Gemcitabine-supported PEG peptide type dendritic macromolecular targeting drug delivery system and preparation method thereof
A technology of dendrimer and gemcitabine, which is applied in pharmaceutical formulas, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc., to achieve small molecular size, high biological safety, and increased specific fragmentation efficiency in vitro Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
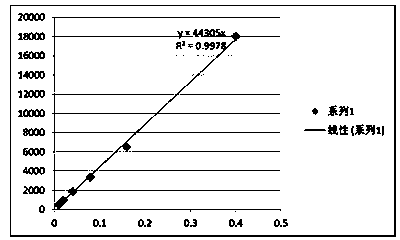
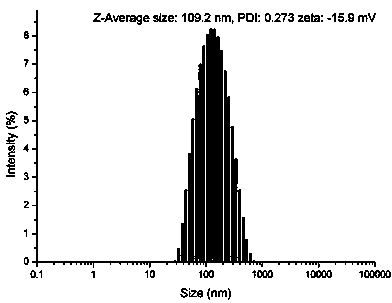
[0064] PEGylated peptide dendrimer targeted drug delivery system loaded with gemcitabine, the drug delivery system is a conjugate of anti-tumor drug gemcitabine, targeting functional factor GFLG and PEGylated peptide dendrimer, its structure is as follows: Figure 10 Shown:
[0065] Conjugate Ⅰ: PEGylated third-generation lysine dendrimer-gemcitabine conjugate with n=10, m=3
[0066] Conjugate II: PEGylated third-generation lysine dendrimer-gemcitabine conjugate with n=1, m=1
[0067] Conjugate Ⅲ: PEGylated third-generation lysine dendrimer-gemcitabine conjugate with n=10, m=1
[0068] Conjugate IV: PEGylated 4th generation lysine dendrimer-gemcitabine conjugate with n=1, m=1
[0069] Conjugate Ⅴ: PEGylated 5th generation lysine dendrimer-gemcitabine conjugate with n=2, m=1
[0070] Conjugate Ⅵ: PEGylated third-generation lysine dendrimer-gemcitabine conjugate with n=5, m=6
[0071] Conjugate VII: PEGylated 4th generation glutamic acid dendrimer-gemcitabine conjugate with ...
Embodiment 2
[0073] The preparation method of the PEGylated polypeptide dendrimer drug delivery system based on GFLG first introduces a methyl vinyl group on the conjugate of gemcitabine and GFLG to obtain the conjugate MA-GFLG-GEM, which is then used as a carrier The peptide dendrimers are alkynylated, and a tritylthio group is introduced into the dendrimers to obtain an alkynyltritylthio dendrimer. After the trityl groups are removed, the conjugated MA -GFLG-GEM undergoes a coupling reaction to obtain a conjugate of an alkynylated dendrimer and gemcitabine, which is finally PEGylated to obtain a finished product.
Embodiment 3
[0075] The specific operation steps of the preparation method of the present invention are as follows:
[0076] A preparation of MA-GFLG-GEM conjugates;
[0077] Using Boc-GFLG-OMe as raw material, react with methyl vinyl chloride to prepare MA-GFLG-OH, and then react MA-GFLG-OH with N,N-diisopropylethylamine and tetrahydrothiazole-2-thione , after the reaction, the reaction product, gemcitabine hydrochloride, and pyridine were dissolved in anhydrous dimethyl sulfoxide, and reacted in the dark, and the reaction product was MA-GFLG-GEM conjugate;
[0078] Synthesis of B Alkynylated Dendrimer
[0079] The dendrimers protected by Boc and Cbz groups are used as raw materials to react with Pb / C in a hydrogen atmosphere. After the reaction is completed, the reaction product is dissolved in DMF, and N,N-diisopropylethylamine is added to the system , hexynoic acid, benzotriazole-N,N,N',N'-tetramethyluronium hexafluorophosphate, 1-hydroxybenzotriazole, react under the protection of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com