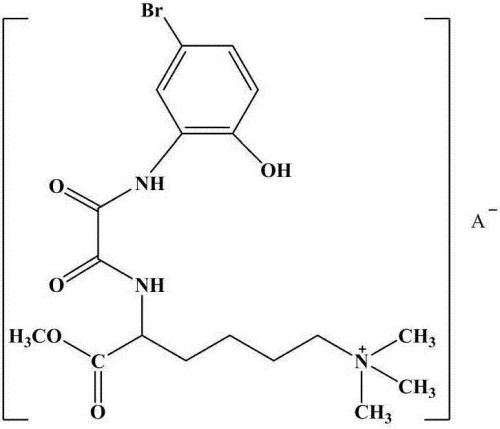
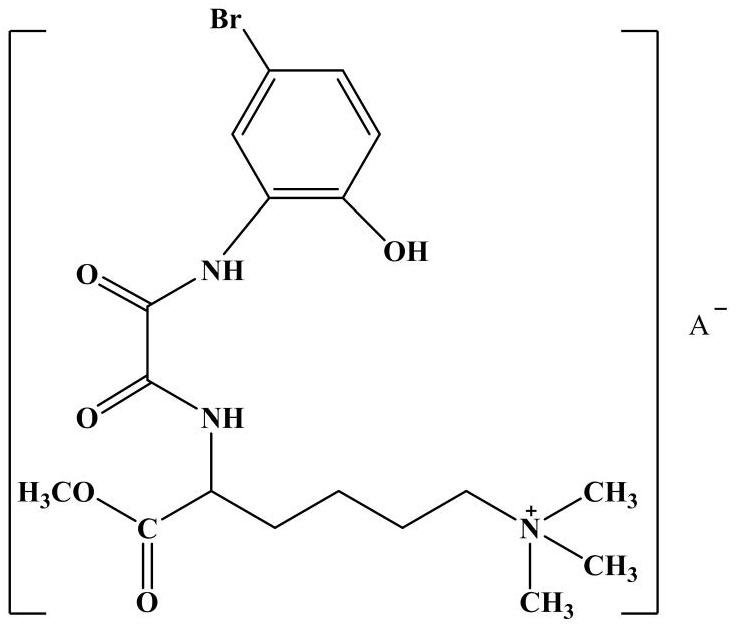
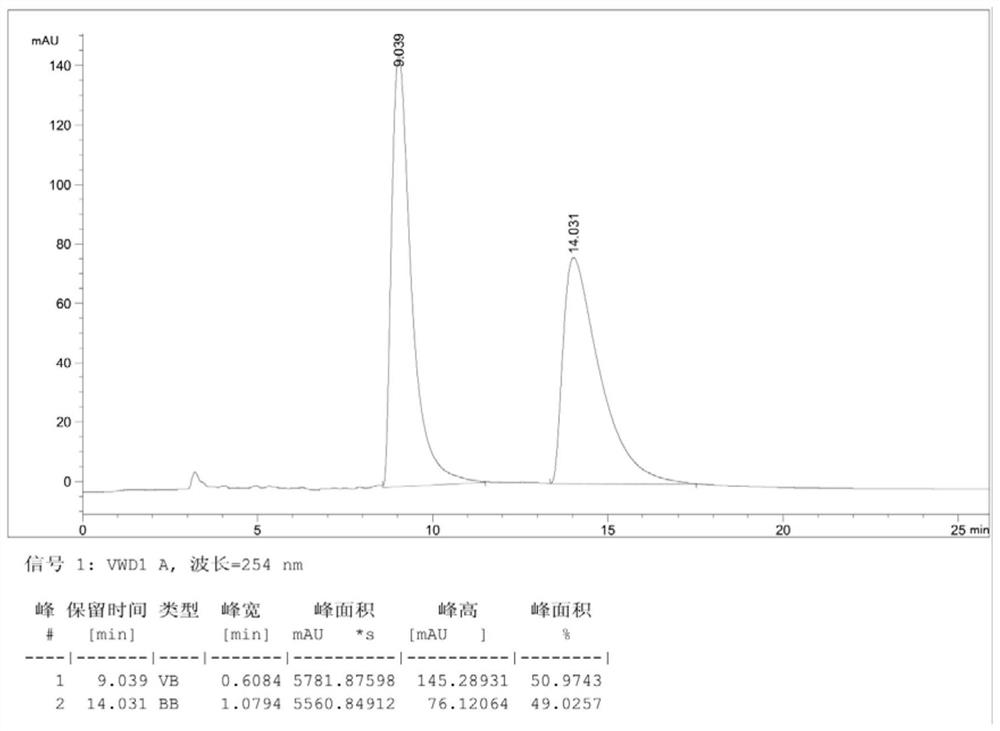
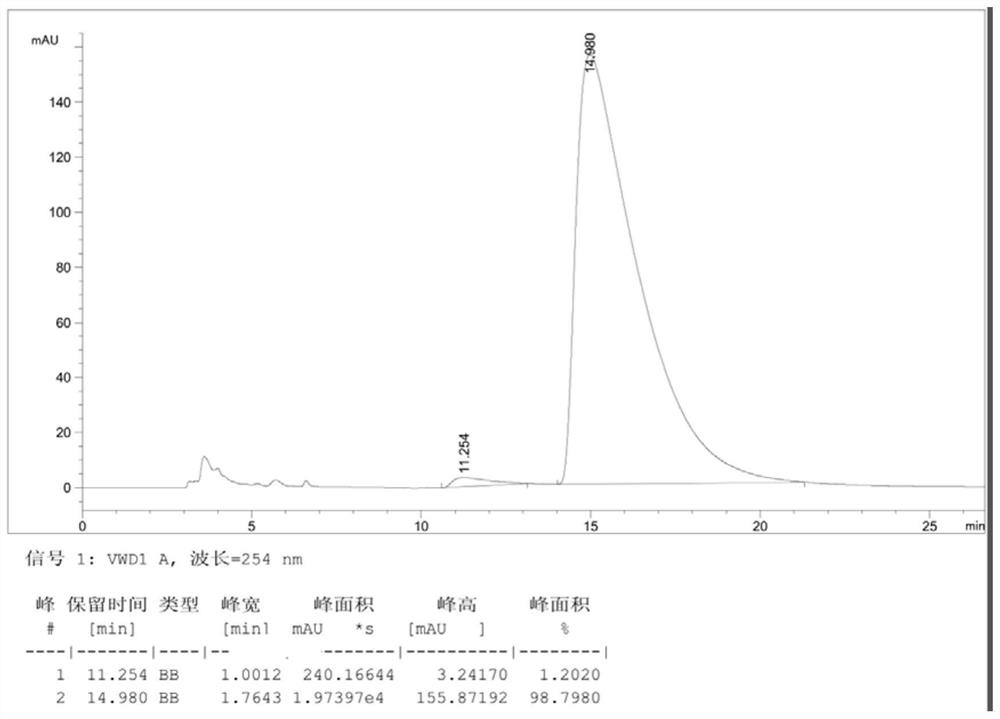
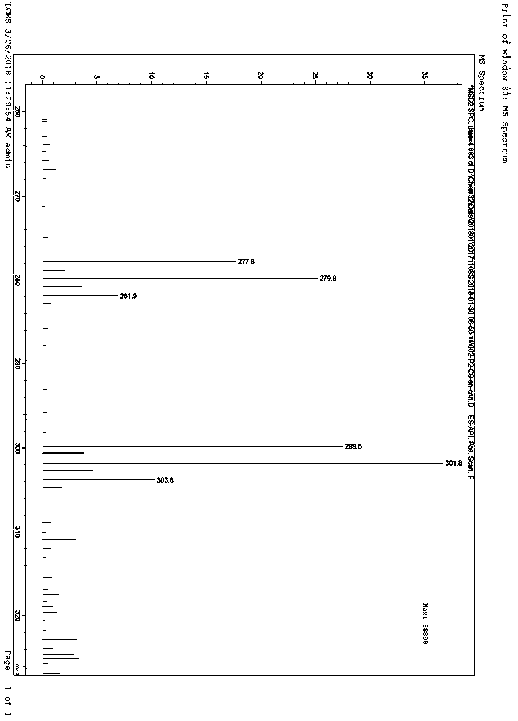
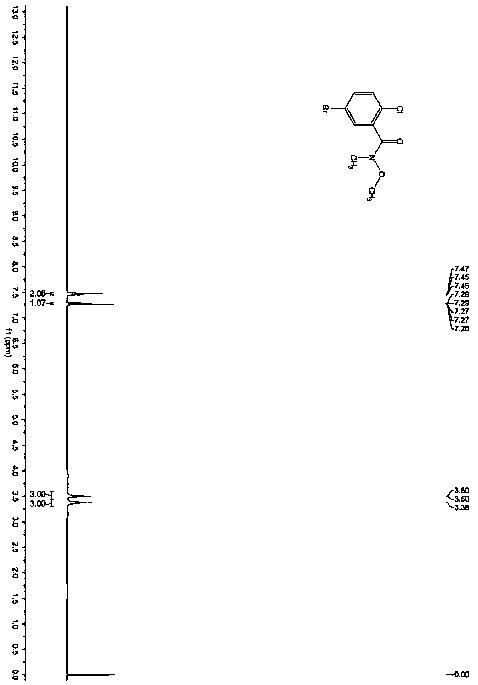
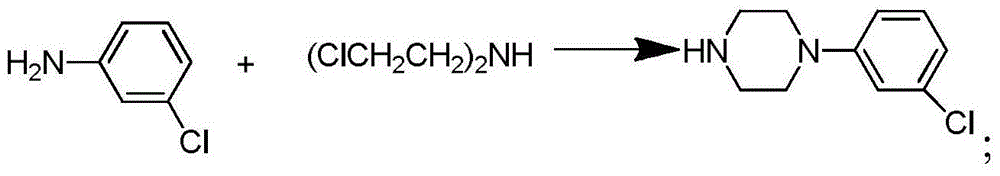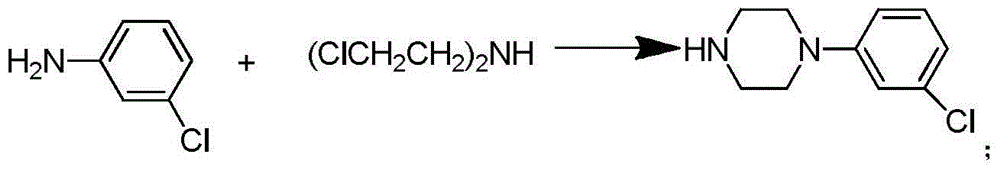Patents
Literature
50 results about "Methylamine hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Methylamine Hydrochloride (DEA List I Chemical) M1242 | 593-51-1 Methylamine Hydrochloride (DEA List I Chemical) from Spectrum Chemical is a colorless organic solid that has an odor similar to that of fish and is used as an essential part for the synthesis of a wide variety of commercial compounds suc.
Synthetic process for Vonoprazan fumarate
PendingCN107778286AReduce contentImprove removal efficiencyOrganic chemistryMethylamine hydrochloridePyridine
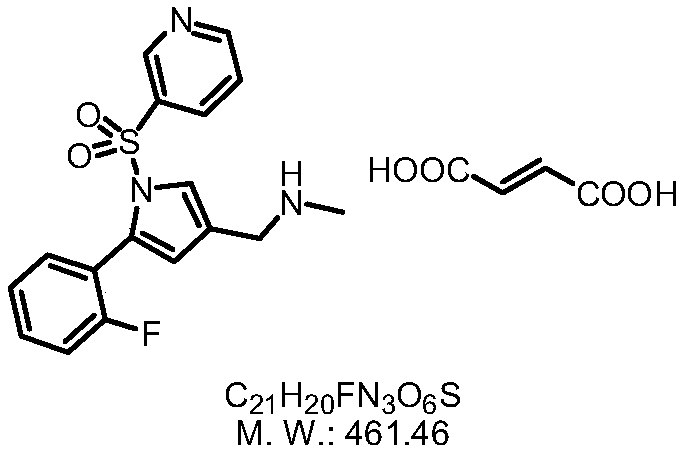
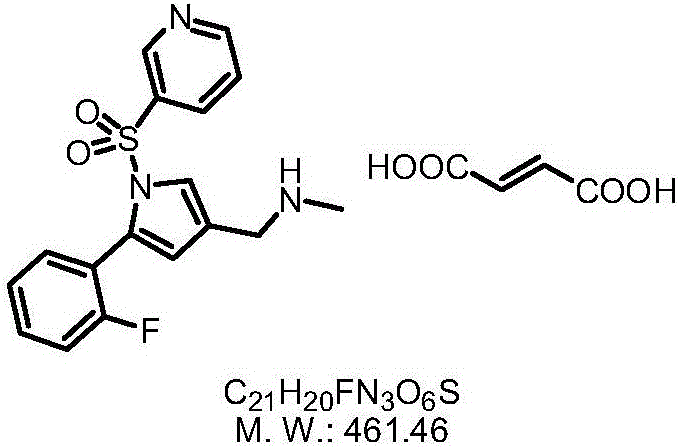
The invention provides a preparation method for preparing high-purity 5-(2-fluorophenyl)-N-methyl-1-(3-pyridine sulfonyl)-1H-pyrrole-3-methylamine fumarate. The method comprises the following steps: taking 5-(2-fluorophenyl)-N-methyl-1-(3-pyridine sulfonyl)-1H-pyrrole-3-methylamine hydrochloride as an intermediate; carrying out alkali treatment, and then salifying the treated intermediate with fumaric acid to obtain a finished product. A final product prepared by adopting the method has high purity and low content of key impurities; in addition, the purification and post treatment steps of fumarate are simplified, and the yield is remarkably improved.
