Preparation method of duloxetine key intermediate
A thienyl and methylamino technology, applied in the field of organic synthesis, can solve the problems of difficult separation and purification, unfavorable long-term use, cumbersome steps, etc., and achieves the effects of fewer reaction steps, economical savings, and simple process route.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
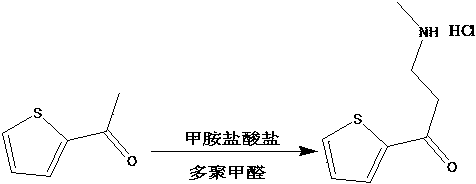
[0020] Example 1 Preparation of 3-N-methylamino-1-(2-thienyl)-1-acetone hydrochloride
[0021] Put 20ml of 2-acetylthiophene into a three-necked flask, add 160-200ml of methanol, 0.01-0.02g of concentrated hydrochloric acid, 16.2-25.7g of methylamine hydrochloride, and 6.8-8.6g of paraformaldehyde, and heat to reflux for reaction. After 9-10 hours, Crystals were precipitated, filtered, and recrystallized with pure water to obtain 32-33 g.
Embodiment 2
[0022] Example 2 Preparation of 3-N-methylamino-1-(2-thienyl)-1-acetone hydrochloride
[0023] Put 20ml of 2-acetylthiophene into a three-necked flask, add 160-200ml of ethanol, 0.01-0.02g of concentrated hydrochloric acid, 16.2-25.7g of methylamine hydrochloride, and 6.8-8.6g of paraformaldehyde, and heat to reflux for reaction. After 8-9 hours, Crystals were precipitated, filtered, and recrystallized with pure water to obtain 34-35 g.
Embodiment 3
[0024] Example 3 Preparation of 3-N-methylamino-1-(2-thienyl)-1-acetone hydrochloride
[0025] Put 20ml of 2-acetylthiophene in a three-necked flask, add 160-200ml of ethanol, 0.01-0.02g of concentrated sulfuric acid, 16.2-25.7g of methylamine hydrochloride, and 6.8-8.6g of paraformaldehyde, and heat to reflux for 9-10 hours. Crystals were precipitated, filtered, and recrystallized with pure water to obtain 35-36 g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com