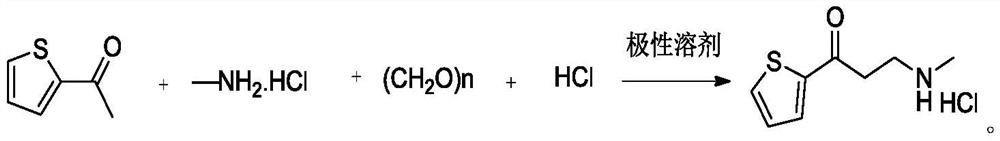Preparation method of duloxetine intermediate
A thienyl and methylamino technology, which is applied in the field of preparation of duloxetine intermediates, can solve the problems of high equipment and operation requirements, strong irritating odor, high environmental protection pressure and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
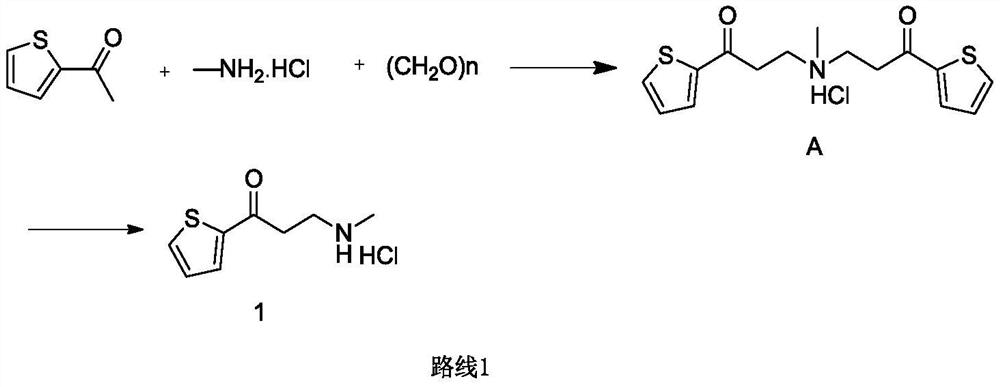
[0036] At room temperature, add 2-acetylthiophene (50.0g, 396mmol), paraformaldehyde (14.3g, 142.6mmol), methylamine hydrochloride (80.3g, 1188mmol), ethanol (500mL), hydrochloric acid (3.6g, 35.6mmol), reacted at 80°C for 16h, after the central control was qualified, added methylamine aqueous solution (7.4g, 59.4mmol), continued the reaction for 4h, lowered the temperature, and filtered to obtain a yellow crude product; the crude product was crystallized with ethanol (150mL), filtered 58 g of the compound of formula (1) was obtained with a purity of 98.6% and a yield of 86%.
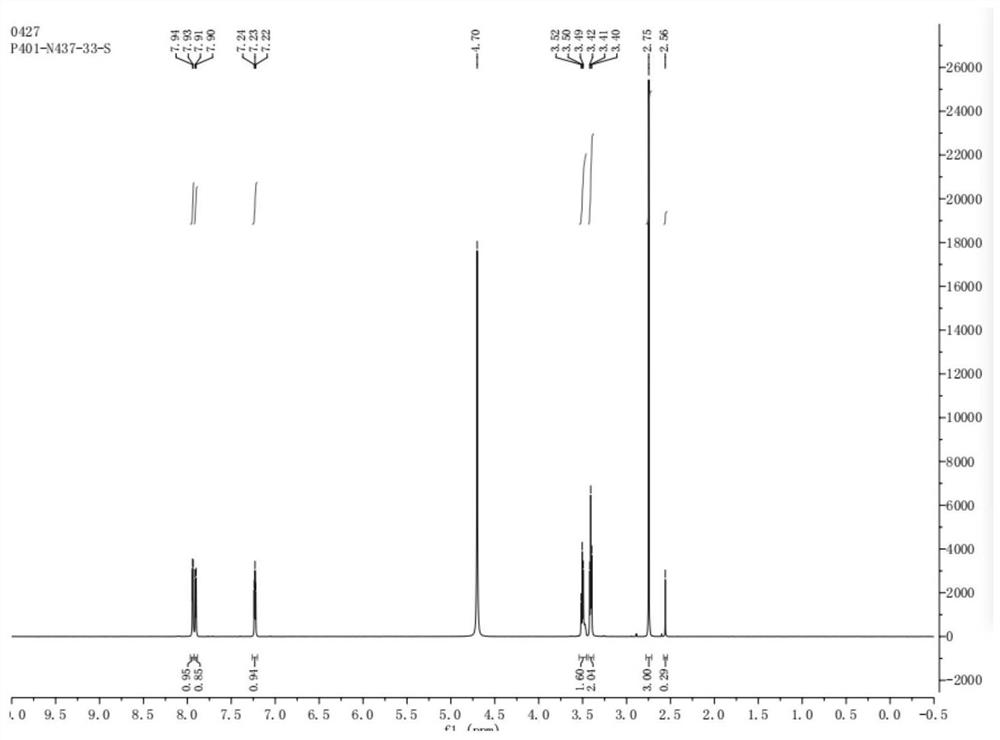
[0037] 1 H-NMR (500MHz, CD3OD) δ7.94~7.93(d,1H), 7.91~7.90(d,1H), 7.24~7.22(q,1H), 3.52~3.49(q,2H), 3.42~3.40( q,2H),2.75(s,3H).
[0038] 3-methylamino-1-(2-thienyl)-1-propanone hydrochloride prepared according to the application 1 H-NMR spectrum as figure 1 shown.
Embodiment 2
[0040] At room temperature, add 2-acetylthiophene (5.0g, 39.6mmol), paraformaldehyde (1.43g, 14.3mmol), methylamine hydrochloride (4.02g, 59.4mmol), ethanol and water in a 100ml reaction flask Mixed solvent (50ml, ethanol: water = 1:2), hydrochloric acid (0.36g, 3.56mmol), react at 80°C for 16h, after passing the central control, add methylamine aqueous solution (0.74g, 5.94mmol), continue the reaction for 4h, cool down , and filtered to obtain a yellow crude product; the crude product was crystallized with ethanol (15 mL), and filtered to obtain 4.6 g of the compound of formula (1), with a purity of 98.4% and a yield of 70%.
Embodiment 3
[0042] At room temperature, add 2-acetylthiophene (5.0g, 39.6mmol), paraformaldehyde (1.43g, 14.3mmol), methylamine hydrochloride (4.02g, 59.4mmol), water (50ml) into a 100ml reaction flask 1. Hydrochloric acid (0.36g, 3.56mmol), react at 80°C for 16h, after the central control is qualified, add methylamine aqueous solution (0.74g, 5.94mmol), continue the reaction for 4h, cool down, filter to obtain a yellow crude product; the crude product is crystallized with ethanol (15mL) , and filtered to obtain 4 g of the compound of formula (1), with a purity of 98.7% and a yield of 60%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com