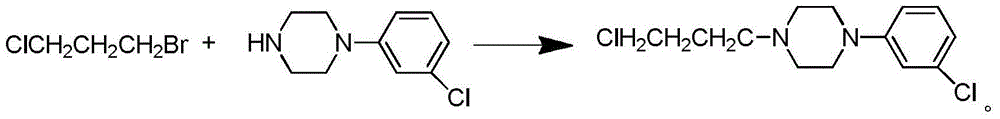
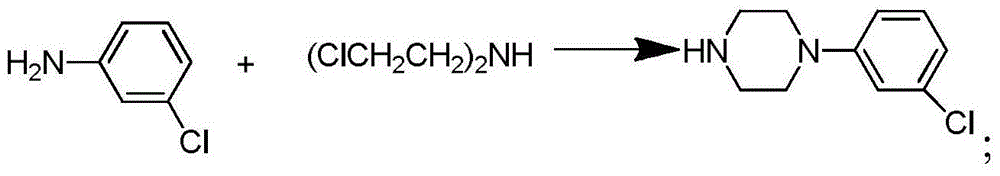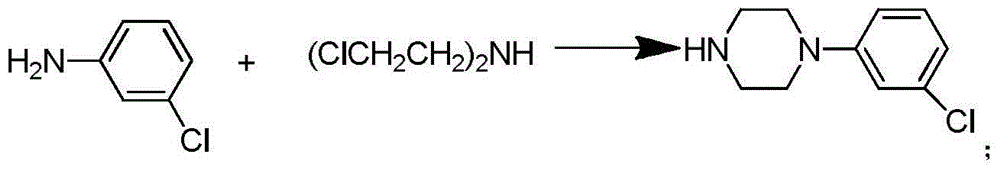Synthetic method of piperazidines drug intermediate
A synthesis method and intermediate technology, which are applied in the synthesis field of piperazine drug intermediates, can solve the problems of low product purity, harsh reaction conditions, complex synthesis route and the like, and achieve high product purity, simple route and simplified post-processing operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0015] The present invention will be described in further detail below in conjunction with specific examples.
[0016] Step (1): Synthesis of bis(2-chloroethyl)methylamine hydrochloride.
[0017] Add 24 g (0.23 mol) diethanolamine to 35 mL CHCl 3 , stirred, slowly dropwise added 65mL (0.92mol) thionyl chloride and 40mL CHCl 3 The mixture was added dropwise in 1 hour, reacted for 2 hours, and removed excess thionyl chloride and CHCl under reduced pressure 3 , to obtain a light yellow solid, which was recrystallized from acetone to obtain bis(2-chloroethyl)methylamine hydrochloride as a white solid, 25.6 g, with a yield of 62%.
[0018] Step (2): Synthesis of 1-(3-chlorophenyl)piperazine hydrochloride.
[0019] 2.2 g (17.2 mmol) of 3-chloroaniline and bis(2-chloroethyl)methylamine hydrochloride (17.2 mmol) were dissolved in 20 mL of xylene. Heat to reflux for 24 hours. Extracted with dichloromethane, dried over anhydrous magnesium sulfate, and distilled off under reduced pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com