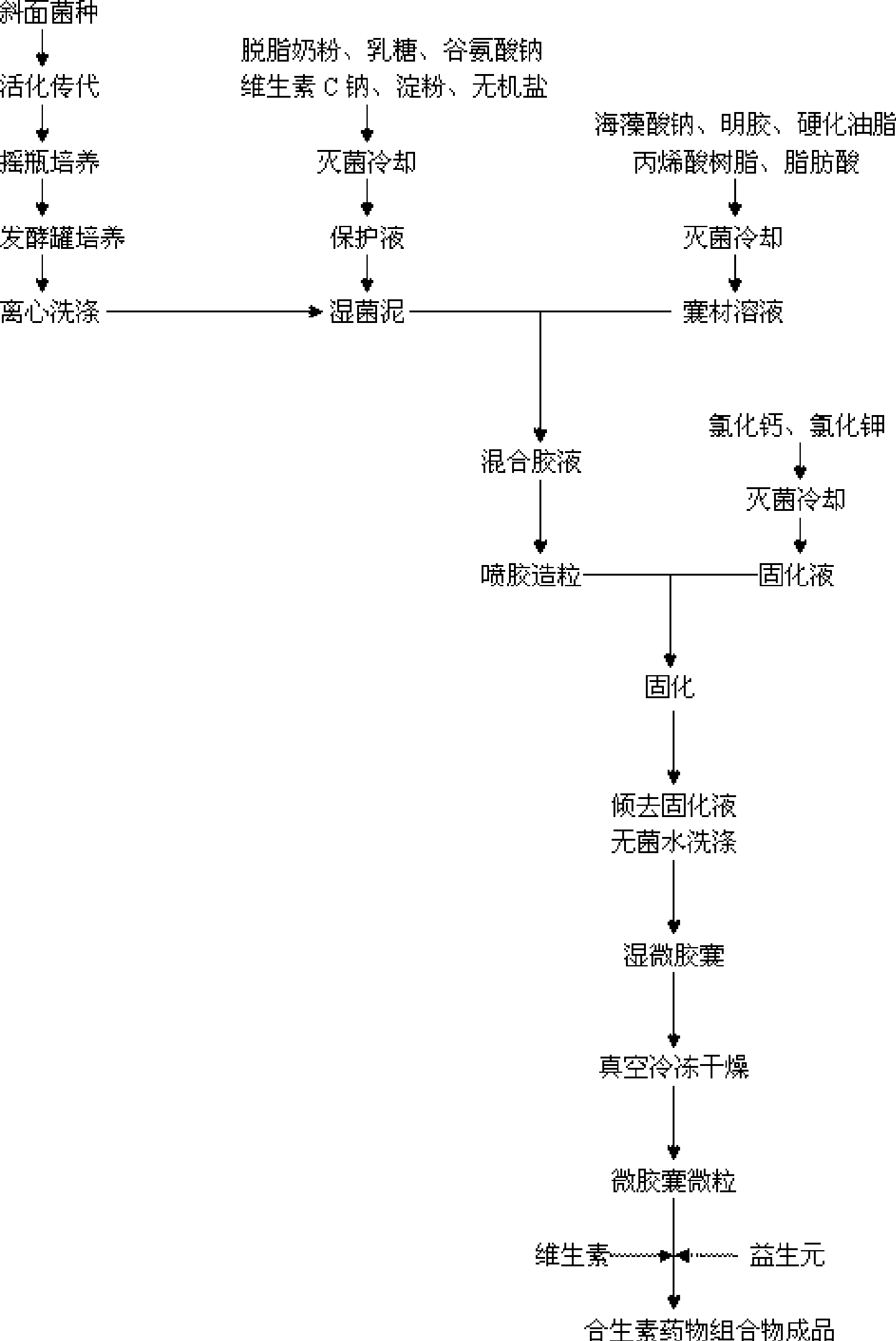
Synbiotics medicament composition
InactiveCN101366734AAdd flavorHigh nutritional valueBacteriaBacteria material medical ingredientsIntestinal structureMicroecosystem
The invention relates to a micro-ecological regulator, which belongs to the category of micro-ecological preparations. The product contains Bifidobacterium bifidum, Bifidobacterium longum, Bifidobacterium adolesentis, lactobacillus acidophilus, fructo-oligosaccharides, galacto-oligosaccharides, vitamins and the like. A concrete production process comprises the following steps: 1. bacterial strains are respectively or jointly subjected to liquid deep high-density cultu, so as to obtain fermentation liquid; 2. after the fermentation liquid is centrifuged, bacterial sludge is collected, added to protective agents and made into freeze-dried powder; 3. the freeze-dried powder is added to microcapsule materials and made into microcapsules through air suspension treatment; and 4. the microcapsules are mixed with oligosaccharides, vitamins and the like and then are made into a synbiotics pharmaceutical composition. By directly replenishing human intestines with Bifidobacterium and lactobacillus acidophilus, and by simultaneously providing prebiotics for ensuring that beneficial bacteria after entering intestines can be activated rapidly and proliferate, the product adjusts and improves intestinal micro-ecosystem, so as to achieve the aims of regulating organism immunity, delaying senility, inhibiting tumors, regulating blood lipid and improving intestines and stomach. A preparation method of the product can solve the technical problem that the prior probiotics medicine is difficult to keep the stability of probiotics at normal temperature and unstable under acidic conditions, and provides a pharmaceutical composition which can be stably stored at normal temperature.
Owner:辽宁大生商贸有限公司
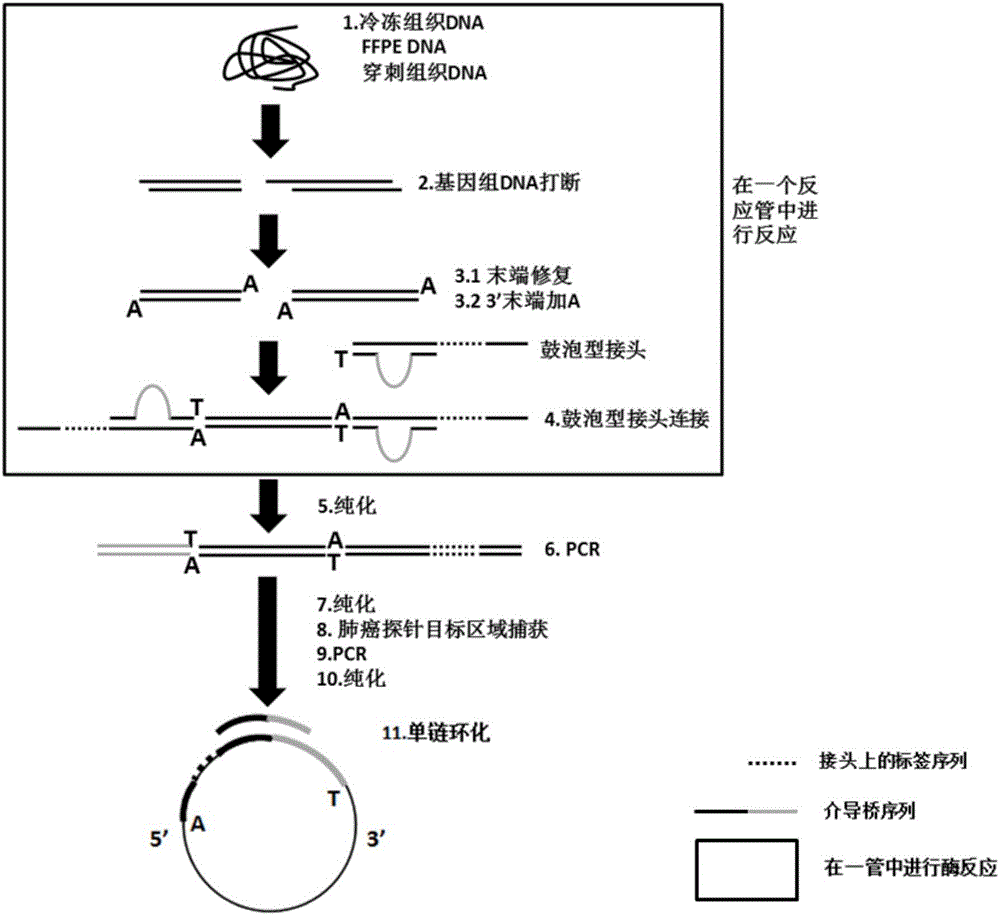
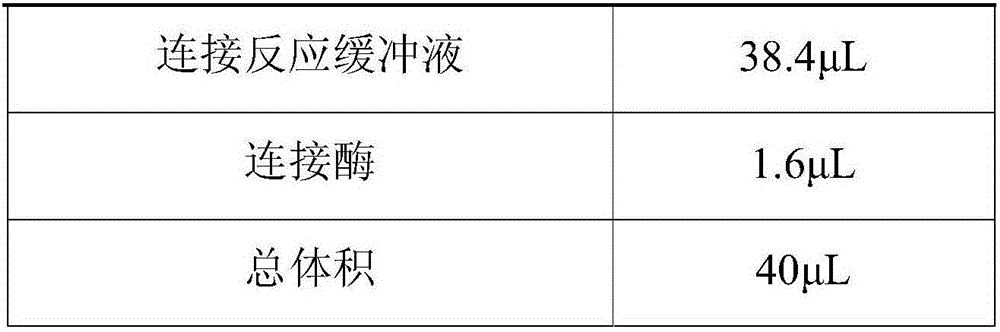
Building method of library for detecting non-small cell lung cancer gene mutation and kit
PendingCN106497920AImprove recycling efficiencyImprove utilization efficiencyMicrobiological testing/measurementLibrary creationGene targetingGene mutation
The invention discloses a building method of a library for detecting non-small cell lung cancer gene mutation and a kit. The method includes: using tubular reaction to complete genome DNA breaking and connector connection, performing hybrid capture on connection products after amplification and non-small cell lung cancer related gene target area probes, and performing BGISEQ-500 / 1000 platform sequencing and data analysis to obtain mutation conditions. The method has the advantages that the experiment flow is optimized greatly by the tubular reaction, operation complexity and time are reduced, and the requirements on clinical sample initial amount are lowered; multiple genes and multiple sites can be detected in one step, point mutation, insertion and deletion, structural variation and copy number variation are covered, the detecting result is accurate and overcomes the defect that a PCR capture method cannot detect the structural variation in one step, and the effectiveness of the high-throughput sequencing applied to the detection of the non-small cell lung cancer gene mutation; the method is wide in coverage, high in cost performance, capable of providing a reference basis for the diagnosing, treatment and drug use performed by doctors, and the method is suitable for being popularized and used in a large-scale manner.