Owner:四川弘远药业有限公司
Improved tadalafil preparation method
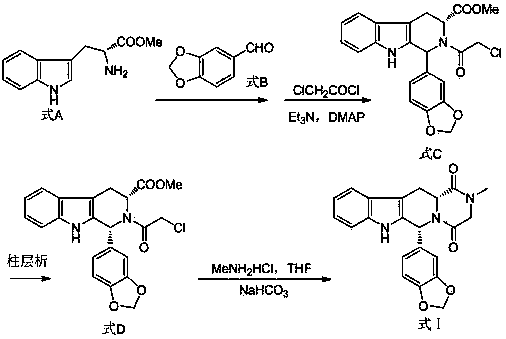
The invention belongs to the field of preparation of chemical raw medicaments, and more in particular relates to an improved preparation method for a phosphodiesterase 5 inhibitor tadalafil. A specific synthesis route is shown in the specification. The method comprises the following steps of performing Pictet-Spengler cyclization reaction and chloroacetyl chloride acylation on starting reactants (D-tryptophan methyl ester hydrochloride and piperonal) to obtain an intermediate product, directly performing subsequent reaction on the intermediate product without purification, preparing an intermediate 1-(1,3-benzodioxol-5-yl)-2-(chloracetyl)-2,3,4,9-tetrahydro-1H-pyridino-[3,4,-B]indol-3-thiophenate methyl by using a one-pot reaction method, performing column chromatography purification to obtain a single cis-isomer, and finally reacting the single cis-isomer with methylamine hydrochloride in the presence of an inorganic base to obtain the tadalafil.
Owner:ANHUI WANBANG MEDICAL TECH
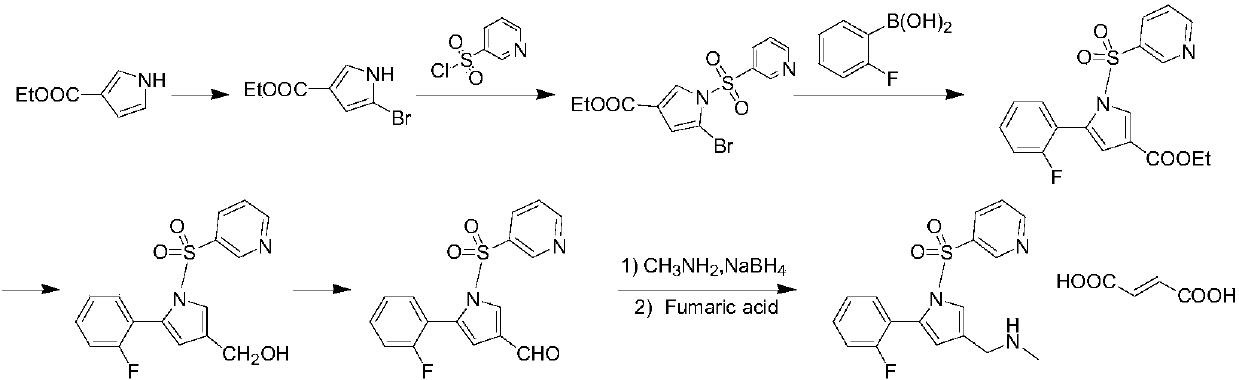
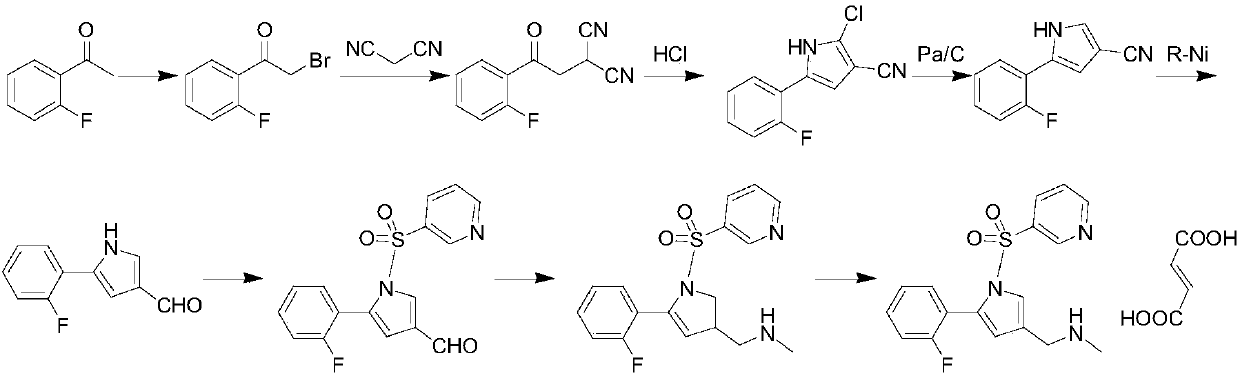
Vonoprazan fumarate preparation method
ActiveCN106366071AAccelerated corrosionHigh yieldCarboxylic acid salt preparationSulfonyl chlorideVonoprazan
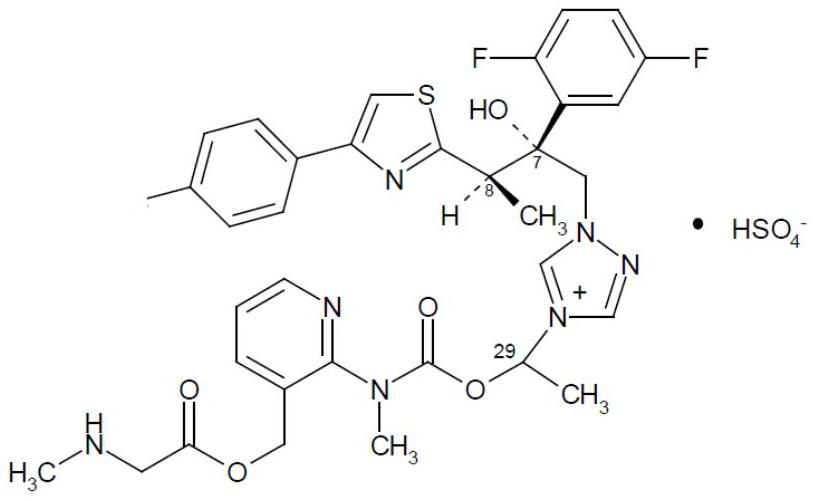
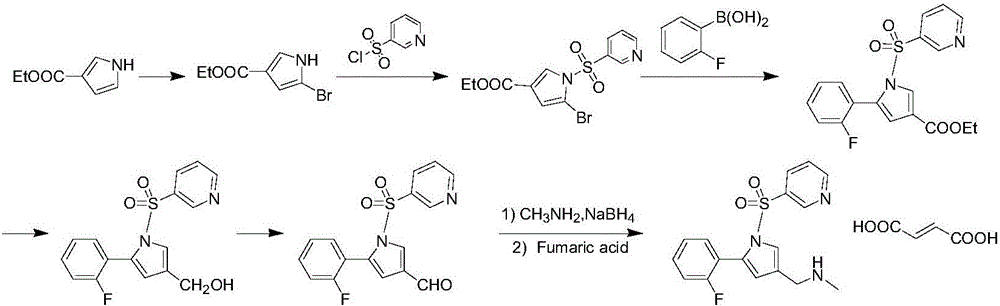
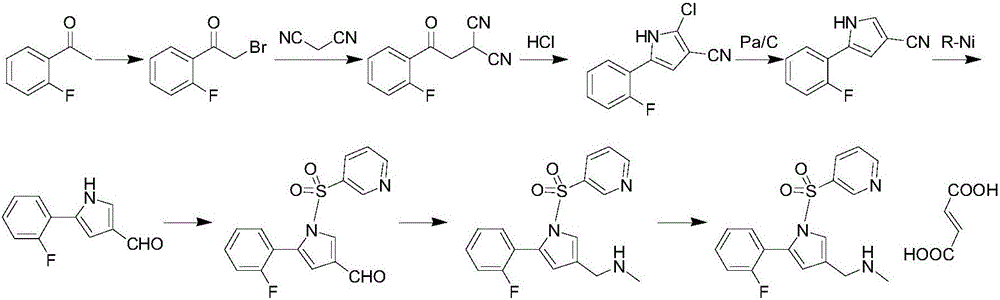
The invention concretely relates to a vonoprazan fumarate preparation method, and belongs to the field of medicines and chemical engineering. The method comprises the following steps: 1, carrying out condensation on 2-fluoroacetophenone used as an initial raw material and allylamine to obtain a compound IV; 2, carrying out a ring closing reaction on the compound IV under the catalysis of a copper catalyst in the presence of a ligand in order to obtain a compound V; 3, carrying out a sulfoamidation reaction on the compound V and pyridine-3-sulfonyl chloride to generate a compound VII; 4, carrying out a bromination reaction on the compound VII by using N-bromosuccimide in order to generate a compound VIII; 5, carrying out an amination reaction on the compound VIII and methylamine hydrochloride under the action of a catalyst and an alkali in order to obtain vonoprazan alkali; and 6, carrying out salt formation on the vonoprazan alkali and fumaric acid in order to obtain vonoprazan fumarate. The preparation method has the advantages of simplicity in operation, mild reaction conditions, high yield and high purity of the product, and easiness in industrial production.
Owner:SHANDONG JINCHENG PHARMACEUTICAL GROUP CO LTD
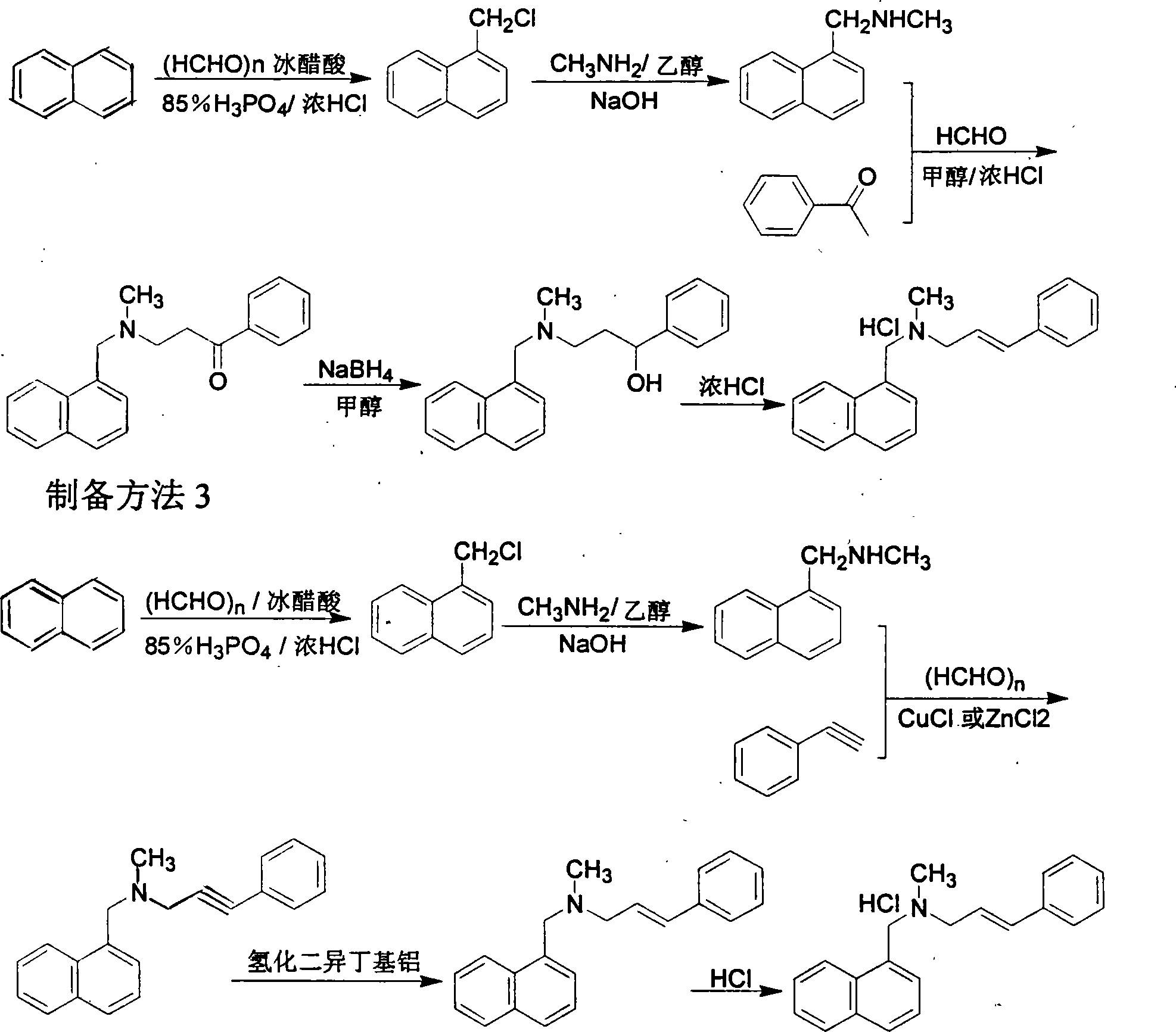
Method for preparing naftifine hydrochloride
ActiveCN101186578AEasy to operateGood removal effectAmino compound preparation by condensation/addition reactionsSynthesis methodsEthyl acetate
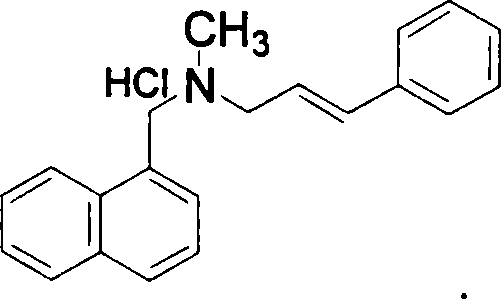
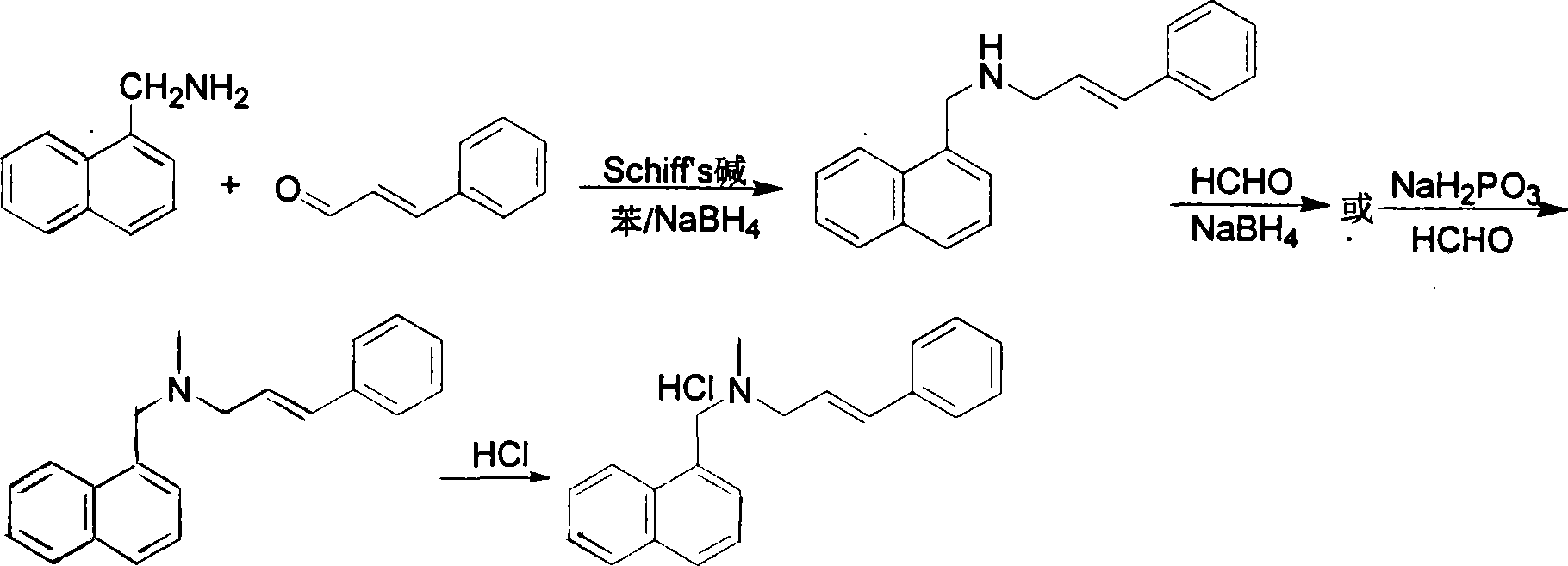
The invention discloses a preparation method of naftifine hydrochloride, which uses N-methyl-1-naphthyl methylamine hydrochloride as raw material, to directly participate reaction without alkalization free purification, uses organic ether solvent as reaction solvent, in the presence of alkali metal carbonate and catalyst, at suitable temperature, to generate crude naftifine hydrochloride via condensation reaction, and feeds acetic ester saturated solution of hydrochloride to generate hydrochlorate, and obtains high-purity naftifine hydrochloride product via recrystallization. The invention completes alkalization free purification, and condensation reaction in one device, with simple operation, high yield, low foreign material content, low cost, and industrialization support, which is better than previous synthesis method.
