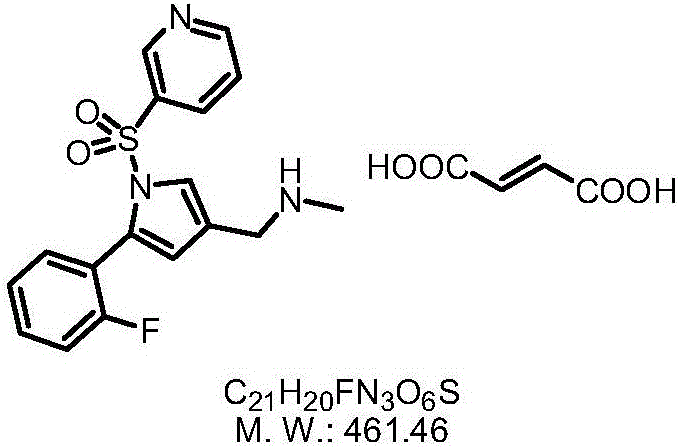
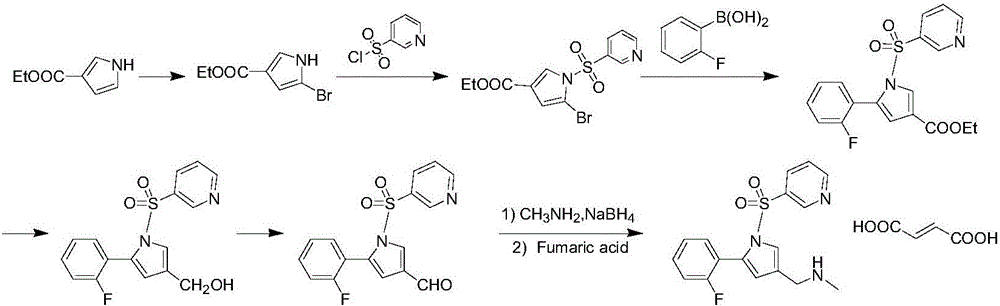
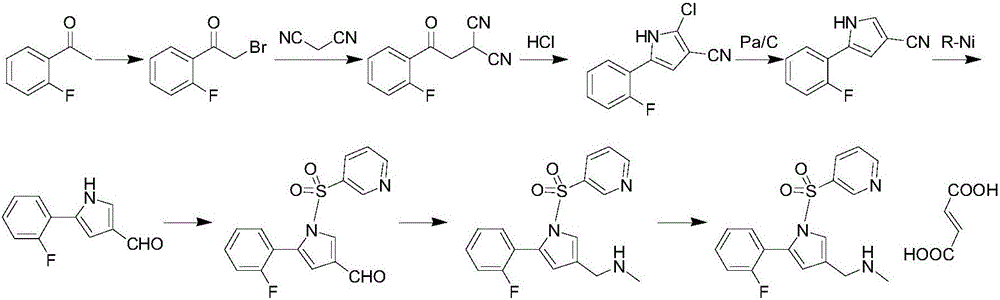
Vonoprazan fumarate preparation method
A technology for vornorazan fumarate and a compound is applied in the field of preparation of vornorazan fumarate, which can solve the problems of difficult operation, high equipment requirements, and high cost, and achieves reduction in the burden of post-reaction treatment and the reduction of The effect of operation difficulty and low equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Preparation of Compound IV:
[0039] In a 2000ml reaction bottle, add 138g of 2-fluoroacetophenone, 114g of allylamine, 966ml of absolute ethanol, and 10ml of glacial acetic acid, heat the reaction system to 50°C, keep it warm for 6h, and cool to At room temperature, a large amount of solids precipitated, stirred for 1 hour, then cooled to 0-10°C, kept warm for 1 hour, filtered with suction, rinsed the filter cake with cold ethanol, and dried to obtain 165 g of white solid, compound IV, with a yield of 93%.
Embodiment 2
[0041] Preparation method 1 of compound V:
[0042] In a 3000ml reaction flask, add 160g of compound IV, 8.5g of cuprous iodide, 285g of triphenylphosphine, and 640ml of dioxane. Under nitrogen protection, stir at 40°C for 16h. TLC detects the progress of the reaction. After the reaction is completed, cool Bring to room temperature, add 1280ml of water, then add 320ml of ethyl acetate to extract three times, combine the organic phase, add 240ml of 1% dilute hydrochloric acid to the organic phase, stir for 15min, separate the liquid, then wash the organic phase with 360ml*3 water for three times, and separate the liquid , the organic phase was concentrated to dryness to obtain a dark brown oil, which was dissolved by adding 240ml of ethanol, and 800ml of water was added dropwise with stirring at room temperature, and a solid precipitated out. The temperature was lowered to 5-10°C, kept for 1h, and suction filtered to obtain 135g of a light yellow solid. That is compound V, the ...
Embodiment 3
[0044] Preparation method 2 of compound V:
[0045]In a 3000ml reaction flask, add 160g of compound IV, 1.7g of cuprous iodide, 474g of triphenylphosphine, and 1600ml of dioxane, under the protection of nitrogen, stir at 60°C for 24h, check the progress of the reaction by TLC, after the completion of the reaction, cool Bring to room temperature, add 1280ml of water, then add 320ml of ethyl acetate to extract three times, combine the organic phase, add 240ml of 1% dilute hydrochloric acid to the organic phase, stir for 15min, separate the liquid, then wash the organic phase with 360ml*3 water for three times, and separate the liquid , the organic phase was concentrated to dryness to obtain a dark brown oil, which was dissolved by adding 240ml of ethanol, and 800ml of water was added dropwise with stirring at room temperature, and a solid precipitated out. The temperature was lowered to 5-10°C, kept for 1h, and suction filtered to obtain 127g of a light yellow solid. That is com...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com