Owner:BGI BIOTECH WUHAN CO LTD
Construction method of plasma free DNA library
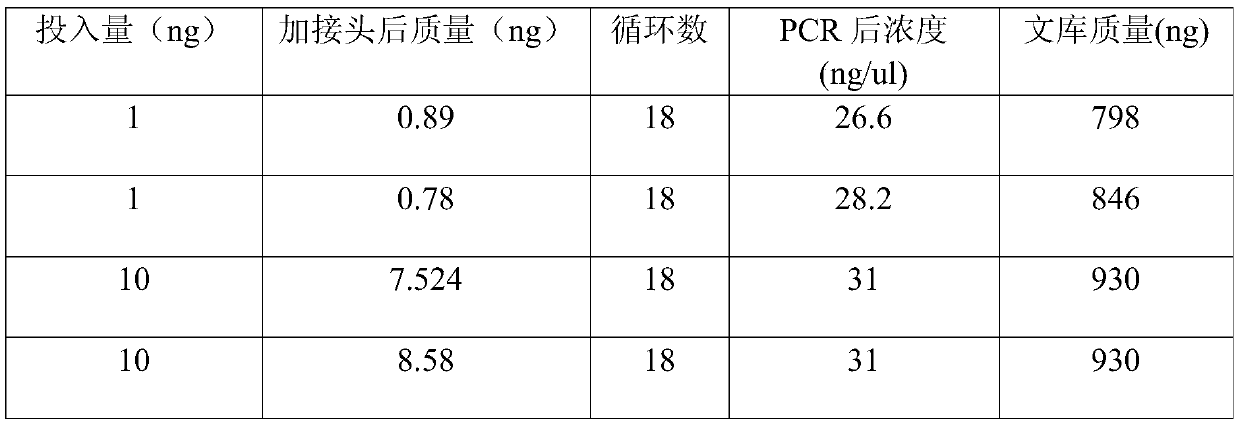
The invention discloses a 'construction method for a small amount of DNA library'. The method comprises the following steps: carrying out end repairing on plasma free DNA or fragmented DNA and simultaneously carrying out phosphorylation modifying on 5' terminal to obtain modified DNA; later, implementing blunt end linking directly to an artificial linker and purifying to obtain relatively pure linker-containing DNA having a gap on joint; then repairing the gap by virtue of polymerase chain reaction (PCR) polymerase before PCR amplification to obtain repaired complete linker-containing DNA; and carrying out PCR amplification reaction and purifying a finished product to obtain a final high-throughput sequencing library. The method is not only simple, convenient and efficient, and is capable of avoiding a purification step and reducing DNA loss in a library construction process, but also can effectively solve a problem of linker self-linking which is easy to occur in a micro-DNA library construction process by virtue of a special linker and blunt end linking way; and meanwhile, by repairing the gap before amplification, sufficient complete templates are provided before amplification for the amplification reaction.
Owner:BEIJING MICROREAD GENE TECH
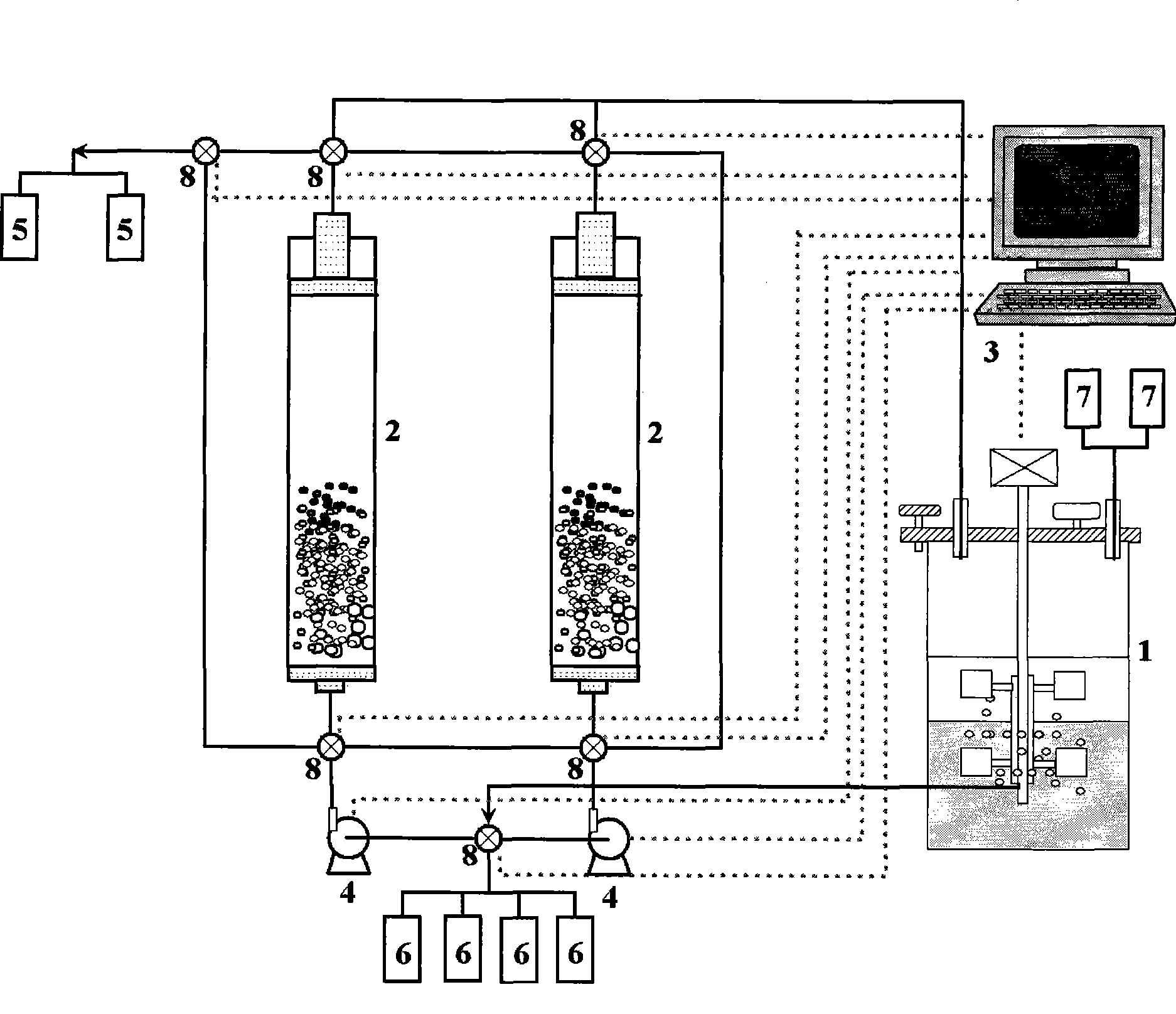
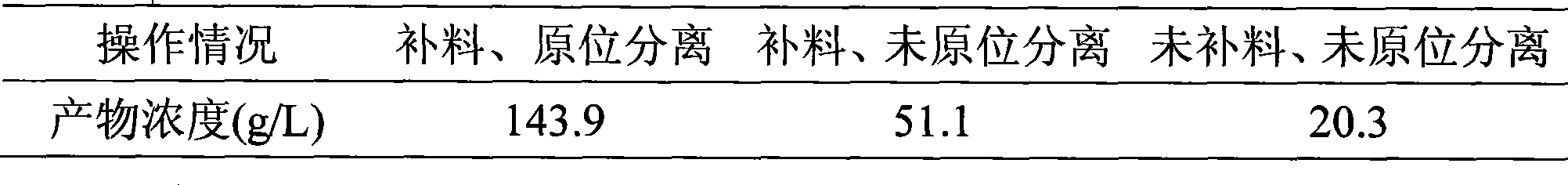
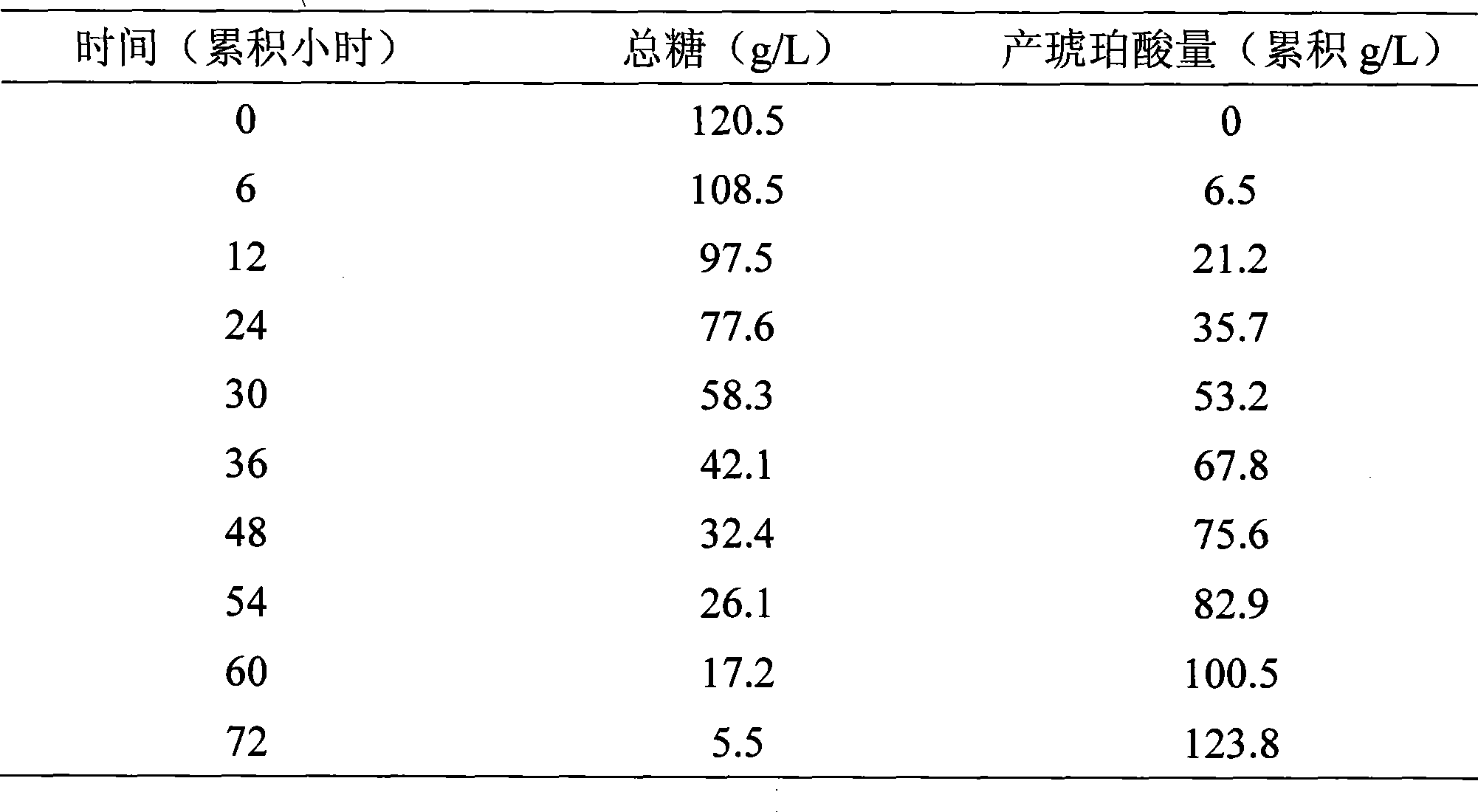
Coupling apparatus and technique for fermentation and separation of succinic acid by expanded bed adsorption and in situ extraction
InactiveCN101386815ASolve the blockageAchieve cloggingBioreactor/fermenter combinationsBiological substance pretreatmentsFixed bedIn situ adsorption
The invention belongs to a coupling device and a coupling technology for in situ extraction of succinic acid by an expanded bed and fermentation and separation of the succinic acid, in particular relates to a technology for producing the succinic acid by the fermentation method, a technology for separating the succinic acid by the expanded bed and a fermentation and separation coupling process. The device is to couple a chromatography column of the expanded bed and a succinic acid bioreactor, so as to extract the succinic acid in situ. The device utilizes the characteristic of the chromatography column of the expanded bed that microbial zymotic liquid provided with solid materials is allowed to directly enter the chromatography column, avoids the blockage problem of a chromatography column of a fixed bed due to direct coupling of the fixed bed and the bioreactor in the prior art, saves an additional membrane separator between the bioreactor and the chromatography column of the expanded bed, realizes direct coupling of the chromatography column of the expanded bed and the bioreactor, and performs in situ extraction of target substances and does not cause blockage of the chromatography column of the expanded bed. Moreover, a coupling system for in situ extraction of the succinic acid by the expanded bed and the fermentation and the separation of the succinic acid is established to meet the requirements of sterilization and pollution-free operation.