Owner:TIANJIN WEIJIE PHARMA
Preparation method of Terbinafine hydrochloride
InactiveCN101870655ARaw materials are cheap and easy to getThorough responseAntimycoticsPhysical/chemical process catalystsSodium iodideAlkyne
The invention relates to a preparation method of Terbinafine hydrochloride, which comprises the following steps: dissolving a certain amount of catalyst, i.e. sodium iodide (or potassium iodide) in an organic solvent, adding 1-chloro-6,6-dimethyl-2-heptylene-4-alkyne, sequentially adding alkali and N-methyl-1-naphthalene methylamine hydrochloride, carrying out condensation at 10-40 DEG C, salifying the obtained product in an alcohol system with hydrogen chloride to obtain the Terbinafine hydrochloride, and carrying out recrystallization in an alcohol / water mixed solution to obtain the qualified product. In the method, the raw materials are cheap and easily-acquired, the reaction is complete, and the reaction time is greatly shortened. Therefore, the method is completely suitable for industrialized mass production.
Owner:YANGTZE RIVER PHARMA GRP BEIJING HAIYAN PHARMA +1
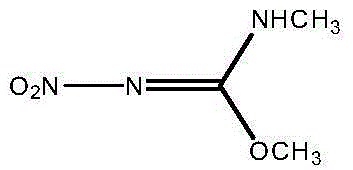
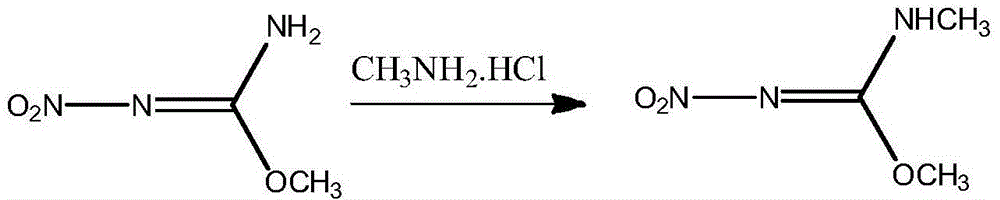
Preparation method of N,O-dimethyl-N'-nitroisourea
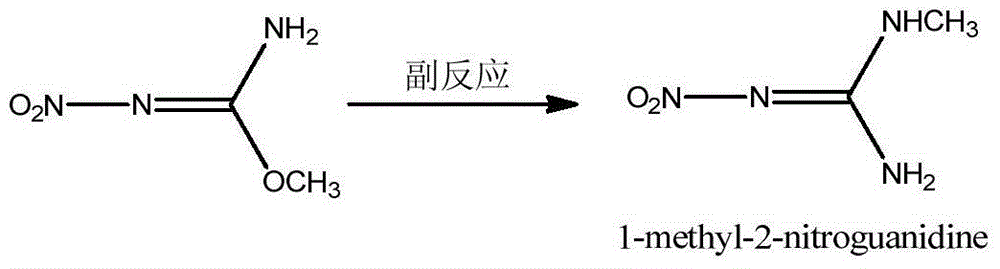
ActiveCN103951590ASuppress generationSimple processUrea derivatives preparationOrganic compound preparationChemical reactionPotassium fluoride
The invention discloses a preparation method of N,O-dimethyl-N'-nitroisourea. According to the preparation method, O-methyl-N-nitroisourea and methylamine hydrochloride are used as a raw material, and water is used as a solvent, so that reaction is performed in a potassium fluoride-water system, wherein the chemical reaction formula is as shown in the specification. The preparation method disclosed by the invention is simple and convenient in process and simple in operation; the water is used as the solvent, the pH value is not needed to be regulated and a catalyst is not needed to be added, so that the generation of secondary reactions can be effectively inhibited. The purity of products can reach more than 99%, and the yield can reach 79.1%; the preparation method is suitable for industrial production.
Owner:湖南海利常德农药化工有限公司
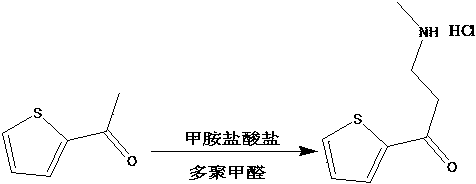
Preparation method of duloxetine intermediate
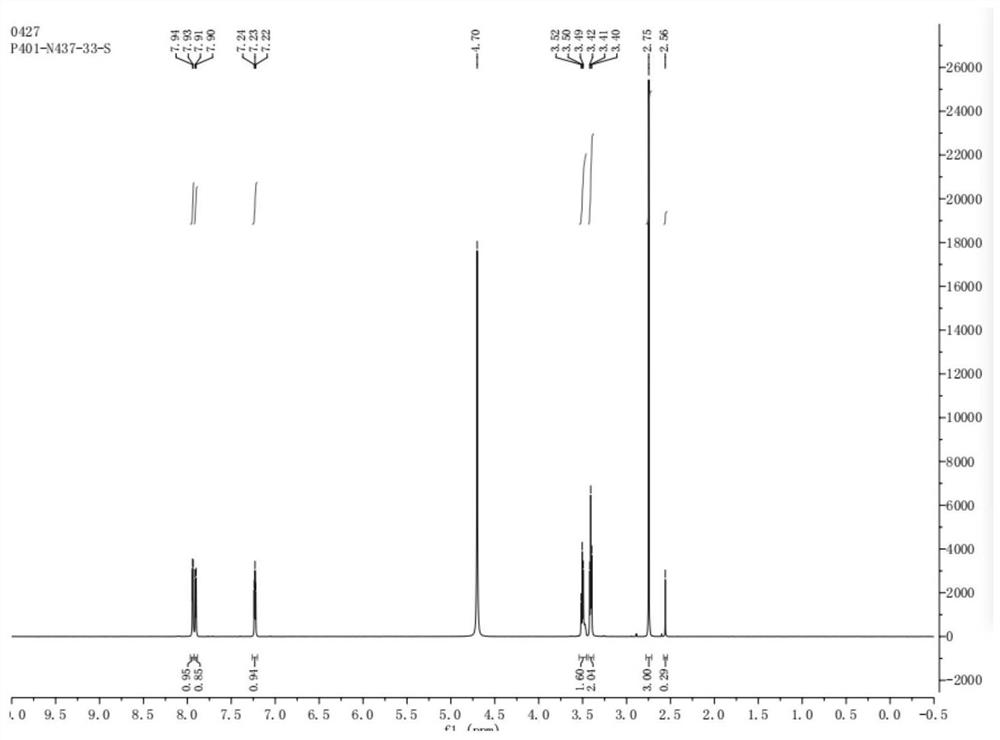
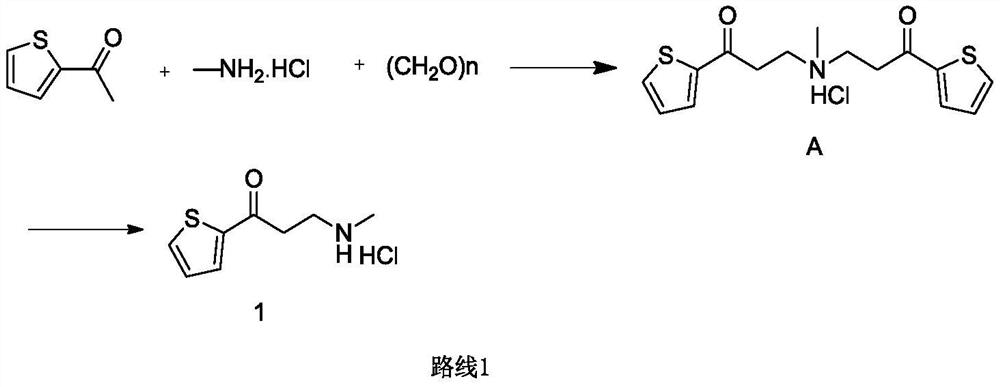
The invention provides a preparation method of a duloxetine intermediate, which comprises the following steps: by using 2-acetylthiophene, methylamine hydrochloride, paraformaldehyde and concentratedhydrochloric acid as raw materials, carrying out a Mannich reaction in a polar solvent to obtain the duloxetine intermediate 3-methylamino-1-(2-thienyl)-1-acetone hydrochloride. The preparation methodhas the advantages of mild reaction conditions and high product purity, and is suitable for industrial production.
Owner:CHEN STONE GUANGZHOU CO LTD
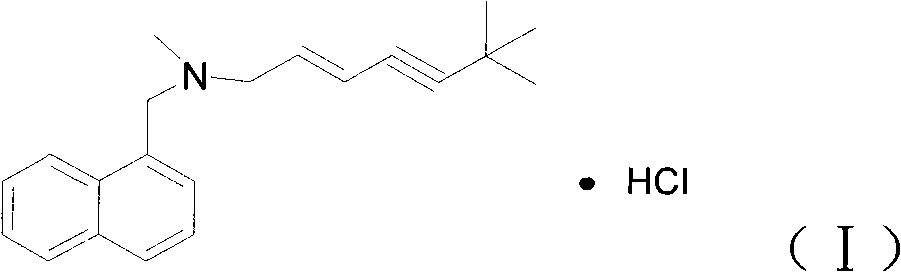
Preparation for preventing and controlling bee fungal diseases
InactiveCN102670664APromote digestion and absorptionHas immunomodulatory functionOrganic active ingredientsAntimycoticsBacillus licheniformisRandom combination
The invention discloses a preparation for preventing and controlling bee fungal diseases. The preparation consists of 0.1 to 2 percent of (E)-N-(6,6-dimethyl heptanoic-2-olefin-4-alkynyl)-N-methyl-1-naphthalene methylamine hydrochloride, 0.1 to 4 percent of beneficial microorganism and 94 to 99.8 percent of soluble starch or saccharides. The beneficial microorganism comprises one or random combination of bacillus subtilis, bacillus licheniformis, bee bifidobacterium, bifidobacterium asteroids, minimum bifidobacterium, bifidobacterium coryneforme, lactobacillus jensenii, lactobacillus amylovorus, lactobacillus plantarum, tiny lactobacillus and lactobacillus bifermentans; and a gram of the beneficial microorganism comprises 10<9> to 5*10<9> viable bacteria. The preparation for preventing and controlling bee fungal diseases is applied to prevention and control of bee or silkworm fungal diseases.
Owner:桑筠
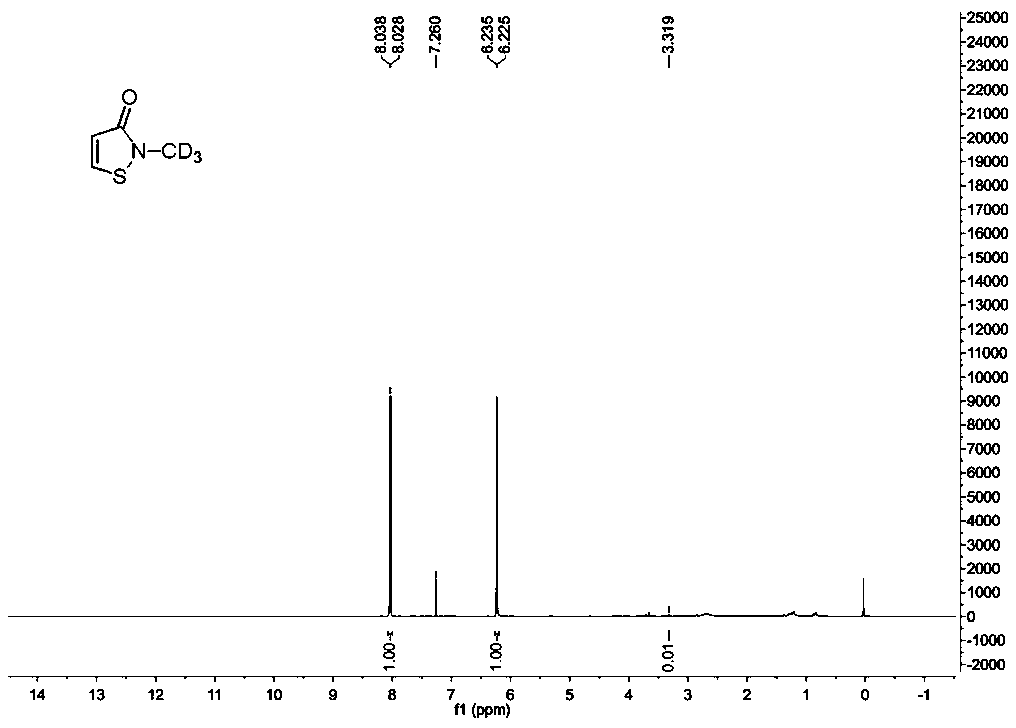
Synthesis method of stable isotope deuterium labeled 2-methyl-4-isothiazole-3-one
The invention provides a synthesis method of stable isotope deuterium labeled 2-methyl-4-isothiazol-3-one, the synthesis method comprises the following steps: step 1, taking 3,3'-dimercaptodipropionicacid and thionyl chloride as raw materials, and obtaining 3,3'-dimercaptodipropionyl chloride through catalytic reaction by a catalyst; step 2, after an alkali and a carbonate are sequentially addedto deuterated methylamine hydrochloride, adding the 3,3'-dimercaptodipropionyl chloride obtained in the step 1 to react to obtain deuterated amide; step 3, adding a reaction solvent and sulfonyl chloride to the deuterated amide obtained in the step 2, continuously stirring the reaction system at 0 DEG C for reaction for 1 hour, and then continuously stirring and reacting at room temperature for 15hours to obtain the stable isotope deuterium labeled 2-methyl-4-isothiazol-3-one, wherein the molar ratio of deuterated amide to sulfonyl chloride is 1: 3. According to the invention, the reaction yield can reach 34%, the purity of the target product can reach 98%, and the deuterium generation rate can reach more than 99%.
