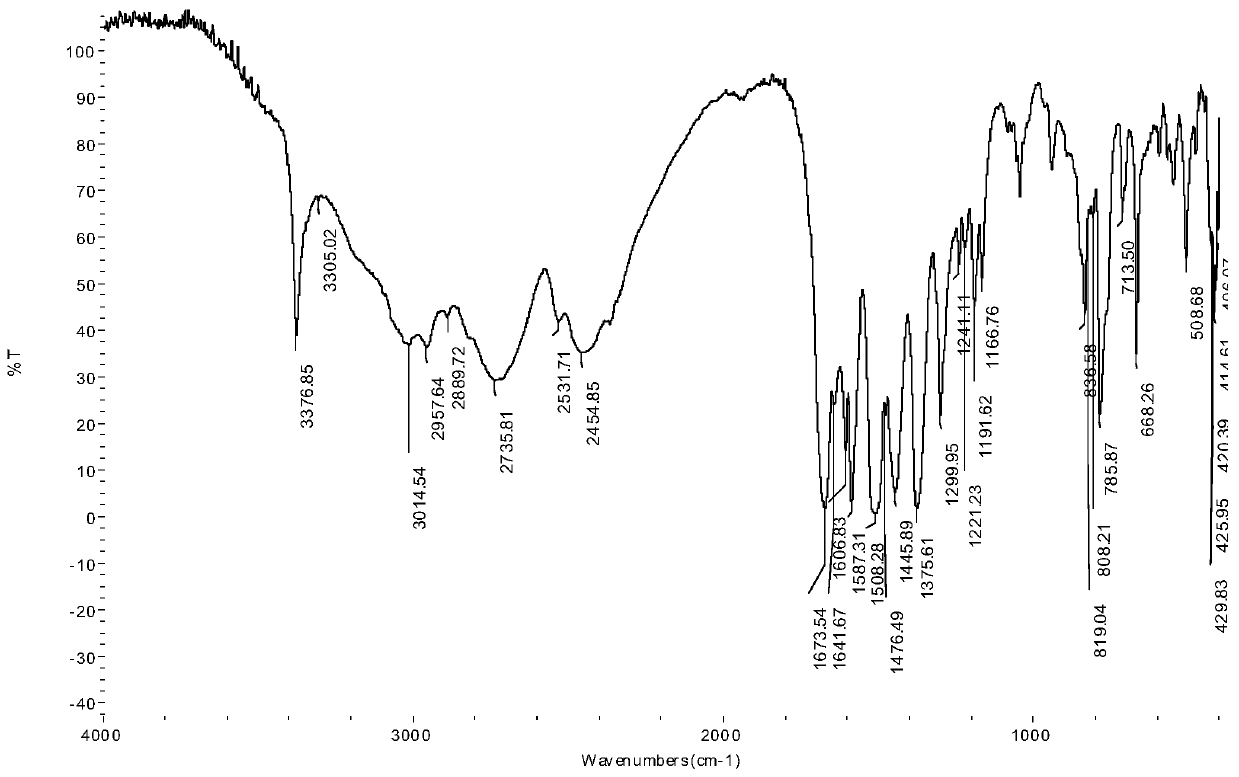
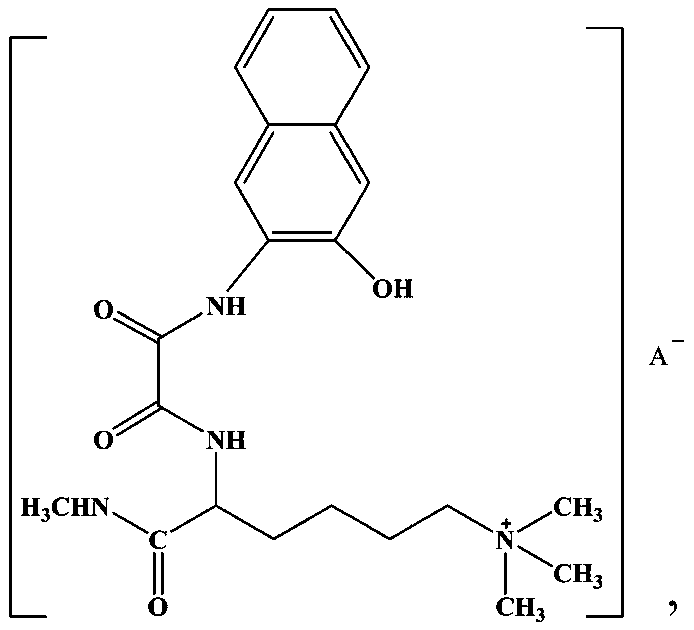
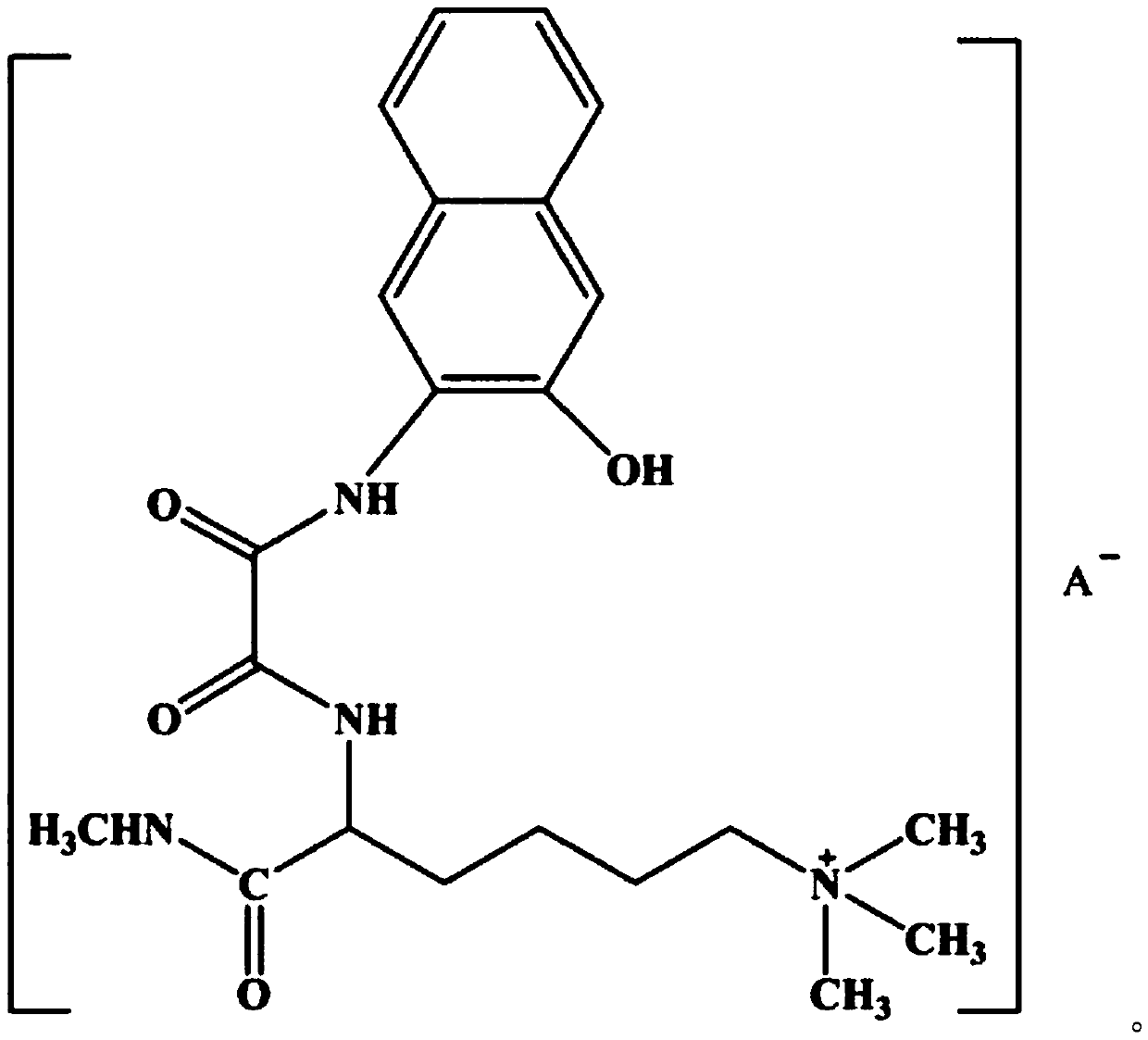
Lamininyl oxalamide with anticancer activity and its synthesis method and application
A technology of lamininate-based oxamide, which is applied in the field of lamininate-based oxalamide and its synthesis, can solve the problems of NIK-3T3 toxicity and other problems, and achieve the effect of expanding high value-added applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] (1) Synthesis of 6-N,N,N-trimethyl-2-carbamoylamide hydrochloride: Reflux 10mmol (2.24g) lamininine hydrochloride in thionyl chloride for 2h, add Dissolve 10mmol of methylamine (0.33g) in 20mL of tetrahydrofuran (THF) solution, continue to reflux for 5h, evaporate the excess solvent, adjust the pH value to neutral with dilute hydrochloric acid, a white precipitate precipitates, filter, and wash with ice ethanol, After washing with diethyl ether several times and drying in vacuo, 1.51 g of a white solid product—6-N,N,N-trimethyl-2-carbamoylamide hydrochloride was obtained, with a yield of 64.1%;