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
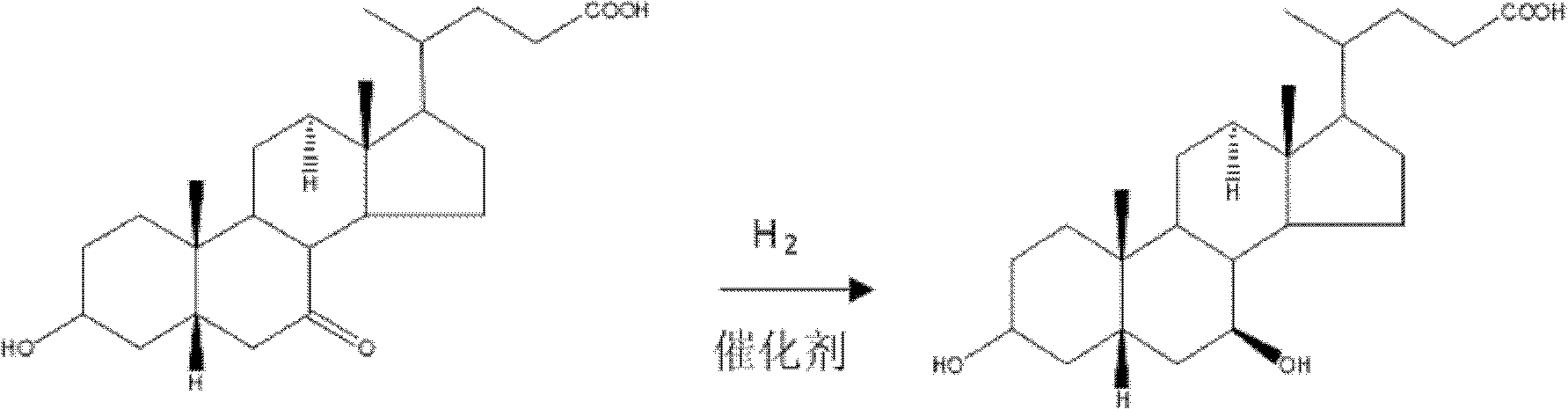
Method for preparing ursodesoxycholic acid by chiral catalytic hydrogenation of 7-ketodesoxycholic acid
InactiveCN102070693AReduce generationReduced purification stepsSteroidsChenodeoxycholic acidDistillation
The invention discloses a method for preparing an ursodesoxycholic acid by the chiral catalytic hydrogenation of a 7-ketodesoxycholic acid, which is characterized by comprising the following steps of: performing oxidation to prepare the 7-ketodesoxycholic acid from a chenodeoxycholic acid serving as an initiative raw material by using a common method; dissolving the 7-ketodesoxycholic acid into a solvent, adding a chiral catalyst, maintaining the pressure of 0 to 20 MPa under alkali condition, introducing nitrogen to perform hydrogenation reduction reaction at 10 to 80 DEG C, and performing distillation after the reaction is finished to remove the solvent; adding purified water in a volume which is 10 to 100 times that of a hydrogenation reduction reaction product, and adding acid liquor to crystallize the hydrogenation reduction reaction product; and separating solids from liquid, and performing washing and drying to obtain solid powder which is the ursodesoxycholic acid. The method for preparing the ursodesoxycholic acid by the chiral catalytic hydrogenation of the 7-ketodesoxycholic acid aims to overcome the shortcomings of the prior art, and ensures a short production flow, high yield and high quality.
Owner:ZHONGSHAN BAILING BIOTECHNOLOGY CO LTD
Synthetic process for Vonoprazan fumarate
PendingCN107778286AReduce contentImprove removal efficiencyOrganic chemistryMethylamine hydrochloridePyridine
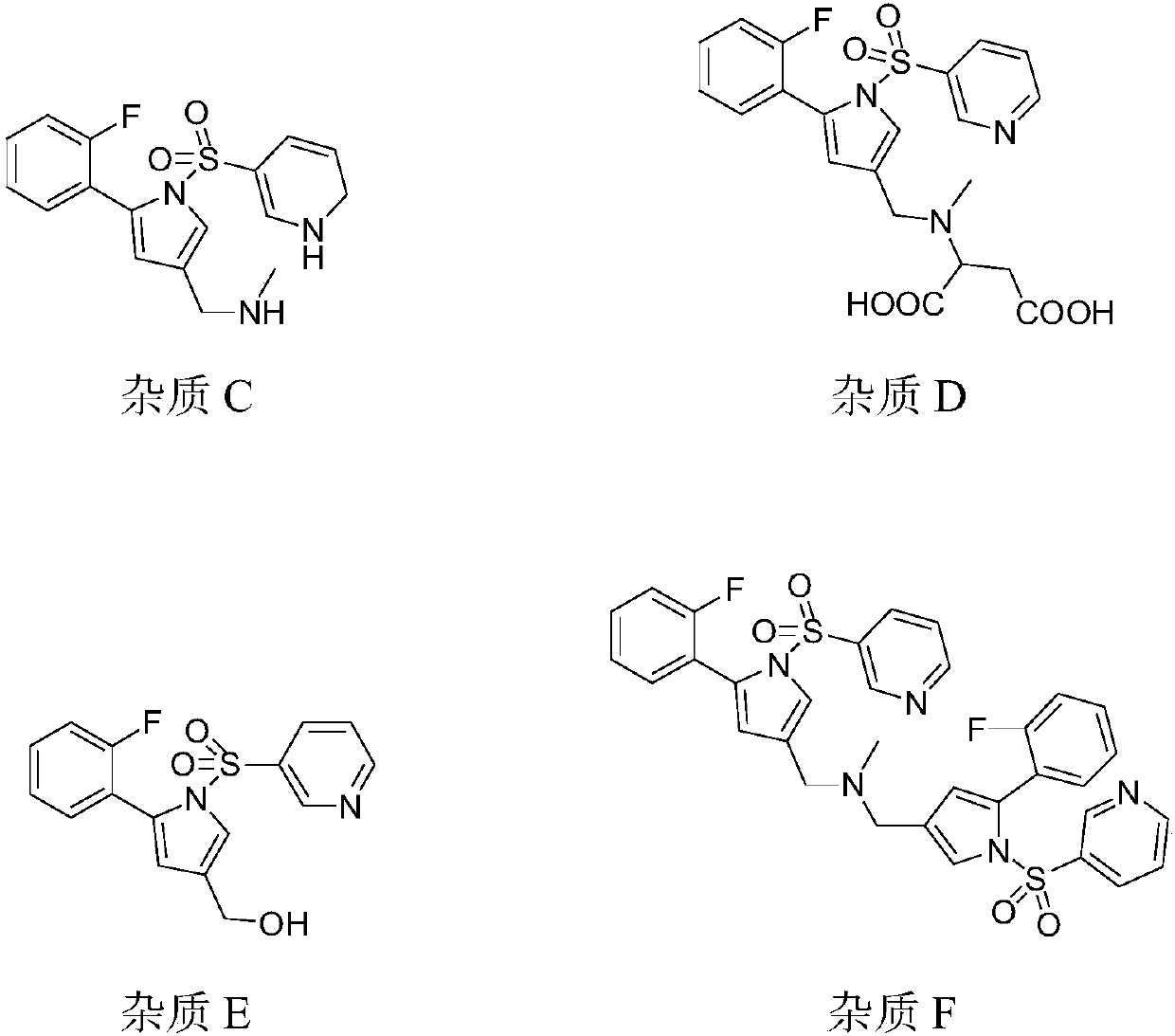
The invention provides a preparation method for preparing high-purity 5-(2-fluorophenyl)-N-methyl-1-(3-pyridine sulfonyl)-1H-pyrrole-3-methylamine fumarate. The method comprises the following steps: taking 5-(2-fluorophenyl)-N-methyl-1-(3-pyridine sulfonyl)-1H-pyrrole-3-methylamine hydrochloride as an intermediate; carrying out alkali treatment, and then salifying the treated intermediate with fumaric acid to obtain a finished product. A final product prepared by adopting the method has high purity and low content of key impurities; in addition, the purification and post treatment steps of fumarate are simplified, and the yield is remarkably improved.