Owner:河南凯美思睿化工科技有限公司
Process for preparing N-methyl homopiperazine from 2-haloethylamine compound
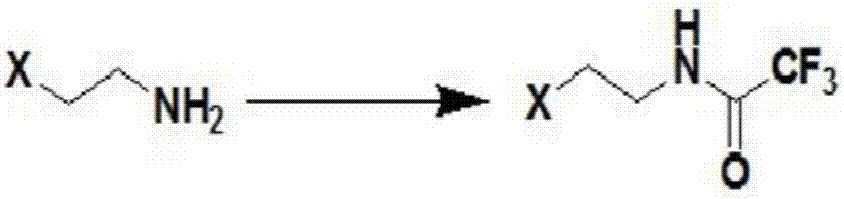
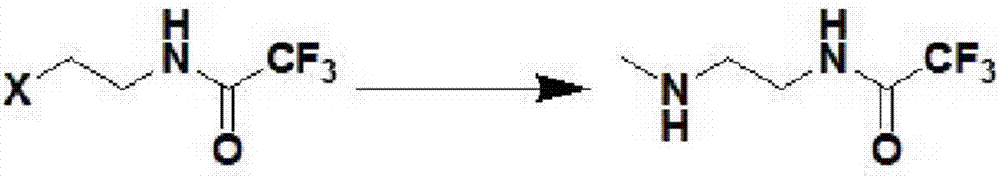
InactiveCN107382883AHigh degree of environmental protectionImprove the safety of production operationsOrganic chemistryEthylenediamineTrifluoroacetic acid
The invention discloses a process for preparing N-methyl homopiperazine from 2-haloethylamine compound, and the process comprises the following steps: Step 1, taking the 2-halogenated ethylamine compound as a raw materia to react with ethyl trifluoroacetate to obtain N-(2-Haloethyl) trifluoroacetamide; Step (2) taking the N-(2-Haloethyl) trifluoroacetamide as a raw material to react with methylamine or methylamine hydrochloride to obtain N-methyl-N'-trifluoroacetyl ethylenediamine; Step (3) taking the N-methyl-N'-trifluoroacetyl ethylenediamine as a raw material to react with 1,3-disubstituted propane compound to obtain N-methyl-N'-trifluoroacetyl homopiperazine; Step (4) taking the N-methyl-N'-trifluoroacetyl homopiperazine as a raw material to react with a hydrogen chloride ethanol solution to obtain N-methyl homopiperazine dihydrochloride; and Step (5) taking the N-methyl homopiperazine dihydrochloride as a raw material to prepare the N-methyl homopiperazine by alkalization. The process has the advantages of simple operation, low cost, high yield, low pollution and suitability for industrialized production.
Owner:SUZHOU BAILINGWEI HYPERFINE MATERIAL
Preparation method of duloxetine key intermediate
A preparation method of a duloxetine key intermediate. The invention discloses a preparation method of 3-N-methylamino-1-(2-thienyl)-1-acetonehydrochloride. The preparation method is characterized in that by using 2-acetylthiophene as an initial raw material and using low-carbon alcohol as a solvent, a typical Mannich reaction is carried out between the initial raw material and methylamine hydrochloride and paraformaldehyde in the presence of catalytic amounts of Lewis acid so as to obtain the product. The yield based on the weight reaches 80-85%. The preparation method has advantages of simple operation, high yield, good purity and the like, and is an excellent technology suitable for industrial production.
Owner:ZHANG JIA GANG VINSCE BIO PHARM
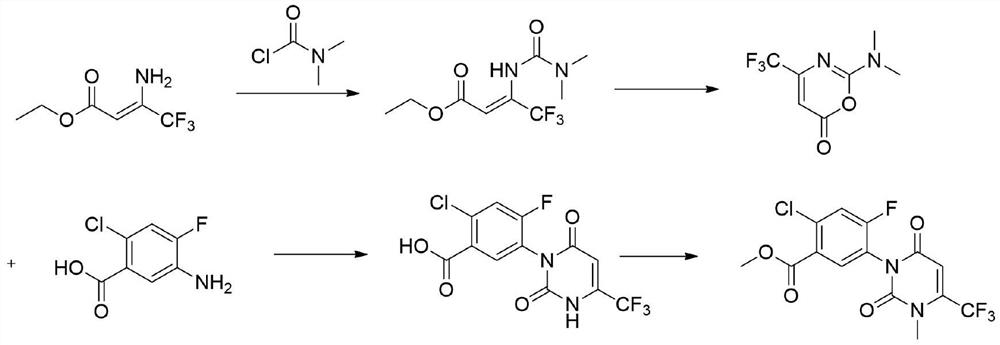
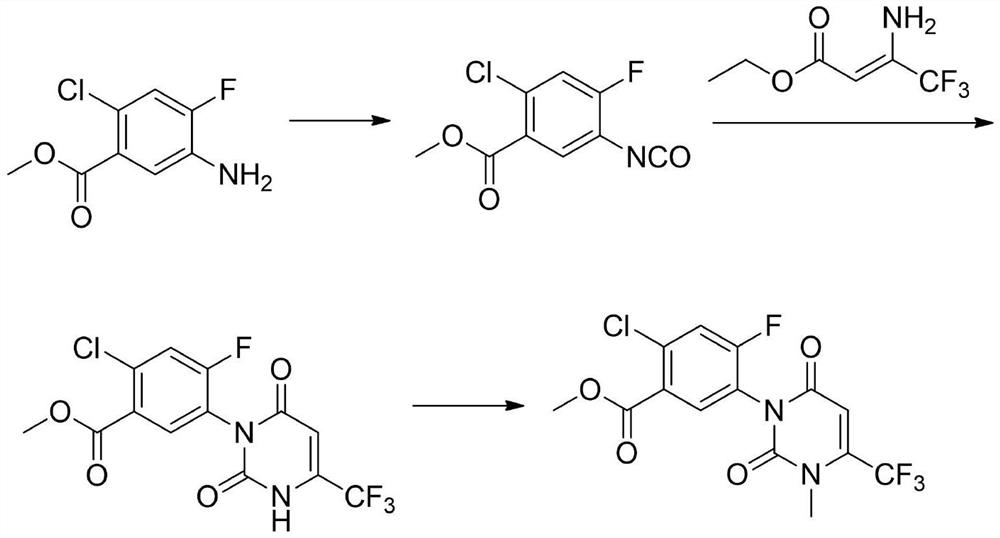
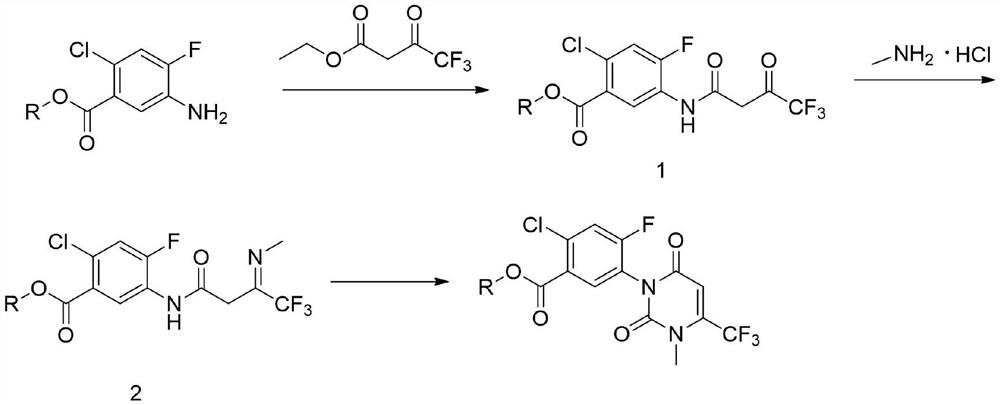
Preparation method of saflufenacil intermediate
The invention relates to the technical field of pesticide organic synthesis, and provides a saflufenacil intermediate preparation method, which comprises: a), carrying out reaction on 2-chloro-4-fluoro-5-aminobenzoate and ethyl 4,4,4-trifluoroacetoacetate to obtain an intermediate (1); b), reacting the intermediate (1) with methylamine hydrochloride to obtain an intermediate (2); and c), reactingthe intermediate (2) with an acylation reagent to obtain the 2-chlorine-4-fluorine-5-(3-methyl-2, 6-diketone-4-trifluoromethyl-2, 3-dihydropyrimidinyl-1(6H)- group) benzoate. The method solves the problems that toxic reagent raw materials with great harm are used in the existing saflufenacil intermediate reaction process, the operation is complicated, and the yield is not high.
Owner:NANJING LYNSCI CHEM
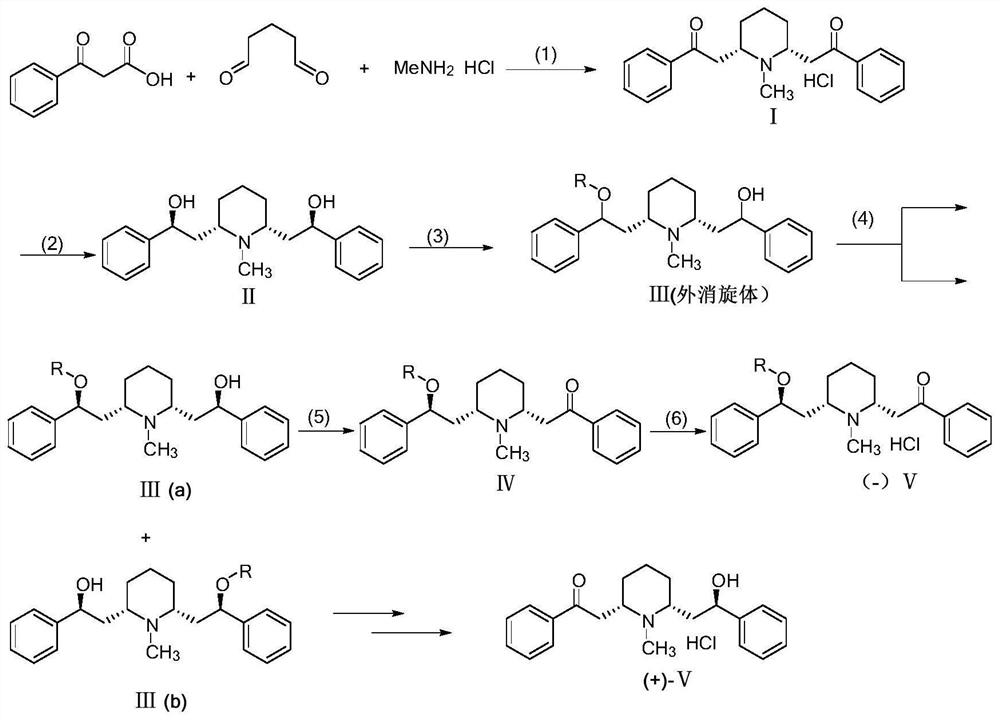
2-[(2R, 6S)-6-[(2S)-2-hydroxy-2-phenethyl]-1-pipecoline]-1-hypnone synthesis method
ActiveCN106496099AChange the status quo that asymmetric synthesis is difficult to achieveSimple processOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsAcetic acidOrganic synthesis
The invention discloses a 2-[(2R, 6S)-6-[(2S)-2-hydroxy-2-phenethyl]-1-pipecoline]-1-hypnone synthesis method, and belongs to the field of organic synthesis. (1S, 2S)-1, 2-diphenyl diaminoethane serving as a raw material is subjected to acylation, substitution and the like to prepare chiral amine catalysts. A main route totally includes two steps: synthesizing glutaraldehyde, benzoyl acetic acid and methylamine hydrochloride to form cis-lobelanine; performing asymmetric selective reduction synthesis of 2-[(2R, 6S)-6-[(2S)-2-hydroxy-2-phenethyl]-1-pipecoline]-1-hypnone under mild reaction conditions and under the action of the catalysts. The raw material in the whole process route is cheap and easy to obtain, the catalysts can be recycled and can continue to be used, and the synthesis method is low in cost, simple in process, mild in reaction condition, convenient in operation and high in total yield.