[0028] (2) Synthesis of lamininic acid ester oxalamide: 27 mmol (6.96 g) of N-(2-hydroxynaphthylamino) oxalyl ethyl ester was dissolved in 30 mL of absolute ethanol solution, and slowly Add it dropwise to anhydrous THF dissolved with 32mmol (7.58g) of 6-N,N,N-trimethyl-2-carbamoylamide hydrochloride to maintain the reaction for 60min, then raise the temperature to 60°C and heat...
Embodiment 2
[0031] (1) Synthesis of 6-N,N,N-trimethyl-2-carbamoylamide hydrochloride: Reflux 10mmol (2.24g) lamininine hydrochloride in thionyl chloride for 3h, add Dissolve 10mmol of methylamine (0.33g) in 20mL of tetrahydrofuran (THF) solution, continue to reflux for 5h, evaporate the excess solvent, adjust the pH value to neutral with dilute hydrochloric acid, a white precipitate precipitates, filter, and wash with ice ethanol, After washing with diethyl ether several times and drying in vacuo, 1.03 g of a white solid product—6-N,N,N-trimethyl-2-carbamoylamide hydrochloride was obtained, with a yield of 43.7%;
[0032] (2) Synthesis of lamininic acid ester oxalamide: 27 mmol (6.96 g) of N-(2-hydroxynaphthylamino) oxalyl ethyl ester was dissolved in 30 mL of absolute ethanol solution, and slowly added dropwise to anhydrous THF dissolved with 6-N,N,N-trimethyl-2-carbamoylamide hydrochloride with a molar ratio of 0.8 to maintain the reaction for 60 minutes, then heated to 30°C for 10 hour...
Embodiment 3
[0034] (1) Synthesis of 6-N,N,N-trimethyl-2-carbamoylamide hydrochloride: Reflux 10mmol (2.24g) lamininine hydrochloride in thionyl chloride for 8h, add Dissolve 10mmol of methylamine (0.33g) in 20mL of tetrahydrofuran (THF) solution, continue to reflux for 5h, evaporate the excess solvent, adjust the pH value to neutral with dilute hydrochloric acid, a white precipitate precipitates, filter, and wash with ice ethanol, After washing with diethyl ether several times and drying in vacuo, 1.03 g of a white solid product—6-N,N,N-trimethyl-2-carbamoylamide hydrochloride was obtained, with a yield of 55.3%;
[0035] (2) Synthesis of lamininic acid ester oxalamide: 27 mmol (6.96 g) of N-(2-hydroxynaphthylamino) oxalyl ethyl ester was dissolved in 30 mL of absolute ethanol solution, and slowly Added dropwise to anhydrous THF dissolved with 6-N,N,N-trimethyl-2-carbamoylamide hydrochloride with a molar ratio of 1.1 to maintain the reaction for 60 minutes, then heated to 90°C for 4 hours, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com