Owner:四川弘远药业有限公司
Gemcitabine-loaded polyethylene glycol (PEG) peptide dendrimer targeting drug-delivery system and preparation method thereof
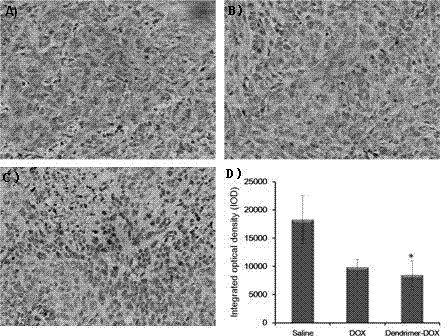
InactiveCN103656667AEasy to catchLarge nano sizeOrganic active ingredientsPharmaceutical non-active ingredientsDendrimerPolyethylene glycol
The invention provides a gemcitabine-loaded polyethylene glycol (PEG) peptide dendrimer targeting drug-delivery system and a preparation method thereof to solve the problem of anti-cancer specificity of a dendrimer drug-delivery system. The drug-delivery system is characterized by being a conjugate of an anti-cancer drug gemcitabine, a targeted functional factor gly-phe-leu-gly (GFLG) and a PEG polypeptide dendrimer, and the gemcitabine is connected with the PEG peptide dendrimer through the GFLG to form a functional dendrimer. The same dose of drug achieves a significant antitumor effect when good biocompatibility is obtained.
Owner:SICHUAN UNIV
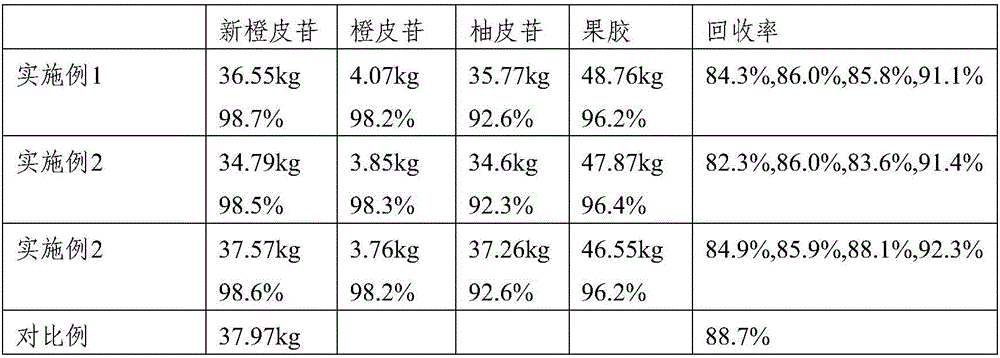
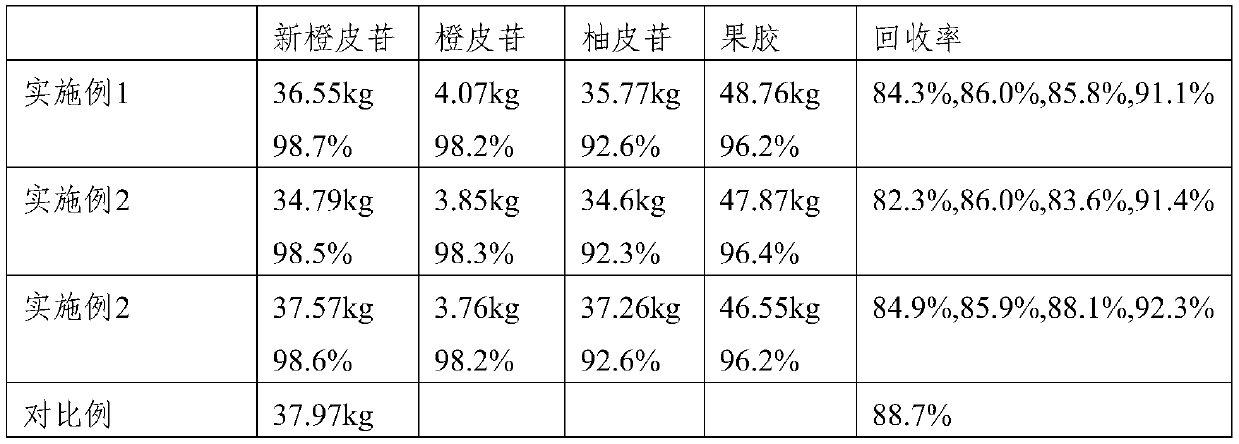
Method for extracting neohesperidin from immature bitter orange and comprehensively utilizing immature bitter orange
ActiveCN106810622AAvoid dependenceReduce manufacturing costSugar derivativesSugar derivatives preparationNaringinOrganic solvent
The invention provides a method for extracting neohesperidin from immature bitter orange and comprehensively utilizing immature bitter orange. The method for comprehensive utilization comprises the following steps: 1) crushing and sieving immature bitter orange; 2) leaching the sieved substance with water at temperature of 55-60 DEG C, and collecting the leachate and filter residue respectively; and 3) purifying the filter residue to obtain hesperidin; and / or separating the leachate to obtain one or more of pectin powder, naringin and neohesperidin. In the method provided by the invention, the immature bitter orange is utilized to the greatest extent; the method mainly adopts warm water for extraction and a little alcohol for separation and purification, and thus the use of organic solvent is minimized; and moreover, the technology is simple and effective and has a wide application value.
Owner:YONGZHOU YIDONG BIOTECH
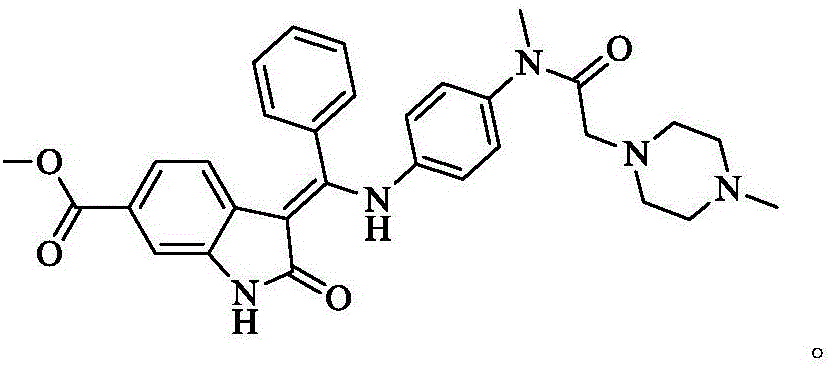
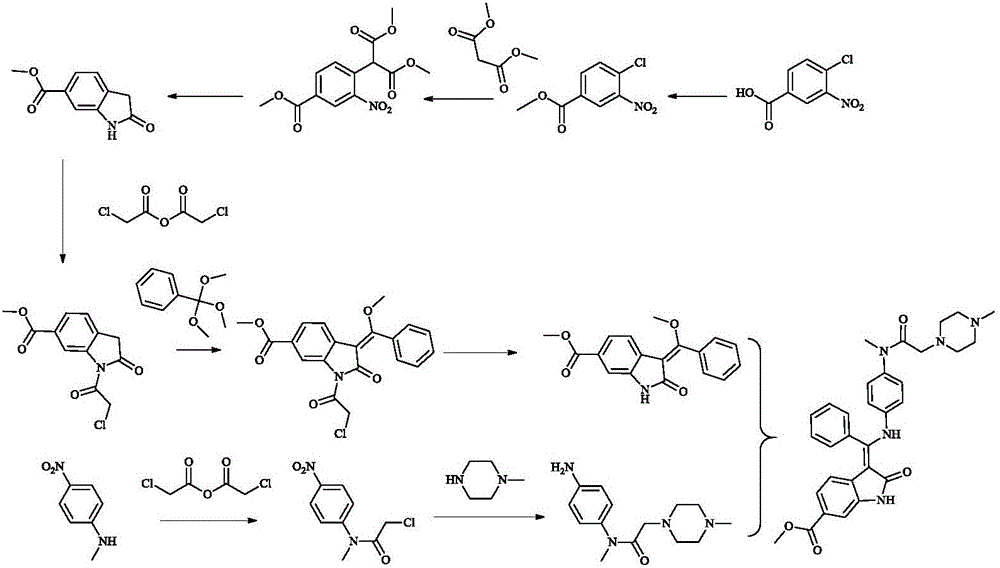
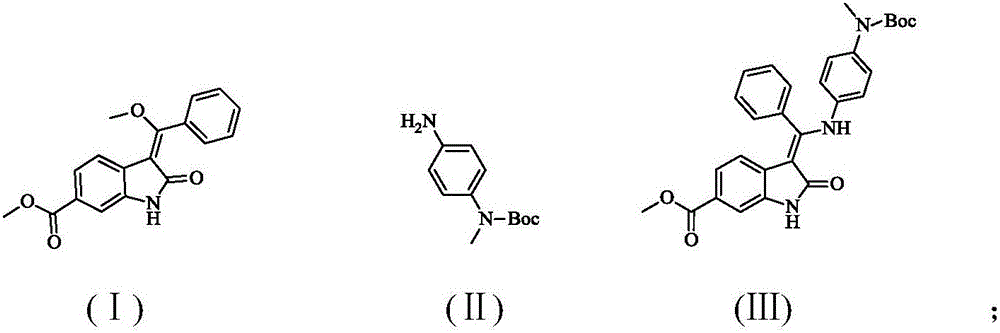
A synthetic method of Nintedanib and an intermediate of Nintedanib
ActiveCN105837493AReasonable designLow reaction temperatureOrganic chemistryAcetic acidChloroacetic acids
A synthetic method of Nintedanib and an intermediate of Nintedanib are disclosed. The method includes reacting a compound shown as a formula (II) and a compound shown as a formula (I) under acidic conditions to produce a compound shown as a formula (III), removing t-butyloxycarboryl of the compound shown as the formula (III) with an acid, adding an alkali, reacting to produce a compound shown as a formula (V), reacting the compound shown as the formula (V) with an activated derivative of chloroacetic acid to produce a compound shown as a formula (VI), and reacting with N-methyl piperazine to produce the Nintedanib. The novel synthetic method with mild reaction conditions for the Nintedanib is provided. The intermediate for synthesizing the Nintedanib is also provided.