Owner:HEADING NANJING PHARMTECH CO LTD
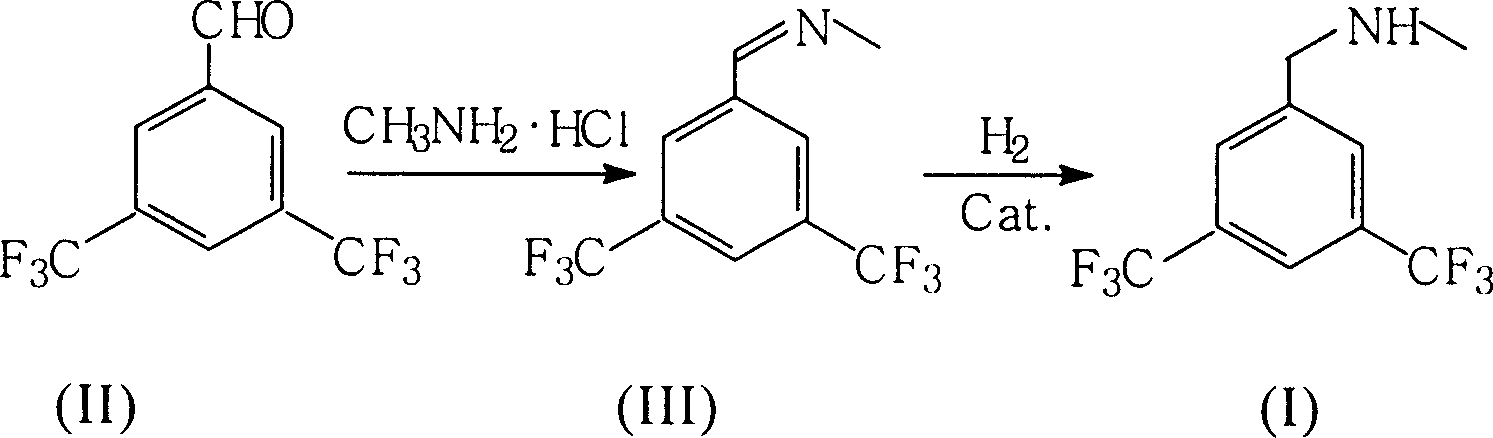
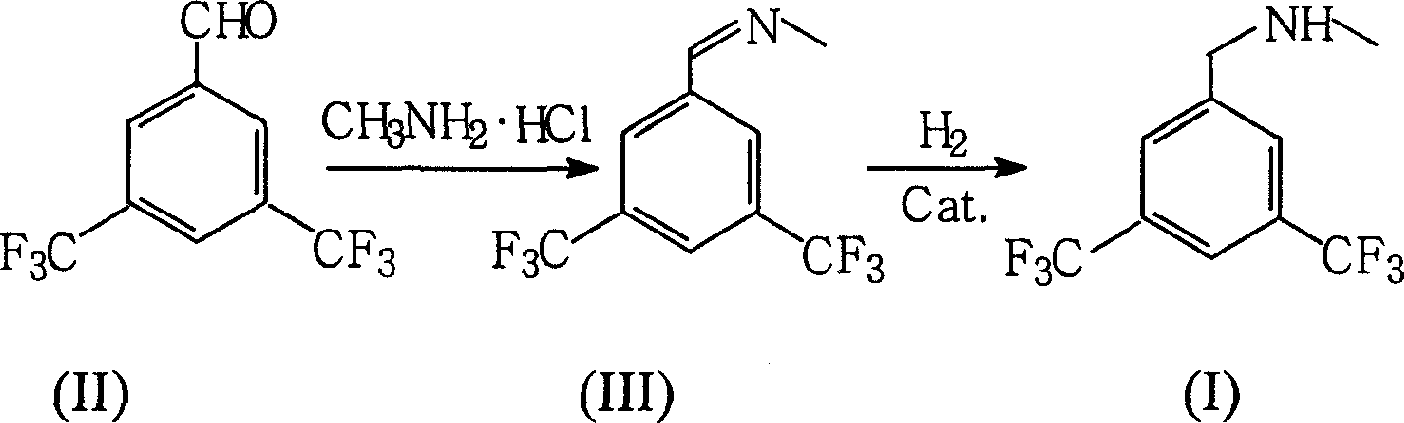
Preparation of N-methy-3,5-ditrifluo-aniline
Production of N-methyl-3,5-bistriflumethylaniline is carried out by oximation reacting 3,5-bistriflutoluyl aldehyde with methylamine hydrochloride, catalyzing and reducing the products. It yields more and is cheap.
Owner:LYNCHEM
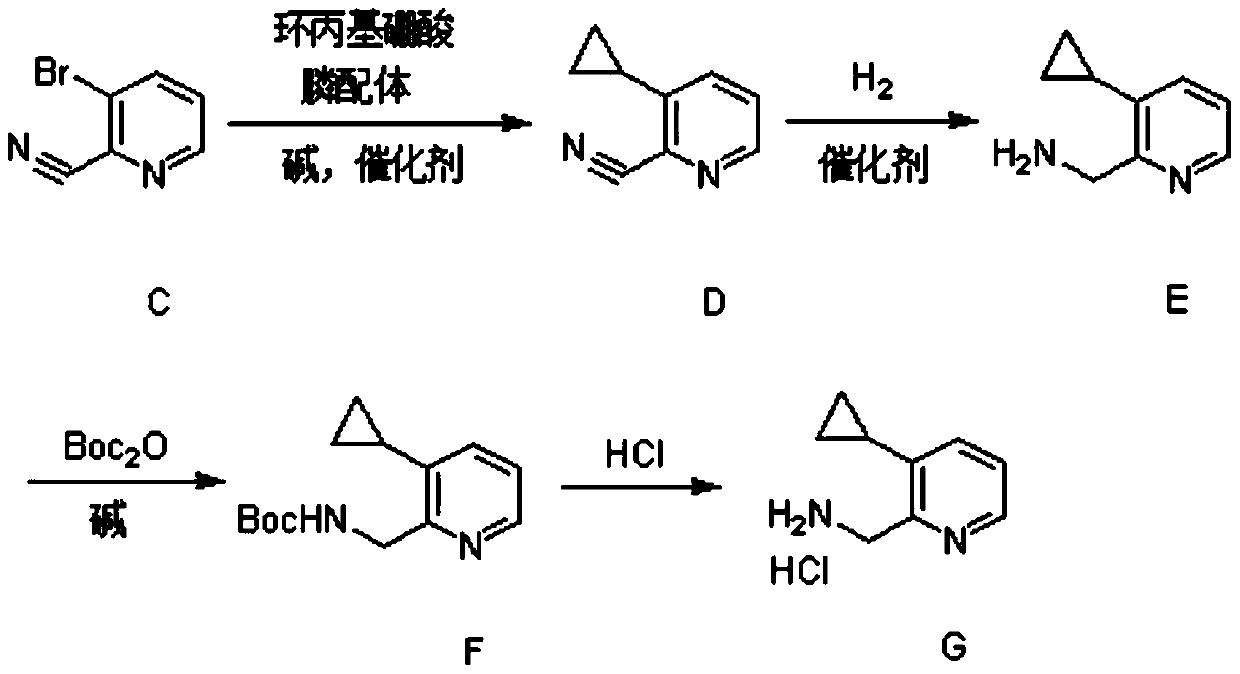
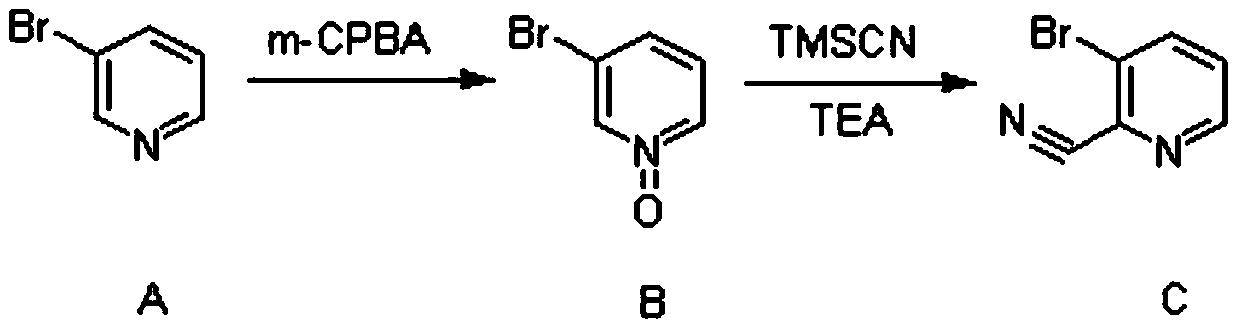
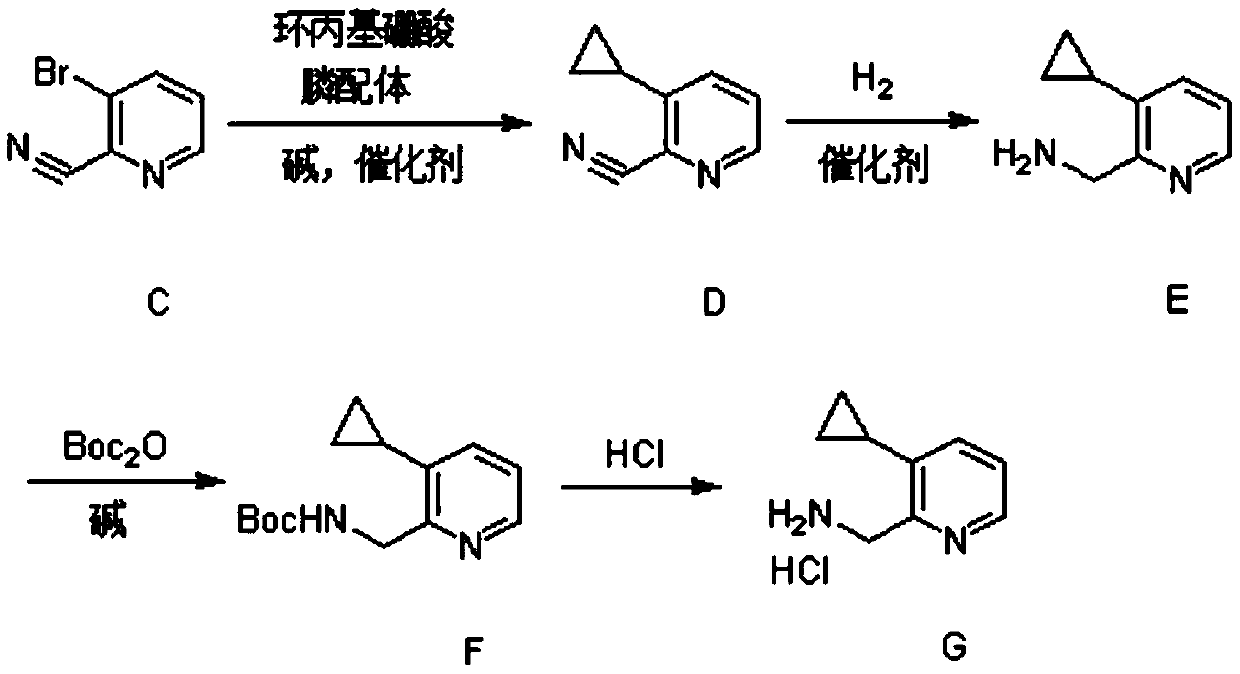
Synthesis method of (3-cyclopropylpyridin-2-yl) methylamine hydrochloride
InactiveCN111285798AReasonable designExperiment operation is simpleOrganic chemistryPtru catalystPhosphine
The invention provides a synthesis method of (3-cyclopropylpyridin-2-yl) methylamine hydrochloride, and belongs to the technical field of synthesis of organic chemical intermediates. The preparation method comprises the following steps: reacting 3-bromo-2-cyanopyridine with cyclopropylboronic acid, a phosphine ligand, an alkali and a catalyst under the protection of nitrogen, then reacting with hydrogen under the action of the catalyst, reacting with di-tert-butyl dicarbonate and alkali, and finally reacting with an organic solvent solution of hydrogen chloride to obtain a target product (3-cyclopropylpyridin-2-yl) methylamine hydrochloride. The 3-bromo-2-cyanopyridine is prepared by taking 3-bromopyridine as a raw material and carrying out nitrogen oxidation and cyanation. The method is reasonable in process design, simple in experimental operation and easy to control.
Owner:阿里生物新材料(常州)有限公司
Method for synthesizing [<2>H3]-1-methylamino-2-phenylpropane
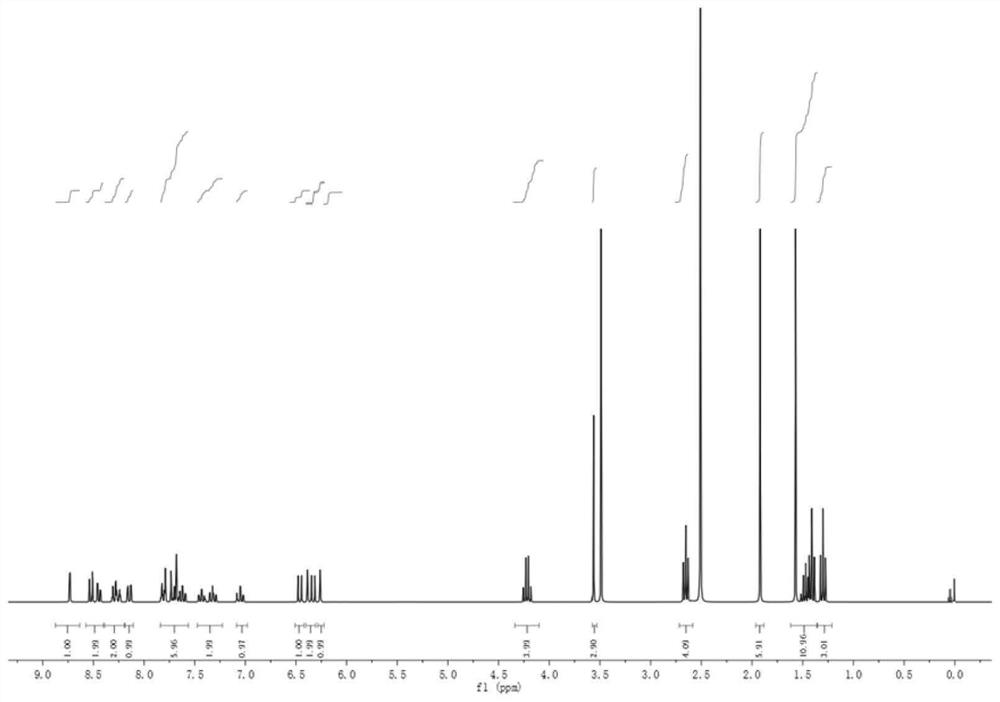
ActiveCN108864177AHigh purityEasy to synthesizeAmino preparation from aminesTitanium organic compoundsOrganic solventFiltration
The invention discloses a method for synthesizing [<2>H3]-1-methylamino-2-phenylpropane. The method is characterized by comprising the following steps: (1) subjecting phenylacetone, deuterated methylamine and tetraalkyl titanate or phenylacetone, deuterated methylamine, tetraalkyl titanate and an acid binding agent or phenylacetone, deuterated methylamine hydrochloride and tetraalkyl titanate to areaction, so as to obtain a first intermediate; (2) subjecting the first intermediate obtained in the step (1) and a reduction reagent to a reaction; (3) adding ammonia water into the step (2) for aquenching reaction, carrying out suction filtration to remove precipitates, carrying out precipitation with an organic solvent, merging liquid, and carrying out extraction and knockout to collect an organic phase, thereby obtaining the [<2>H3]-1-methylamino-2-phenylpropane. The method has the advantages that the synthesis route is short, the operation is simple and safe, the reaction time is short, and the yield exceeds 50%. Through further purification, both the purity and deuterated rate of the [<2>H3]-1-methylamino-2-phenylpropane can reach 99.5% or more, and thus, the [<2>H3]-1-methylamino-2-phenylpropane completely can serve as a deuterated internal reference substance for a mass-spectrum internal reference quantitative analysis method.
