Laminbutinyl bromooxamide derivatives with anticancer activity and composition thereof
A technology of bromoxamide and laminarin ester, which can be used in the field of drug synthesis and can solve problems such as NIK-3T3 toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
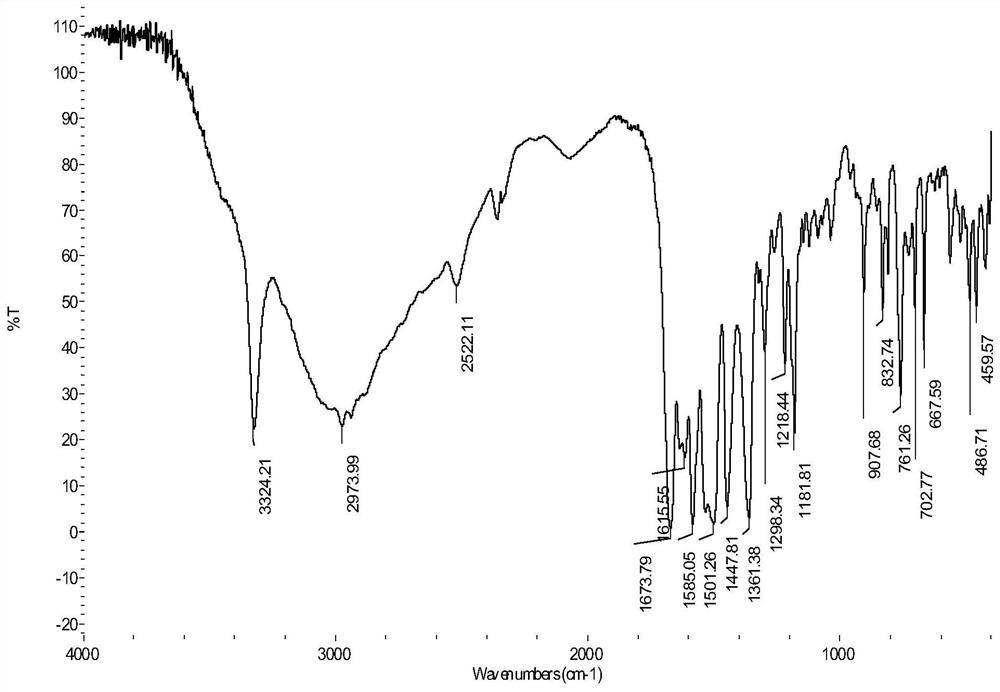
[0023] (1) Synthesis of 6-N,N,N-trimethyl-2-carbamic acid methyl ester hydrochloride: reflux 10mmol (2.24g) lamininine hydrochloride in thionyl chloride for 2h, add 50mmol (1.60g) Methanol, continue to reflux for 8h, evaporate the excess solvent, adjust the pH value to neutral with dilute hydrochloric acid, a white precipitate precipitates, filter, wash with ice ethanol and ether for several times, and dry in vacuum to obtain a white solid 1.62 g, yield 68.1%.
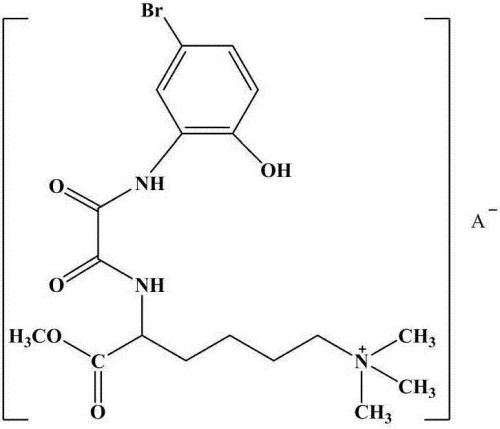
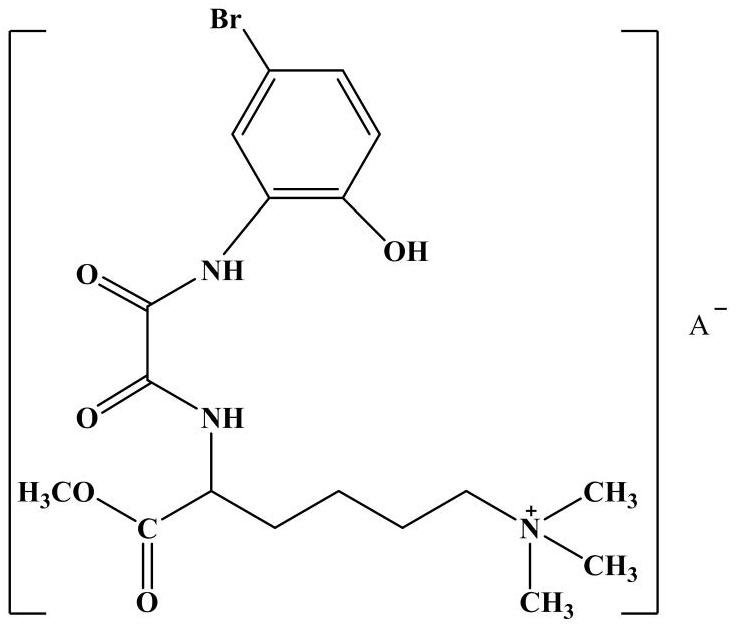
[0024] (2) Synthesis of lamininate-based bromooxamide derivatives: 29 mmol (8.19 g) of N-(5-bromo-2-hydroxyanilino) oxalyl ethyl ester was dissolved in 30 mL of absolute ethanol solution, Under ice-bath conditions, slowly dropwise added 32mmol (7.58g) of 6-N,N,N-trimethyl-2-carbamoylamide hydrochloride in anhydrous THF to maintain the reaction for 60min, The temperature was raised to 85°C and the reaction was heated for 6 hours. The pH value of the solution was adjusted to neutral with dilute hydrochloric acid, and a ...
Embodiment 2
[0027] (1) Synthesis of 6-N, N, N-trimethyl-2-carbamic acid methyl ester hydrochloride: same as Example 1, only the reflux time of lamininine hydrochloride in sulfur oxychloride is changed to 3h, the reflux reaction time after adding methanol was changed to 3h. 1.04 g of the target product was obtained with a yield of 43.7%.
[0028] (2) Synthesis of lamininic acid ester bromooxamide derivatives: with embodiment 1, only N-(5-bromo-2-hydroxyanilino) oxalyl ethyl ester and 6-N,N,N- The molar ratio of trimethyl-2-carbamoylamide hydrochloride was changed to 1:0.8, the reaction temperature was set at 35° C., and the reaction time was set at 10 h to obtain 4.68 g of the target product with a yield of 33.7%.
Embodiment 3
[0030] (1) Synthesis of 6-N, N, N-trimethyl-2-carbamic acid methyl ester hydrochloride: same as Example 1, only the reflux time of lamininine hydrochloride in sulfur oxychloride is changed to 2.4h, the reflux reaction time after adding methanol was changed to 8h. 1.36 g of the target product was obtained with a yield of 57.3%.
[0031] (2) Synthesis of lamininic acid ester bromooxamide derivatives: with embodiment 1, only N-(5-bromo-2-hydroxyanilino) oxalyl ethyl ester and 6-N,N,N- The molar ratio of trimethyl-2-carbamoylamide hydrochloride was set at 1:1, the reaction temperature was set at 85°C, and the reaction time was set at 4h. 7.17 g of the target product was obtained with a yield of 51.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com