Owner:SOUTHEAST UNIV
Synthetic method of polystyrene block copolymer for ultramicro filter membrane
The invention discloses a synthetic method of a polystyrene block copolymer for an ultramicro filter membrane. The method comprises the following steps: mixing a first monomer, an initiator, a chain transfer agent and a solvent A to form a homogeneous solution, polymerizing in an inert atmosphere for a certain time, adding styrene and a second monomer, continuously polymerizing for a proper time, and settling a polymer solution to obtain the polystyrene block copolymer. The prepared block copolymer has the molecular weight of 5,000-750,000g / mol and the polydispersity index of 1.05-1.55, and can be used for preparing a homopore membrane, a nanofiber membrane, a self-repair ultramicro filter membrane and a composite membrane material.
Owner:ZHEJIANG UNIV
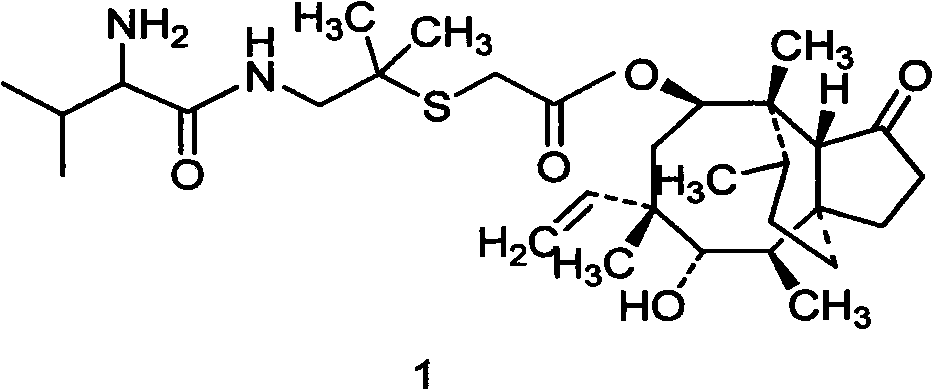
Preparation method of valnemulin and hydrochloride thereof
The invention belongs to preparation of antibiotic, in particular to preparation method of Valnemulin and hydrochloride thereof.D-hydroxy valine Dane salt is added into more than ten weight of tetrahydrofuran and is suspended by stirring at 0-20 DEG C, and triethylamine, N, N-dimethyl formamide (acetamide) and N-methyl morpholine are added until reaction liquid is clear; methyl (ethyl) chloroformate molar weight of which is not more than that of D-hydroxy valine Dane salt is added; (2-amino-1, 1-dimethyl ethyl) mercaptoacetic acid, (3aS, 4R, 5S, 6S, 8R, 9R, 9aR, 10R)-octohydrogen-5, 8-dyhydroxy-4, 6, 9,10-tetramethyl-6-ethenyl-3a, 9-propane-3aH-cyclopentene (cyclooctene)-1(4H)-keto-8-ester the molar weight of which is not more than that of D-hydroxy valine Dane salt is dissolved in equal mass of tetrahydrofuran, the reaction liquid is dripped in and stirred at 0-10 DEG C, and reaction is carried out for 2-5h at 0-20 DEG C; the organic solvent is removed, the residue is added with water and hydrochloric acid is used for adjusting pH value, heating up, stirring and decontaminating are carried out, and the filtration is freeze dried to obtain Valnemulin hydrochloride. The invention solves the problems of low purity and high reaction condition of the existing product and has the advantages of simple technology and high product purity.
Owner:河北远征禾木药业有限公司
Method for preparing ammonium bifluoride with high purity
InactiveCN101671037AReduced purification stepsReasonably control the decomposition temperatureAmmonium halidesDecompositionAmmonium fluoride
The invention discloses a method for preparing an ammonium bifluoride with high purity. The ammonium fluoride with high purity is taken as a raw material and is decomposed at the temperature of 130-148.5 DEG C, thus generating ammonia gas; the ammonia gas generated by decomposition is absorbed by a dilute hydrochloric acid solution with the mass concentration of 5-12%; the ammonium fluoride crystals obtained by the decomposition are sublimed under the temperature of 222-230 DEG C; and the ammonium bifluoride vapor is collected, thus obtaining the ammonium bifluoride product. The method has high yield and high product purity and can meet high requirement.
Owner:WENGFU (GRP) CO LTD
Adriamycin-loaded PEGylated peptide dendrimer targeted drug delivery system and preparation method thereof
InactiveCN103768612AMonodisperseEasy to catchOrganic active ingredientsPharmaceutical non-active ingredientsDendrimerMedicine
The invention provides an adriamycin-loaded PEGylated peptide dendrimer targeted drug delivery system and a preparation method thereof to solve the problem about anticancer specificity of a dendrimer drug delivery system. The targeted drug delivery system and the preparation method thereof are characterized in that the drug delivery system is a conjugate of antitumor drugs, namely adriamycin, an targeted functional factor GFLG (Gly-Phe-Leu-Gly) and a PEGylated peptide dendrimer, and the adriamycin and PEGylated peptide dendrimer are connected through the GFLG to form a functional dendrimer, so that excellent biocompatibility is achieved, and a low-dose medicine is enabled to achieve the obvious antitumor efficacy.
Owner:SICHUAN UNIV
Tolerance-improved mutant Taq DNA polymerase as well as preparation method and application thereof
ActiveCN110684752AImprove toleranceHigh polymerization amplification abilityBacteriaMicrobiological testing/measurementPHA polymeraseMutant
The invention discloses tolerance-improved mutant Taq DNA polymerase as well as a preparation method and application thereof. According to the tolerance-improved mutant Taq DNA polymerase, one or moreamino acids are inserted, substituted or deleted in an amino acid sequence of Taq DNA polymerase shown in SEQ ID NO.1, and compared with the Taq DNA polymerase shown in SEQ ID NO.1, the amino acid sequence has significantly enhanced impurity tolerance. The recombinant Taq DNA polymerase mutant has remarkably enhanced tolerance to blood, fluorochrome and high ion strength, and can directly performPCR detection on blood samples, save time and avoid false negative.
Owner:VAZYME BIOTECH NANJING
Method for preparing 2-hydroxybenzeneboronic acid
ActiveCN103804403AReduce manufacturing costMeet the needs of large-scale productionGroup 3/13 element organic compoundsN-ButyllithiumBoric acid
The invention provides a method for preparing 2-hydroxybenzeneboronic acid, which relates to the technical field of industrial production for 2-hydroxybenzeneboronic acid. The method is characterized by comprising the following steps of: selecting raw material phenol which is already commercialized in the market as an initial raw material, in a normal-temperature condition, protecting hydroxyl at first, and then directly dripping n-butyllithium in the mixture of protected hydroxyl intermediate and borate compounds without separating crude products, and after the reaction is finished, hydrolyzing to prepare 2-hydroxybenzeneboronic acid. The method is easily-available in raw materials, high in both the purity and yield of the reaction product, stable in process conditions, simple to operate and suitable for large-scale production, and provides novel thinking and method for preparing 2-hydroxybenzeneboronic acid.