Owner:INST OF FORENSIC SCI OF MIN OF PUBLIC SECURITY
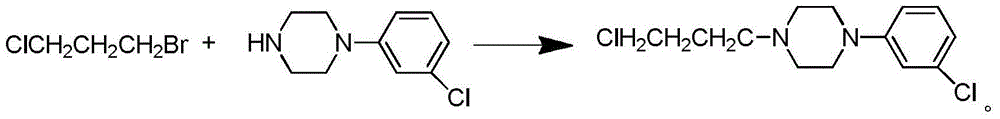
Synthetic method of piperazidines drug intermediate
The invention discloses a synthetic method of a piperazidines drug intermediate. The synthetic method comprises the following steps: step one, reacting diethanol amine with thionyl chloride to prepare di(2-chloroethyl) methylamine hydrochloride; step two, reacting 3-chloroaniline with di(2-chloroethyl) methylamine hydrochloride to prepare 1-(3-chlorphenyl) piperazine hydrochlorid; step three, reacting 1-(3-chlorphenyl) piperazine hydrochloride with 1-bromine-3-chloropropane to prepare 1-(3-chlorphenyl)-4-(3-chloropropyl) piperazine hydrochloride. The line is simple, convenient and easy to perform, the aftertreatment operation is also greatly simplified, the reaction conditions are mild, and the product purity is high.
Owner:SUZHOU JONATHAN NEW MATERIALS TECH
Method for recycling methylamine hydrochloride
InactiveCN101891652AHigh yieldSimple processIsocyanic acid derivatives preparationCarbamic acid derivatives preparationOrganic solventFiltration
The invention discloses a method for recycling methylamine hydrochloride to prepare methylamino formyl chloride. The methylamino formyl chloride is prepared by adopting a high-pressure reaction, using an organic solvent as a reaction solvent, and reacting the methylamine hydrochloride with phosgene, wherein the yield in terms of the methylamine hydrochloride is more than or equal to 90.0 percent. The method for recycling the methylamine hydrochloride has a simple process and a mild reaction condition; the used solvent is suitable for industrialized requirements; and reaction solution is directly used without purification after filtration and phosgene removal, and the three-waste amount is greatly reduced.
Owner:HUNAN CHEM RES INST
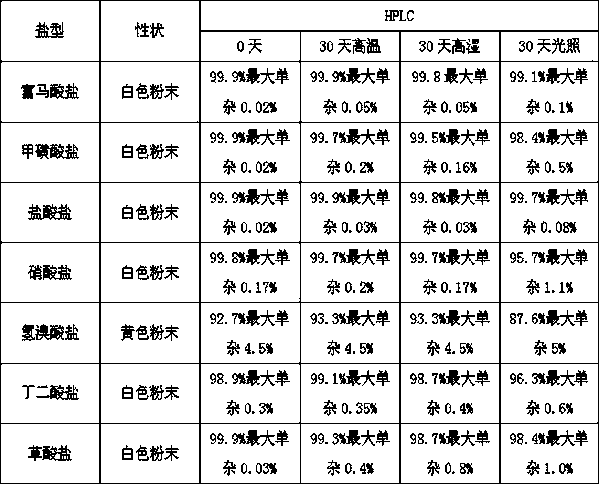
Preparation of pyrrole sulfonic acid compound salt type
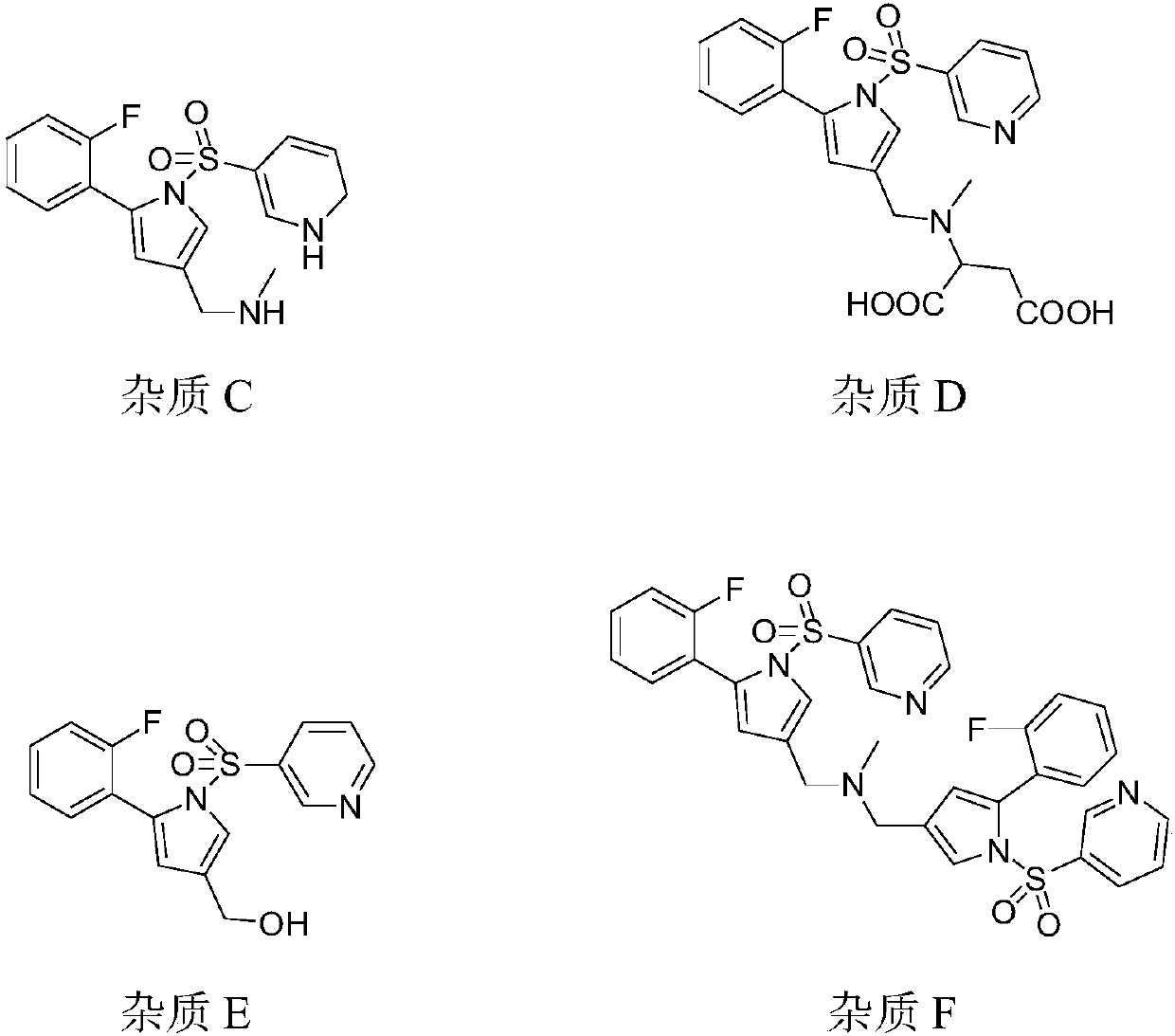
The invention provides a 1-(5-(2-fluorophenyl)-1-3-(3-methoxypropoxy)benzenesulfonyl chloride)-1H-pyrrol-3-yl)-N-methylamine salt type. The salt type contains organic acid salt and inorganic acid salt. By mainly screening various organic acids and inorganic acids, 1-(5-(2-fluorophenyl)-1-3-(3-methoxypropoxy)benzenesulfonyl chloride)-1H-pyrrol-3-yl)-N-methylamine hydrochloride and 1-(5-(2-fluorophenyl)-1-3-(3-methoxypropoxy)benzenesulfonyl chloride)-1H-pyrrol-3-yl)-N-methylamine fumarate which have relatively high purities and relatively good stabilities can be obtained and can be used for treating erosive esophagitis, gastric ulcer, duodenal ulcer and helicobacter pylori, eradicating adaptation diseases and treating relevant diseases caused by hyperacidity.
Owner:JIANGSU CAREFREE PHARM CO LTD
Preparation process of benzofuran with amide side chain
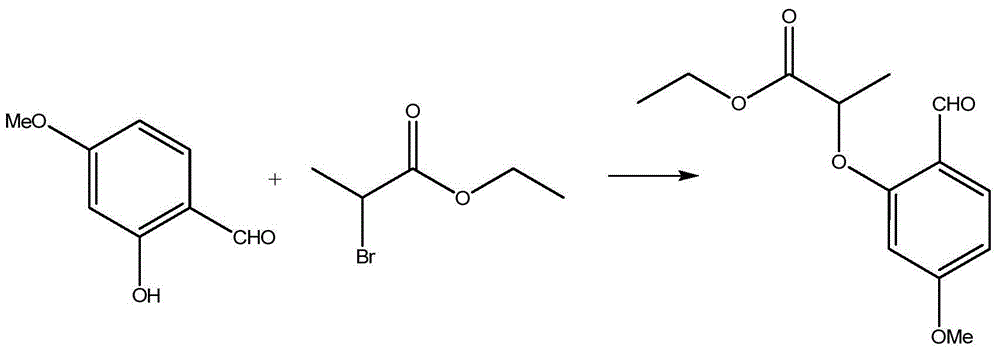
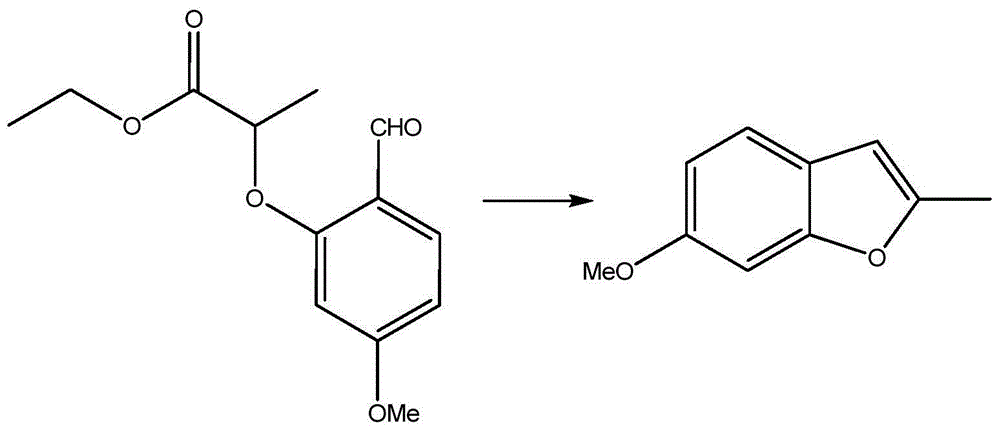
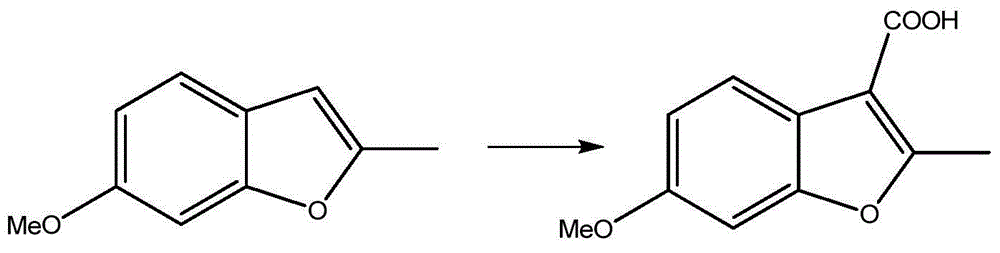
The invention relates to a preparation process of benzofuran with amide side chain. The preparation process comprises the following steps: reacting adjacent hydroxyl compound, 2-halogen substituendum, solvent and alkali to obtain 2-(2-formyl-5-methoxy-phenoxyl)-ethyl propionate; reacting a first product, dioxane and sodium salt to obtain 2-methyl-6-methoxy benzofuran; mixing dichloromethane, aluminium chloride, oxalyl chloride and a second product to obtain 2-methyl-3-carboxyl-6-methoxy benzofuran; carrying out backflow on a third product, dichloromethane, thionyl chloride and dimethyl formamide to obtain 2-methyl-3-chloroformy-6-methoxy benzofuran; reacting a first product, tetrahydrofuran, methylamine hydrochloride and alkali to obtain 6-methoxyl-N,2-dimethyl benzofuran-3-formamide; and reacting a fifth product, dichloromethane and boron tribromide to obtain the benzofuran with amide side chain. The yield reaches 97%.