Owner:BENGBU CHINA SYNCHEM TECH CO LTD
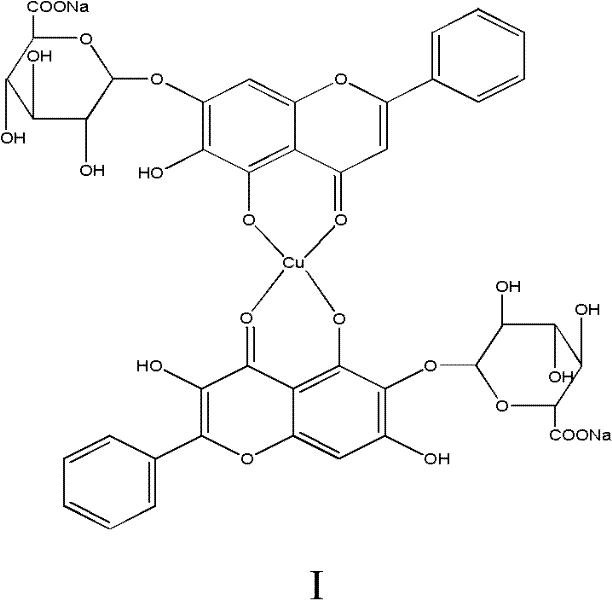
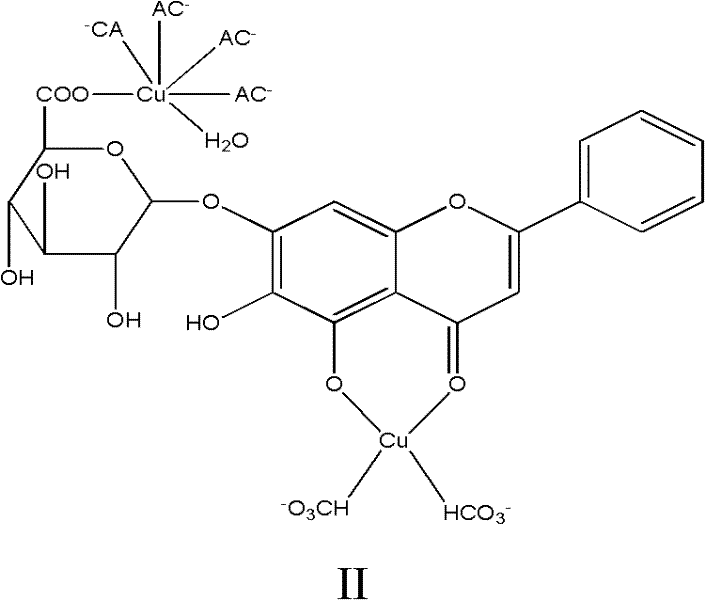
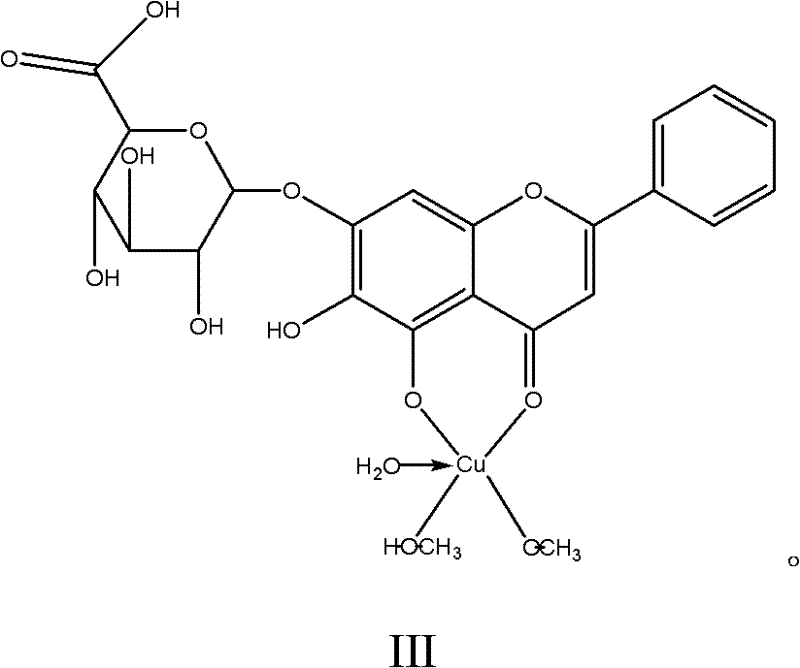
Baicalin-copper complexes and preparation methods thereof
InactiveCN102336773AAvoid it happening againHigh purityAntibacterial agentsCopper organic compoundsPharmacyFeed additive
The invention relates to baicalin-copper complexes and preparation methods thereof, and belongs to the field of chemical pharmacy. Two baicalin-copper complexes with novel structures are prepared from different raw materials in different molar ratios; after the complexes are subjected to in-vitro bacteriostatic experiments, results indicate that the complexes have a high bacteriostatic function; and compared with baicalin-metal complexes disclosed in the prior art, the baicalin-copper complexes have better medicinal effect and can serve as feed additives. The invention also provides preparation methods for the baicalin-copper complexes. The methods are simple, and easy to control; and the final products have high purity.
Owner:BEIJING HUAMU GREAT EXPLOIT SCI & TECH
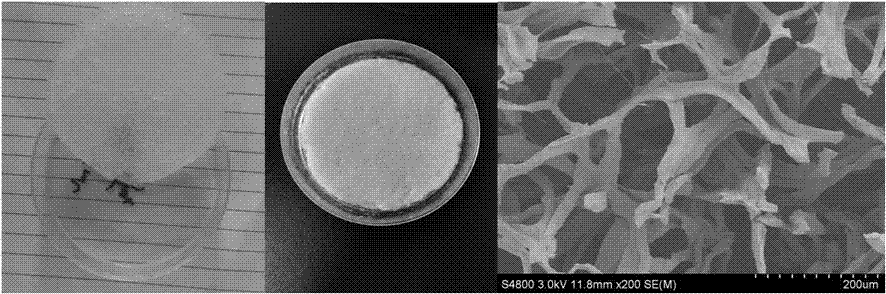
Fibroin protein fiber scaffold and preparation method thereof
The invention relates to a fibroin protein fiber scaffold and a preparation method thereof, after degumming of silk, the silk is soaked in an acid solution for dispersion to obtain a silk fiber dispersion solution; the silk fiber dispersion solution is injected into a mold for freezing to obtain a silk fiber frozen body; the silk fiber frozen body is immersed in an organic solvent to remove formic acid, and soaked and washed with deionized water to obtain a wet-state fibroin protein fiber scaffold; the-state fibroin protein fiber scaffold is coldly treated to obtain a frozen body, and the frozen body is frozen and dried to obtain the fibroin protein fiber scaffold. The internal structure of the fibroin protein fiber scaffold mainly comprises fiber, and the fibroin protein fiber scaffold has high porosity, high penetration rate and excellent mechanical properties, is very conducive to nutrient transport, cell migration and tissue growth, and is an ideal tissue engineering scaffold.
Owner:上海丝波敦生物科技有限公司
Separating and purifying method for new recombinant human interferon alpha2b
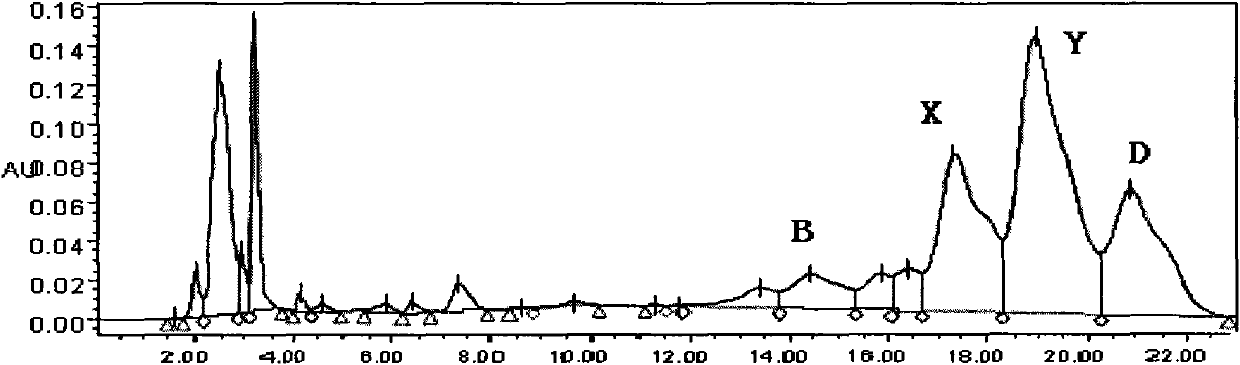
ActiveCN1749274AReduced purification stepsSmooth processPeptide preparation methodsInterferonsChromatographic separationMolecular sieve
The present invention relates to the separation and purification method of new recombinant human interferon alpha-2b. The separation and purification method includes the following steps: crushing recombinant human interferon alpha-2b colibacillus as engineering bacillus, dissolving in urea solution, Tris-HCl washing, cracking with guanidine hydrochloride, boric acid renaturing, regulating pH value, dissolving in salt solution, chromatographic separation with hydrophobic column M1, chromatographic separation with CM-Sepharose column, chromatographic separation with hydrophobic column M2, and chromatographic separation with S-100 molecular sieve. The present invention has less purification steps and integrated purification process, and may be used in large scale automatic production.
Owner:BEIJING KAWIN TECH SHARE HLDG
Process of extracting volatile oils and alpinetin from Alpinia katsumadai
InactiveCN102477354AHigh yieldIncrease profitOrganic chemistryEssential-oils/perfumesAlpinetinAlcohol
The invention relates to a process of extracting volatile oils and alpinetin from Alpinia katsumadai. The process comprises the following steps of: grinding Alpinia katsumadai material, loading to an extraction kettle; performing supercritical CO2 extraction; filtering to obtain a volatile oil product; mixing the extraction residues with an appropriate amount of entrainers; performing supercritical CO2 extraction again to obtain flavonoids; diluting the flavonoid extract; subjecting to membrane separation; purifying by polyamide column chromatography; eluting with alcohols; concentrating to crystallize the eluate; re-crystallizing with anhydrous methanol; filtering out crystals; and drying to obtain an alpinetin product. The process is simple, has less pollution and low energy consumption, and is better in extraction rate and purity.
Owner:苏州宝泽堂医药科技有限公司
A method for extracting neohesperidin from Citrus aurantium and comprehensive utilization of Citrus aurantium
ActiveCN106810622BAvoid dependenceReduce manufacturing costSugar derivativesSugar derivatives preparationNaringinOrganic solvent
The invention provides a method for extracting neohesperidin from immature bitter orange and comprehensively utilizing immature bitter orange. The method for comprehensive utilization comprises the following steps: 1) crushing and sieving immature bitter orange; 2) leaching the sieved substance with water at temperature of 55-60 DEG C, and collecting the leachate and filter residue respectively; and 3) purifying the filter residue to obtain hesperidin; and / or separating the leachate to obtain one or more of pectin powder, naringin and neohesperidin. In the method provided by the invention, the immature bitter orange is utilized to the greatest extent; the method mainly adopts warm water for extraction and a little alcohol for separation and purification, and thus the use of organic solvent is minimized; and moreover, the technology is simple and effective and has a wide application value.