Owner:NANTONG HENGSHENG FINE CHEM
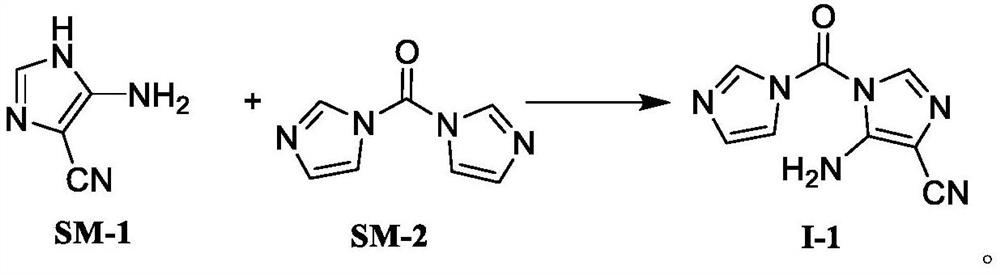
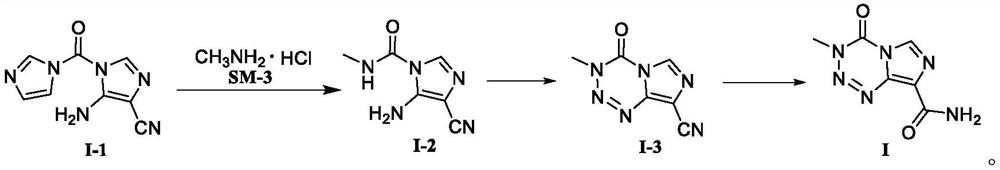
Temozolomide intermediate compound
PendingCN114591247AHigh selectivityReduce usageOrganic chemistryBulk chemical productionMuzolimineHydrolysis
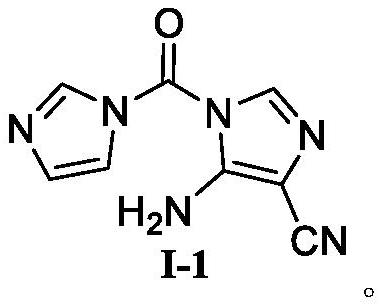
The invention belongs to the technical field of medicine synthesis, and particularly relates to a temozolomide intermediate compound. According to the preparation method, 5-amino-1H-imidazole-4-nitrile is taken as an initial raw material and reacts with N, N '-carbonyldiimidazole, and the new temozolomide intermediate 5-amino-1-(1H-imidazole-1-carbonyl)-1H-imidazole-4-nitrile is obtained. Meanwhile, the invention provides a method for preparing temozolomide by using the novel intermediate compound. The method comprises the following steps: reacting 5-amino-1-(1H-imidazole-1-carbonyl)-1H-imidazole-4-nitrile with methylamine hydrochloride, diazotizing the obtained product, cyclizing, and hydrolyzing cyano to obtain temozolomide. The synthesis method of the novel intermediate provided by the invention is simple, the reaction of the novel intermediate and methylamine hydrochloride can effectively avoid the use of a highly toxic reagent methyl isocyanate and methylamino formyl chloride with higher toxicity and irritation, diazotization and ring-closure reaction are realized in one step, and the separation of unstable diazonium salt is avoided. The synthetic route is short, the yield is high, the reaction condition is mild, the process is stable, and the method is suitable for large-scale industrial production.
Owner:LUNAN PHARMA GROUP CORPORATION
Preparation of N-methy-3,5-ditrifluo-aniline
Production of N-methyl-3,5-bistriflumethylaniline is carried out by oximation reacting 3,5-bistriflutoluyl aldehyde with methylamine hydrochloride, catalyzing and reducing the products. It yields more and is cheap.
Owner:LYNCHEM
Laminbutinyl bromooxamide derivatives with anticancer activity and composition thereof
ActiveCN107540569BExpand high value-added applicationsMaintain cationic propertiesOrganic compound preparationCarboxylic acid amides preparationSolventAniline
The invention discloses a laminine ester bromo oxamide derivative with anticancer activity and a composition thereof. The preparation method includes the steps of conducting backflow on laminine hydrochloride in thionyl chloride, adding methyl alcohol to conduct backflow again, steaming excess solvent, and regulating to neutral until white precipitate is dissolved out; filtering, using glacial alcohol and diethyl ether to wash, and conducting vacuum drying to obtain a white solid product; dissolving N-(5-bromine-2-hydroxyl aniline) ethyl oxalyl in an absolute ethyl alcohol solution, slowly adding drop by drop into anhydrous THF which dissolves prepared 6-N,N,N-trimethyl-2-carbamyl methylamine hydrochloride, conducting temperature reaction, and regulating the solution to neutral with dilutehydrochloric acid or a dilute hydrobromic acid solution until white precipitate is dissolved out; filtering, taking the precipitate to conduct recrystallization with ethyl alcohol, and conducting vacuum drying to obtain a target product. According to the laminine ester bromo oxamide derivative with anticancer activity and the composition thereof, through the introduction of a brominated ring structure, the cytotoxic activity of laminine to tumor cell strains in vitro can be enhanced.
Owner:QINGDAO UNIV
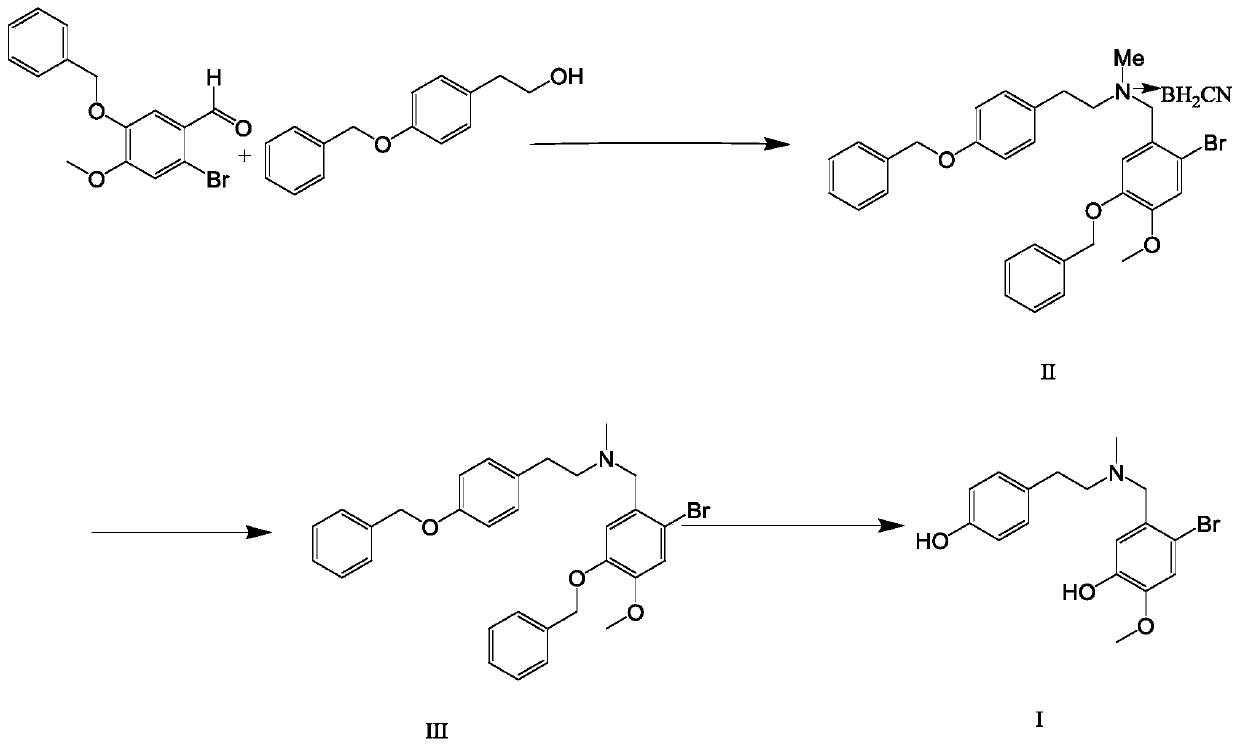
A kind of preparation method of galantamine key intermediate
ActiveCN106977412BLow production costEasy to operateOrganic compound preparationGroup 3/13 element organic compoundsMethylamine hydrochloride4-methoxybenzylamine
The invention discloses a preparation method of galanthamine key intermediate -N-methyl(4-leptodactyline)-2-bromo-5-hydroxyl-4-methoxybenzylamine. The preparation method comprises the following steps: by taking 2-bromo-5-benzyloxy-4-methoxybenzaldehyde, benzyloxyphenyl ethanol and methylamine hydrochloride as raw materials, condensing, degrading and performing debenylation reaction to prepare N-methyl(4-leptodactyline)-2-bromo-5-hydroxyl-4-methoxybenzylamine. Cheap and easily available raw materials are adopted in the method, so that the production cost can be effectively controlled, the operation is simple, and the reaction conditions are mild. The intermediate is used for industrial synthesis of galanthamine, and the problem that the yield from the industrial production of galanthamine is low can be effectively solved.
Owner:ZHANG JIA GANG VINSCE BIO PHARM
Preparation method of isavuconazonium sulfate intermediate
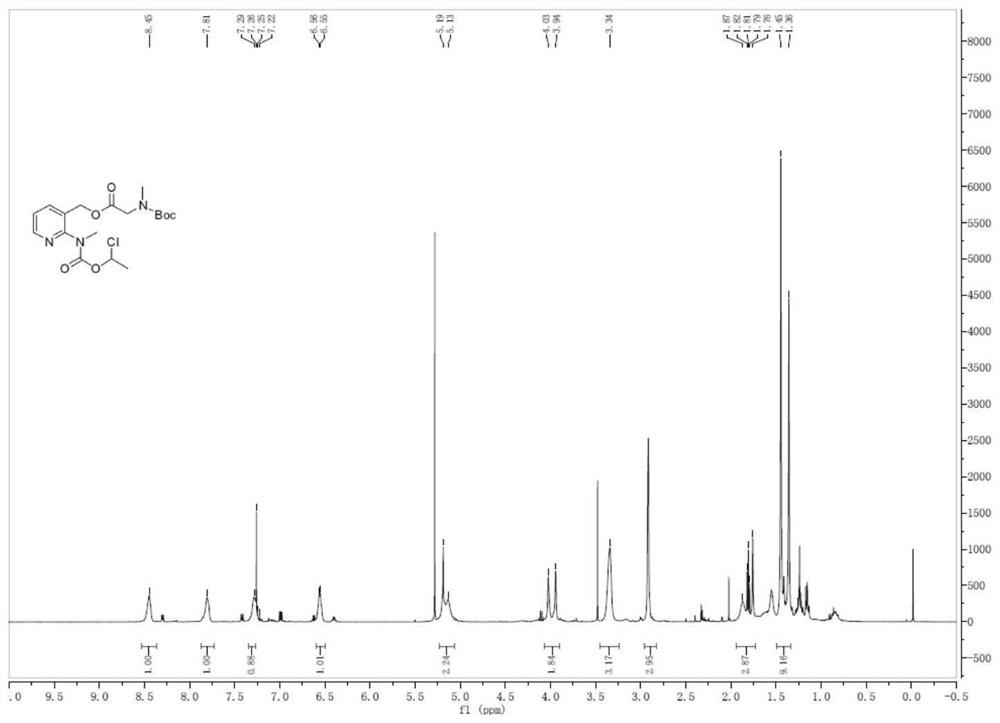
InactiveCN113861107AReduce consumptionMild reaction conditionsOrganic chemistryMethylcarbamic acidSarcosine
The invention discloses a preparation method of an isavuconazonium sulfate intermediate. The preparation method comprises the following steps: firstly, reacting absolute ethyl alcohol, sodium ethoxide and methylamine hydrochloride, cooling an obtained reaction solution, adding an ethanol solution of 1-chloroethyl methylcarbamate for continuous reaction, and conducting filtering to obtain an ethanol solution of methyl carbamic acid-1-chloroethyl ester; cooling the obtained solution, adding an ethanol solution of 2-chloro-3-pyridinemethanol for stirring reaction, filtering a reactant, removing ethanol, adding dichloromethane for dissolving, conducting washing after dissolving, and taking an organic phase, namely a dichloromethane solution of 1-chloro-ethyl (3-hydroxymethyl-pyridin-2-yl)-methylcarbamate; and firstly adding BOC-sarcosine into the obtained solution, then adding carbodiimide and 4-dimethylaminopyridine in batches for a reaction, and performing treatment after the reaction, so as to obtain the isavuconazole onium sulfate intermediate. The isavuconazonium sulfate is prepared by using the technical scheme of the invention, the side reaction of the process is less, and the purity of the obtained product is high.
Owner:KAIFENG MINGREN PHARMA
A preparation method and application of a fluorescent molecular probe for detecting glutathione thiol transferase
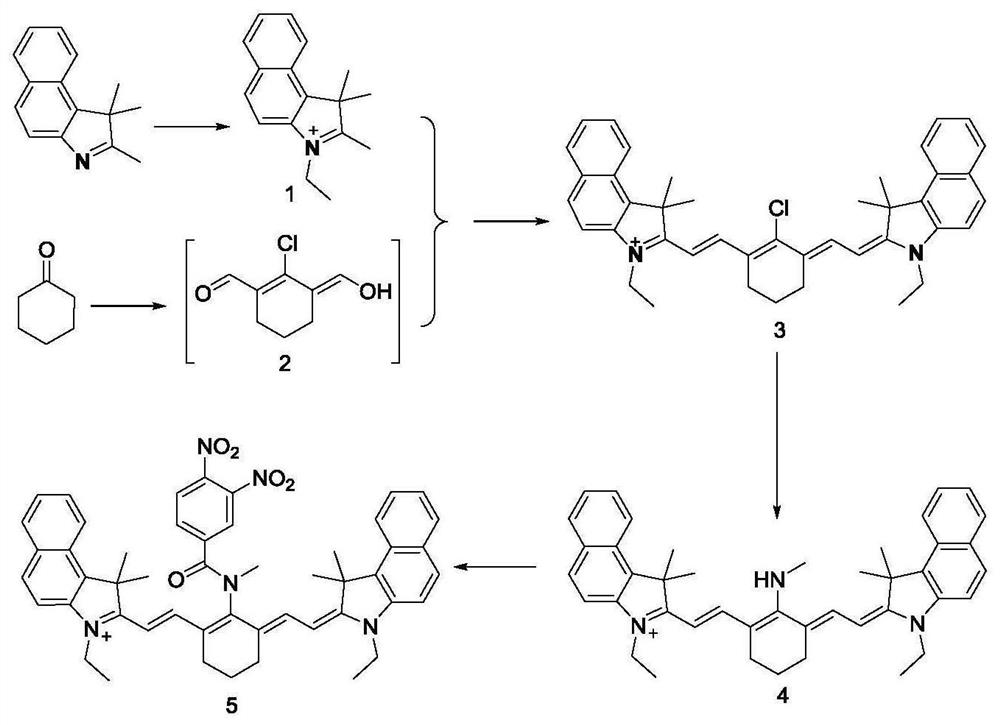
ActiveCN109776390BHigh sensitivityThe detection process is fastOrganic chemistryFluorescence/phosphorescenceCyclohexanoneTrimethyl benzene
The invention belongs to the field of analytical chemistry, and the fluorescent molecular probe for detecting glutathione thiol transferase is prepared by the following steps: dissolving 2,3,3-trimethyl-3H-benzindole and ethyl iodide React in anhydrous acetonitrile to obtain product 1; mix anhydrous N,N-dimethylformamide and anhydrous dichloromethane, add dropwise phosphorus oxychloride and anhydrous dichloromethane, add cyclohexanone, pour into Product 2 was collected on ice; product 1 and product 2 were dissolved in n-butanol and benzene solution to obtain product 3; product 3, methylamine hydrochloride and triethylamine were dissolved in anhydrous N,N-dimethylformamide 3,4-dinitrobenzoic acid and triphosgene were dissolved in anhydrous dichloromethane and mixed, N,N-diisopropylethylamine was added to react, and the obtained residue was dissolved in anhydrous Add N,N-diisopropylethylamine and 4-dimethylaminopyridine to dichloromethane water, dissolve the product 4 in dichloromethane and add the above mixture to obtain the molecular probe.
Owner:BINZHOU MEDICAL COLLEGE
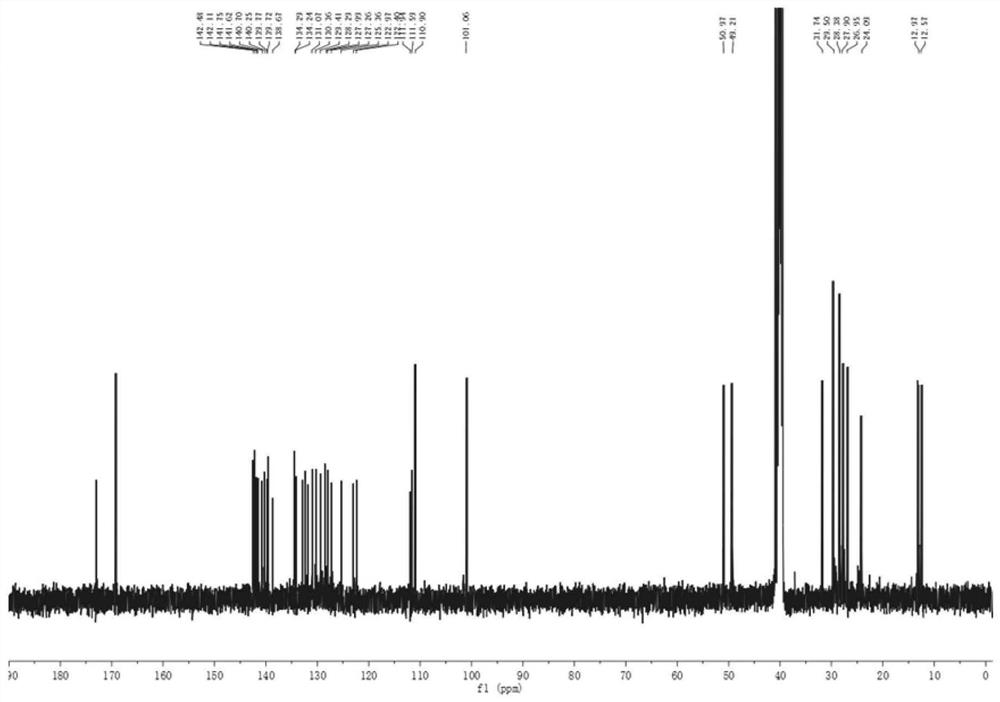
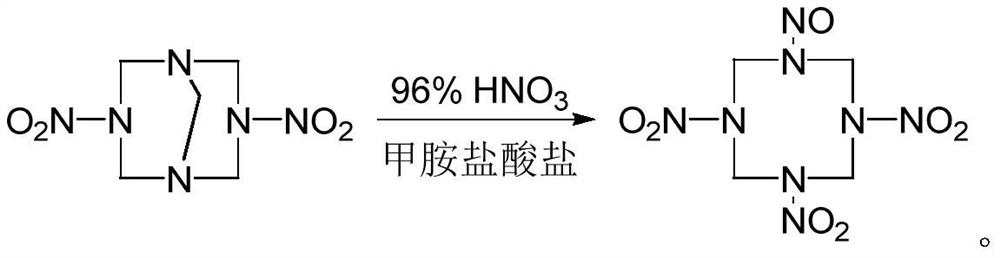
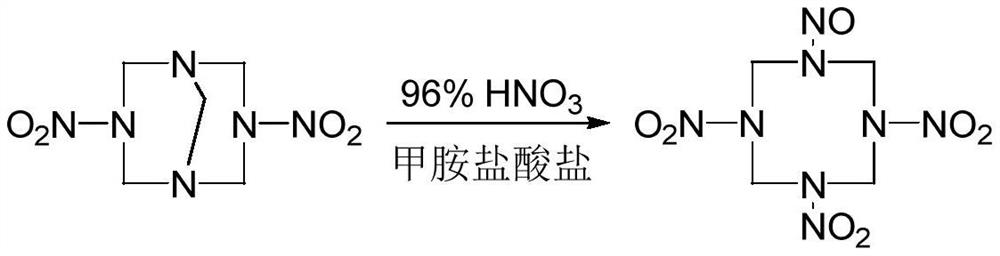
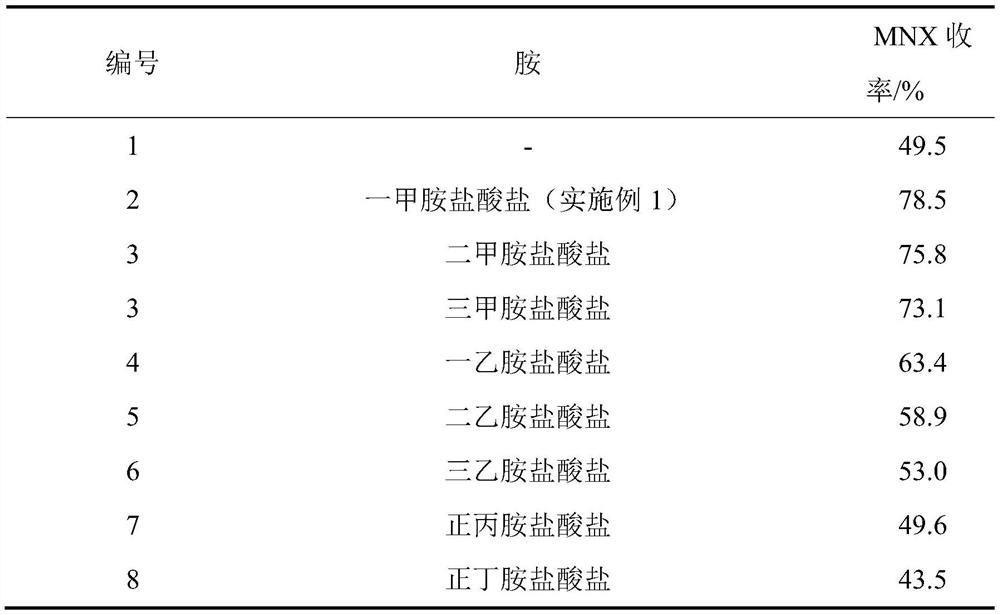
A kind of synthetic method of 1-nitroso-3,5,7-trinitro-1,3,5,7-tetraazacyclooctane
The invention discloses a method for synthesizing 1-nitroso-3,5,7-trinitro-1,3,5,7-tetraazacyclooctane. Its steps are: first at low temperature 3,7-dinitro-1,3,5,7-tetraazabicyclo [3.3.1] nonane in methylamine hydrochloride and mass fraction of 96% fume Carry out nitrolysis reaction and nitrolysis reaction in nitric acid system; slowly add water dropwise, filter, wash with water, and dry to obtain 1‑nitroso‑3,5,7‑trinitroso‑1,3,5,7‑tetraaza Cyclooctane. The method avoids the use of ammonium nitrate, the obtained product has high purity, and the yield reaches 78.5%; it also avoids the use of sodium nitrite or dinitrogen tetroxide, etc., which simplifies the production process, reduces production costs, and reduces environmental pollution; improves production safety .
Owner:NANJING UNIV OF SCI & TECH
Preparation method of vonorazan fumarate
ActiveCN106366071BAccelerated corrosionHigh yieldCarboxylic acid salt preparationSulfonyl chlorideVonoprazan
The invention concretely relates to a vonoprazan fumarate preparation method, and belongs to the field of medicines and chemical engineering. The method comprises the following steps: 1, carrying out condensation on 2-fluoroacetophenone used as an initial raw material and allylamine to obtain a compound IV; 2, carrying out a ring closing reaction on the compound IV under the catalysis of a copper catalyst in the presence of a ligand in order to obtain a compound V; 3, carrying out a sulfoamidation reaction on the compound V and pyridine-3-sulfonyl chloride to generate a compound VII; 4, carrying out a bromination reaction on the compound VII by using N-bromosuccimide in order to generate a compound VIII; 5, carrying out an amination reaction on the compound VIII and methylamine hydrochloride under the action of a catalyst and an alkali in order to obtain vonoprazan alkali; and 6, carrying out salt formation on the vonoprazan alkali and fumaric acid in order to obtain vonoprazan fumarate. The preparation method has the advantages of simplicity in operation, mild reaction conditions, high yield and high purity of the product, and easiness in industrial production.
Owner:SHANDONG JINCHENG PHARMACEUTICAL GROUP CO LTD
Preparation method of optically pure lobeline hydrochloride and enantiomer thereof
PendingCN113582912AHigh optical purityReduce processing costsOrganic chemistry methodsBulk chemical productionChemical synthesisPtru catalyst
The invention belongs to the technical field of chemical synthesis, and particularly relates to a preparation method of optically pure lobeline hydrochloride and an enantiomer of the optically pure lobeline hydrochloride. According to the method, benzoylacetic acid, methylamine hydrochloride and glutaraldehyde are used as raw materials, and a target product can be prepared through condensation, reduction, acylation, resolution, oxidation and hydrolysis reactions in sequence. According to the method, meso-racemic lobeline is derived into raceme through acylation by adopting a desymmetry strategy, so that conditions are created for further resolution, and the use of a chiral catalyst which is high in price and tedious in preparation is avoided. In the splitting step, the product is filtered, the obtained filtrate is subjected to hydrolysis and other treatments to obtain an intermediate compound II, and the intermediate compound II can be recycled, so that the process cost is greatly saved; and moreover, the whole preparation process flow is simple to operate, mild in condition, high in optical purity of the product and very suitable for large-scale industrial production.
Owner:GUANGDONG PHARMA UNIV
Preparation method of Dapagliflozin isomer impurities I
ActiveCN108314613ASpecific responseEasy to operateOrganic compound preparationCarbonyl compound preparation by condensationSolventCompound c
The invention discloses a preparation method of Dapagliflozin isomer impurities I. The preparation method comprises the following steps that a, 2-chlorine-5-bromobenzoic acid is dissolved in a first solvent; an acylation reagent is added; reaction is performed for 1 to 8h at 20 to 60 DEG C to generate a compound A; b, methoxyl methylamine hydrochloride is dissolved in a second solvent; alkali is added at -10 to 10 DEG C; then, the compound A capable of being dissolved in the second solvent is dropwise added; the temperature is maintained; stirring reaction is performed for 1 to 4h to obtain acompound B; c, a compound C is dissolved in a third solvent; a hexane solution of n-butyllithium is added at -78 DEG C; then, the compound B capable of being dissolved in the third solvent is dropwiseadded; after reaction, treatment is performed to obtain the Dapagliflozin isomer impurities I. The carbonyl substitution reaction is used, so that the reaction is exclusive; by-products are few; theoperation is simple; the process is stable; the repeatability is high; the total yield of the product is improved by 90 percent or higher; the cost is reduced; the commercialized production and the batch supply are facilitated.
Owner:SHENZHEN SUNGENING BIO-MEDICAL CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

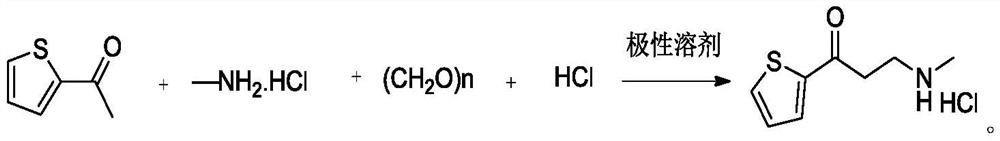
![2-[(2R, 6S)-6-[(2S)-2-hydroxy-2-phenethyl]-1-pipecoline]-1-hypnone synthesis method 2-[(2R, 6S)-6-[(2S)-2-hydroxy-2-phenethyl]-1-pipecoline]-1-hypnone synthesis method](https://images-eureka.patsnap.com/patent_img/277dcd7b-14f0-4e15-bb06-76cdb68e2149/HDA0001129246290000011.png)
![2-[(2R, 6S)-6-[(2S)-2-hydroxy-2-phenethyl]-1-pipecoline]-1-hypnone synthesis method 2-[(2R, 6S)-6-[(2S)-2-hydroxy-2-phenethyl]-1-pipecoline]-1-hypnone synthesis method](https://images-eureka.patsnap.com/patent_img/277dcd7b-14f0-4e15-bb06-76cdb68e2149/HDA0001129246290000021.png)
![2-[(2R, 6S)-6-[(2S)-2-hydroxy-2-phenethyl]-1-pipecoline]-1-hypnone synthesis method 2-[(2R, 6S)-6-[(2S)-2-hydroxy-2-phenethyl]-1-pipecoline]-1-hypnone synthesis method](https://images-eureka.patsnap.com/patent_img/277dcd7b-14f0-4e15-bb06-76cdb68e2149/HDA0001129246290000031.png)
![Method for synthesizing [<2>H3]-1-methylamino-2-phenylpropane Method for synthesizing [<2>H3]-1-methylamino-2-phenylpropane](https://images-eureka.patsnap.com/patent_img/9e93e590-12af-45c2-95dc-26116205556e/HDA0001635442650000011.png)
![Method for synthesizing [<2>H3]-1-methylamino-2-phenylpropane Method for synthesizing [<2>H3]-1-methylamino-2-phenylpropane](https://images-eureka.patsnap.com/patent_img/9e93e590-12af-45c2-95dc-26116205556e/HDA0001635442650000012.png)
![Method for synthesizing [<2>H3]-1-methylamino-2-phenylpropane Method for synthesizing [<2>H3]-1-methylamino-2-phenylpropane](https://images-eureka.patsnap.com/patent_img/9e93e590-12af-45c2-95dc-26116205556e/HDA0001635442650000021.png)