Owner:YONGZHOU YIDONG BIOTECH
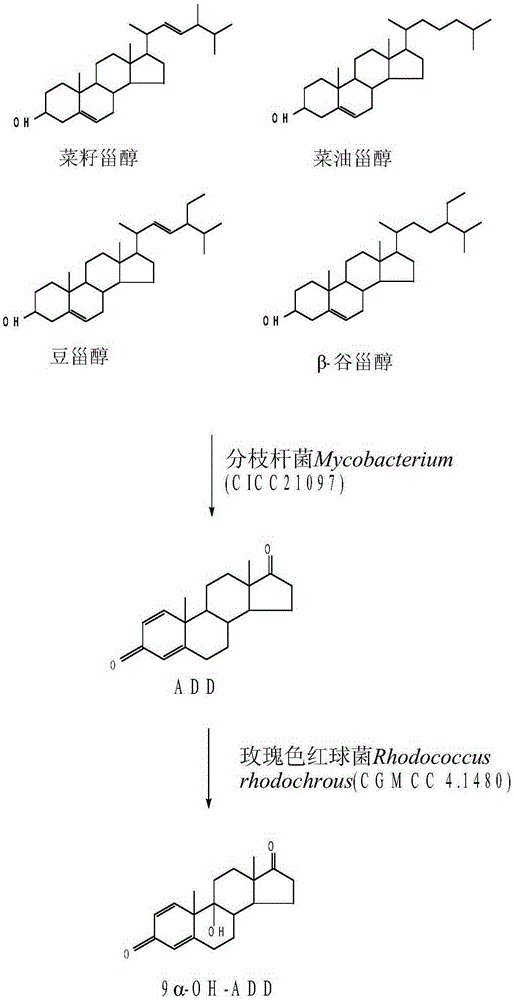
Method for preparing 9 alpha-hydroxy-androstane-1,4-diene-3,17-dione
InactiveCN105219829AImprove utilization efficiencyReduce solvent usageSteroidsFermentationAndrostaneSolvent
The invention discloses a method for preparing 9 alpha-hydroxy-androstane-1,4-diene-3,17-dione. The method takes phytosterol as a substrate, utilizes two microbial mixed bacteria, namely Mycobacterium (CICC 21097) and Rhodococcus rhodochrous (CGMCC 4.1480) for fermentation to prepare 9 alpha-hydroxy-androstane-1,4-diene-3,17-dione. Besides, a compound dissolution promotor is added into a fermentation culture medium to ensure the highest rate of molar conversion from phytosterol to 9 alpha-OH-ADD reaches 84.3 percent. Meanwhile, purification in a two-step 9 alpha-hydroxy-androstane-1,4-diene-3,17-dione preparation method is omitted, and the consumption of a solvent is reduced.
Owner:SHANGHAI APPLIED TECHNOLOGIES COLLEGE
A kind of preparation method of ZSM-35 molecular sieve
ActiveCN104418357BEasy to prepareReduced purification stepsFerrierite aluminosilicate zeoliteMolecular sieveCrystallinity
Owner:CHINA PETROLEUM & CHEM CORP +1
Low-initial-quantity plasma free DNA methylation library-building kit and method
PendingCN110669824AAvoid damageReduce inputMicrobiological testing/measurementLibrary creationEngineeringDNA damage
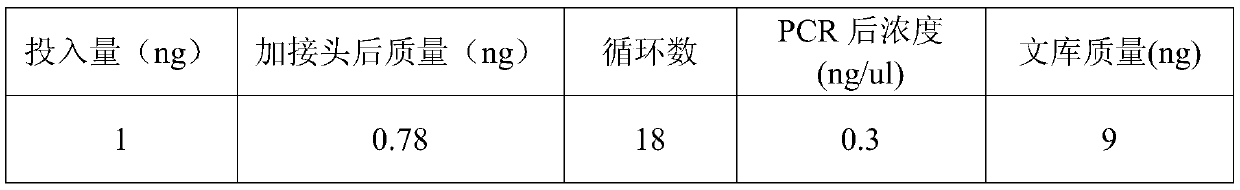
The invention discloses an enzymatic methylation library-building method. According to the method, through TET enzyme methylation treatment, DNA damage in the methylation library-building process is lowered to a certain extent, and thus the requirement for the input amount of DNA in a nucleic acid sample is lowered; besides, by further cooperating with a one-step reagent for terminal repair and Aaddition reaction, one-tube library building can be realized, and the purification step is reduced; and by further cooperating DNA ligase enhancing liquid, the connection efficiency can be improved, and the library-building quality and the sequencing quality are improved; and the method provided by the invention is low in library-building initial quantity, the DNA input amount can be as low as to1 ng, and the method is suitable for trace library building including cfDNA.
Owner:广州迈格基因科技有限公司
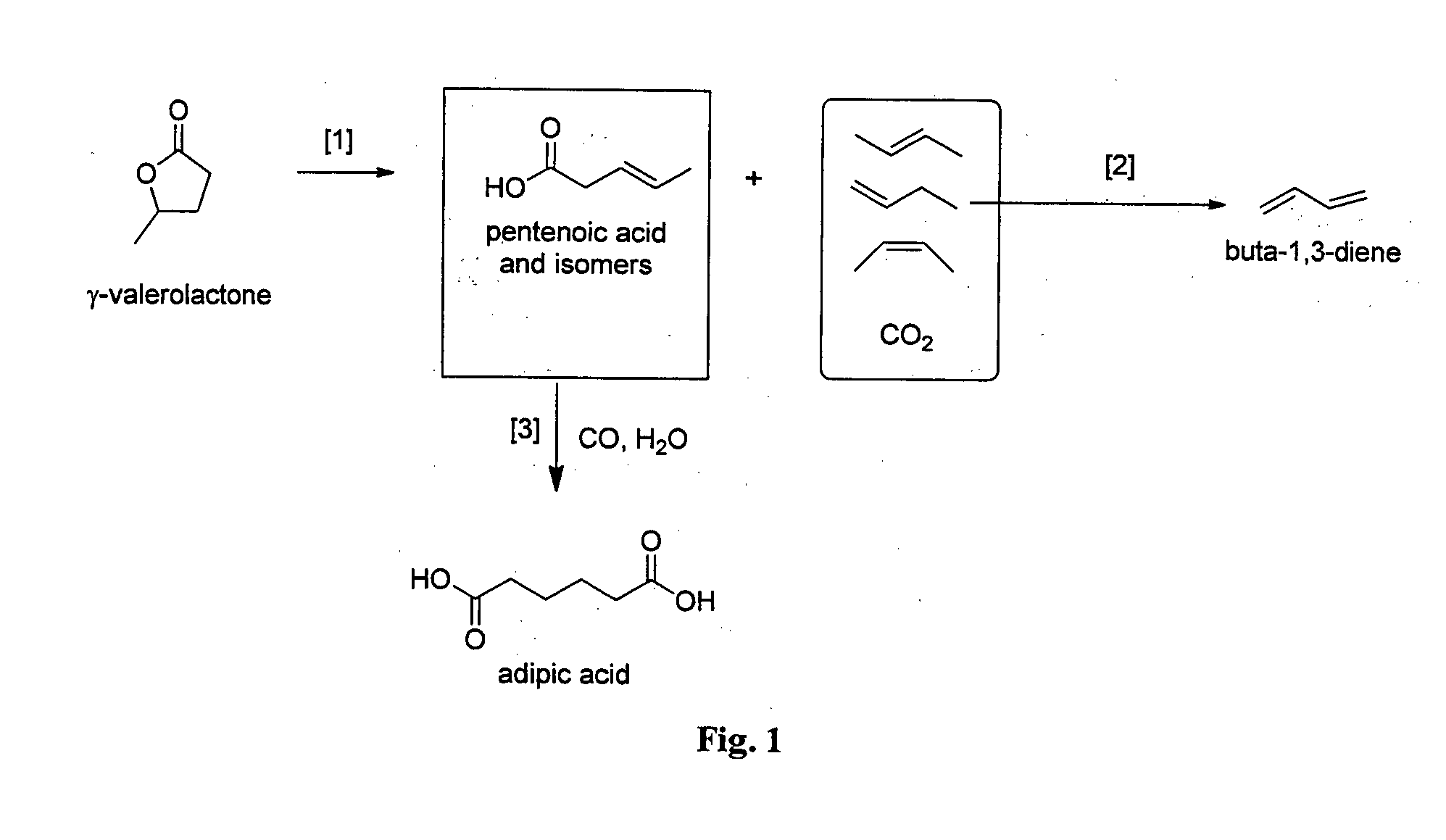
Process to produce a diene from a lactone
InactiveUS20150133685A1Reduce intermediate purification stepReduce wasteOrganic compound preparationCatalystsPolymer scienceAlkene
Owner:AGENCY FOR SCI TECH & RES
Preparation method for ursodesoxycholic acid
The invention discloses a preparation method for ursodesoxycholic acid, and relates to the field of biochemical pharmacy. A preparation method for ursodesoxycholic acid in the prior art is relatively poor in stereoselectivity during a hydrogenation reduction reaction, and the method is relatively long in production line, not high in product yield and relatively poor in production quality. The preparation method comprises the following steps: dissolving 7-ketodesoxycholic acid in a solvent, adding a chiral catalyst, under an alkaline condition, maintaining the pressure to be 0-20 MPa, introducing hydrogen at 15-81 DEG C to carry out a hydrogenation reduction reaction, and performing distillation to remove the solvent after the reaction is completed; adding 13-95 times of purified water, and then adding acid liquor, so that a product after the hydrogenation reduction reaction is crystallized; separating solid and liquid, washing and drying the solid to obtain solid powder, namely the ursodesoxycholic acid. The preparation method is short in product line, high in product yield and good in product quality.
Owner:CHANGDE YUNGANG BIOTECHNOLOGY CO LTD
Gemcitabine-supported PEG peptide type dendritic macromolecular targeting drug delivery system and preparation method thereof
InactiveCN104288779AReduced risk of spillage to tumor siteExtend cycle timeOrganic active ingredientsPowder deliveryDendrimerMedicine
The invention provides a gemcitabine-loaded polyethylene glycol (PEG) peptide dendrimer targeting drug-delivery system and a preparation method thereof to solve the problem of anti-cancer specificity of a dendrimer drug-delivery system. The drug-delivery system is characterized by being a conjugate of an anti-cancer drug gemcitabine, a targeted functional factor gly-phe-leu-gly (GFLG) and a PEG polypeptide dendrimer, and the gemcitabine is connected with the PEG peptide dendrimer through the GFLG to form a functional dendrimer. The same dose of drug achieves a significant antitumor effect when good biocompatibility is obtained.
Owner:SICHUAN UNIV
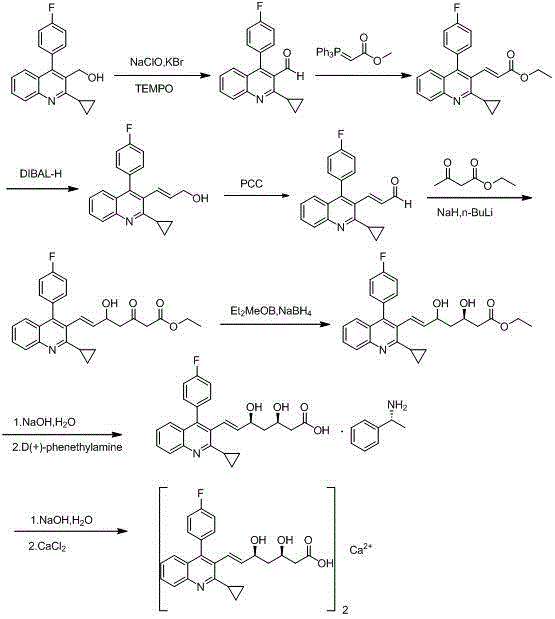
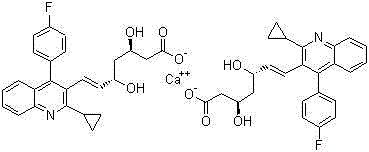
Method for preparing intermediate of pitavastatin calcium
InactiveCN104016916AReduced purification stepsThe reaction steps are simpleOrganic chemistryMethyl aldehydePitavastatin
The invention discloses a method for preparing an intermediate of pitavastatin calcium. The method comprises the steps of reacting 2-cyclopropyl-4-(4-fluorophenyl) quinoline-3-methyl aldehyde with (triphenylphosphoranyl) methyl acetate to obtain an intermediate 1; reacting the intermediate 1 in presence of a strong reducing agent to obtain an intermediate 2; reacting the intermediate 2 in the presence of an oxidant to obtain an intermediate 3; reacting the intermediate 3 with ethyl acetoacetate under the action of a strong base reagent to obtain an intermediate 4; carrying out chiral reduction on the intermediate 4 and a carbonyl group under the action of a reducing agent to obtain an intermediate 5; and hydrolyzing the intermediate 5 under the action of an alkali to obtain pitavastatin heptenoic acid, and then reacting pitavastatin heptenoic acid with a chiral reagent, carrying out chiral resolution and hydrolyzing to obtain the pitavastatin heptenoic acid product. The method disclosed by the invention has the beneficial effects that the disadvantage of the basic patent route is overcome, no risk reagent is used, the purification step is reduced and the reaction steps are simplified and the method is applicable in large-scale industrial production.
Owner:NANTONG CHANGYOO PHARMATECH CO LTD
Rabies virus SNK-CTN strain and application thereof
InactiveCN104357406AReduce contentReduce extractionMicroorganism based processesAntiviralsRabies virus strainDiploid cells
The invention relates to the field of microbes and biological pharmacy, and particularly relates to an adapted strain of a rabies virus strain (a CTN-1V strain) in a diploid cell MRC-5 strain. The preparation method of the rabies virus strain comprises the following steps: 1) taking MRC-5 cells in a cell bank for resuscitation, cultivating for 3 days for forming single layer cells, pouring away a cell culture fluid in an MRC-5 cell culture flask, adopting a 0.25% of trypsin digestive juice for digestion, dispersing into uniform cells, inoculating a rabies virus CTN-1V5 strain suspension according to the dosage of 0.01-1.0 MOI, cultivating at 37 DEG C for 2-3 days, replacing the suspension with a maintenance medium containing 2-4% of bovine serum, cultivating at 33-35 DEG C for 3-5 days to obtain a virus liquid, and cryopreserving at -70 DEG C to prepare a virus suspension; 2) adopting the method, and continuously subculturing for 30-32 generations on MRC-5 cells. The preservation code of the rabies vaccine virus strain (an SNK-CTN strain) is CGCC No8887.
Owner:SHINAIKE JIANGSU BIOLOGICAL PHARMA
Preparation method of deoxy analog of Echinocandin B
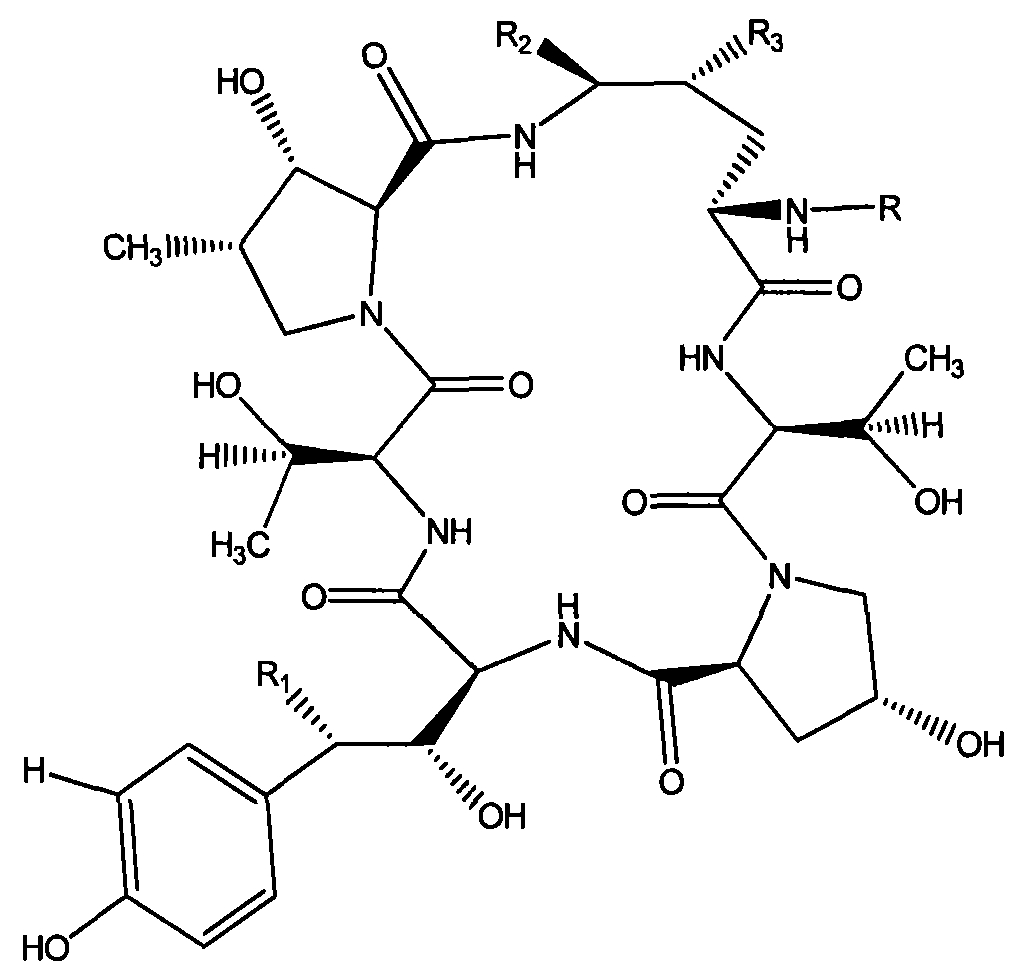
InactiveCN101993477AReduced purification stepsLower conversion costsPeptide preparation methodsEchinocandin BEthyl acetate
The invention discloses a preparation method of a deoxy analog of Echinocandin B. The method comprises the following steps of: (1) mixing a fermenting mixture of Echinocandin B with an organic solvent to obtain a solution 1; (2) mixing the solution 1 with an acid water solution to obtain a solution 2; and (3) standing the solution 2 at the temperature of 30-50 DEG C for 12-60h to obtain the deoxy analog of a compound shown as formula I, in the formula, R is linolenic acyl, R1=OH, R2=OH, and R3=OH; the organic solvent is selected from methanol, ethanol, ethyl acetate, acetone or chloroform; acid in the acid water solution is selected from hydrochloric acid, acetic acid or citric acid, wherein the concentration of the acid is 1-3mol / L; and pH of the solution 2 is 1-6.
Owner:SHANGHAI INST OF PHARMA IND
GFLG-based PEGylated peptide dendrimer drug delivery system and preparation method thereof
InactiveCN103768613AHigh biosecurityReduced risk of spillage to tumor siteOrganic active ingredientsPharmaceutical non-active ingredientsDrug deliveryPeptide
The invention aims to solve the problem about the anticancer specificity of a PEGylated dendrimer drug delivery system and provides a preparation method of a GFLG-based PEGylated peptide dendrimer drug delivery system. The preparation method is characterized in that the drug delivery system is a conjugate of the PEGylated peptide dendrimer, a targeted functionality factor GFLG and an anticancer therapeutic factor; GFLG is adopted to connect the therapeutic factor and the PEGylated peptide dendrimer to form a functionality dendrimer, so that excellent biocompatibility is obtained, and the remarkable anticancer curative effect is achieved by using medicine of the same or lower dosage.
Owner:SICHUAN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com